Abstract
Diffuse large B-cell lymphoma (DLBCL) includes two prognostically-important subtypes, the germinal center B-cell (GCB) and the non-GCB types. The aim of this study was to evaluate immunohistochemical approaches for predicting the survival of patients with DLBCL following autologous hematopoietic stem cell transplantation (AHSCT). We identified 62 patients with DLBCL who either had an initial complete remission (17 patients) or received salvage chemotherapy for relapsed or refractory disease (45 patients), followed by AHSCT. Tissue microarrays were immunostained with monoclonal antibodies against GCET1, CD10, BCL6, MUM1, FOXP1 and LMO2. Using the Hans algorithm, we classified 50% of the cases as GCB type, whereas the Choi algorithm classified 58% as GCB type and LMO2 was positive in 69%. However, no significant differences were found in the five-year overall or event-free survivals using any of these approaches. In conclusion, cell-of-origin fails to predict survival of DLBCL patients treated with AHSCT.
Keywords: cell-of-origin, algorithm, immunophenotype, diffuse large B-cell lymphoma, autologous hematopoietic stem cell transplantation, survival
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) worldwide [1]. Morphological, biological and clinical studies have subdivided DLBCL into a variety of morphological variants and distinct disease entities [2]. However, a large number of cases do not belong to a specific disease entity and are classified as DLBCL, not otherwise specified. This group of lymphomas is biologically heterogeneous and constitutes 25-30% of adult NHL in western countries, and a higher percentage in developing countries [2].
Currently, the International Prognostic Index (IPI) is the most important tool used to predict the response to treatment and prognosis of patients with DLBCL [3]. However, even in the IPI risk groups, substantial variability in outcome has been observed. Gene expression profiling (GEP) for the cell-of-origin also has prognostic value in DLBCL independent of the IPI [4-6]. Patients with a GEP resembling that of germinal center B-cells (GCB) have a better outcome than those with a profile resembling activated B-cells (ABC). A cell-of-origin algorithm using only three immunostains can also successfully translate GEP data into practical application and subdivide DLBCL patients into similar prognostic groups, the GCB and the non-GCB types [7]. This algorithm is also useful when rituximab is added to standard chemotherapy [8]. Recently, a new immunostain algorithm using five antibodies was developed to improve the accuracy of this classification [9].
In addition to these cell-of-origin algorithms, studies looking for new prognostic markers have been conducted. One of these markers, LMO2, has been found to be a promising predictor of survival in de novo DLBCL [10]. LMO2 is a cysteine-rich LIM domain-containing transcription factor which plays a role in erythropoiesis, and is frequently involved in a chromosomal translocation in childhood T-cell acute lymphoblastic leukemia [11,12]. LMO2 is not expressed in normal T lymphocytes [13], but is expressed at high levels in germinal center B cells [4]. LMO2 expression is reported to be a predictor of survival in DLBCL, with positive immunostaining (>30%) correlating with longer survival [10].
Addition of rituximab (R) to the standard CHOP regimen (cyclophosphamide, adriamycin, vincristine and prednisone) increases the complete remission (CR) rate and improves the event-free survival (EFS) and overall survival (OS) of patients with DLBCL [14-16]. Both of the cell-of-origin algorithms and LMO2 expression have prognostic value for DLBCL patients receiving R-CHOP or similar regimens [8,17]. However, patients who are resistant to initial treatment or whose disease recurs after conventional therapy have smaller chance of a durable remission with salvage therapy [18-20]. In patients with a relapse of chemotherapy-sensitive DLBCL, high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) can lead to a cure in 40 -60% of cases and currently constitutes the standard of care [21].
The aim of this study was to evaluate the use of immunohistochemical approaches including the cell-of-origin algorithms of Hans et al [7] and Choi et al [9], and LMO2 expression alone [10], for predicting the survival of patients with DLBCL following AHSCT.
Materials and Methods
Patients
We identified patients diagnosed with DLBCL who received CHOP or R-CHOP chemotherapy as the first-line treatment. These patients either had an initial CR, with or without a subsequent relapse, or had primary refractory disease (primary induction failure – PIF). They were all later treated with high-dose salvage therapy followed by AHSCT. A total of 179 patients with DLBCL underwent AHSCT from 1996 through 2007 at the University of Nebraska Medical Center and met the above inclusion criteria. However, tissue blocks were only available from 85 patients. These patients were divided into three groups based on their treatment response prior to AHSCT. In Group 1, the patients were either in initial CR or had a relapse post-CR that was sensitive to salvage therapy. In Group 2, the patients had PIF with initial chemotherapy but were sensitive to salvage therapy. In Group 3, the patients had PIF or relapsed post-CR and were resistant to salvage therapy. The Institutional Review Board of the University of Nebraska Medical Center approved this study.
Immunohistochemistry on tissue microarrays
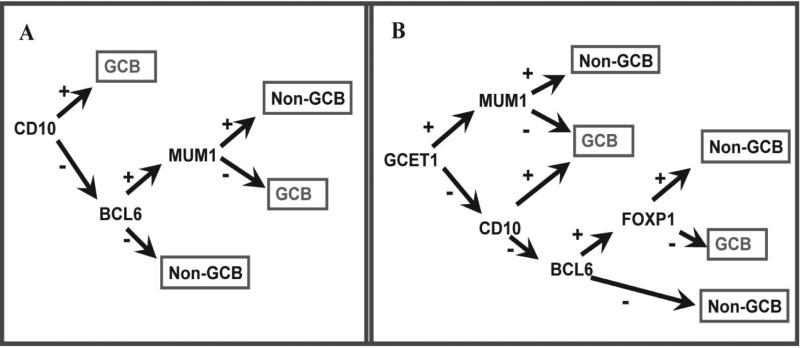
Tissue microarrays were prepared with three representative 0.6 mm tissue cores obtained from formalin-fixed, paraffin-embedded tissue blocks from each of the 85 cases with available blocks, as described previously [22]. Briefly, hematoxylin and eosin-stained slides from each tissue block were reviewed to define diagnostic areas of DLBCL. PAX5 and CD3 immunostains were used to aid in the interpretation of immunostains performed with monoclonal antibodies against GCET1, CD10, BCL6, MUM1, FOXP1 and LMO2 on tissue microarray sections using previously described methods [9,10]. Classification of DLBCL into the GCB and non-GCB types was performed using the algorithm of Hans et al [7], and the algorithm recently proposed by Choi et al [9] (Figure 1). Insert Figure 1. The positive cutoff for the antibodies in the Hans algorithm (CD10, BCL6 and MUM1) was 30%; for the Choi algorithm, we used a 30% cutoff for CD10 and BCL6, and 80% for the other stains (GCET1, FOXP1 and MUM1). A positive cutoff of 30% was used for LMO2 [10]. Among the 85 cases, only 62 cases yielded interpretable immunostaining results for all of these antibodies, and the rest of the cases were excluded from further analysis. In 50 cases (81%), tissue was available from the initial diagnostic biopsy for immunostaining. In the remaining 12 cases, the initial diagnostic tissue was not available and tissue from a later biopsy was used.
Figure 1.
Diagrams of the Hans (A) and Choi (B) algorithms based on the expression of three (CD10, BCL6 and MUM1) and five(CD10, BCL6, MUM1, GCET1 and FOXP1) markers, respectively
Statistical analysis
Patient characteristics were compared by clinical group, cell-of-origin subtype (GCB and non-GCB), and LMO2 expression using the ANOVA model and Fisher's exact test. Cumulative incidence estimation methods were used to estimate the two-year progression rates by group, which were compared with the log-rank test. The Kaplan-Meier method was used to estimate the OS and EFS distributions. OS was calculated as the time since transplantation to the date of death or last contact. Patients who were alive at last contact were treated as censored for OS analysis. EFS was calculated as the time since transplantation to the date of progression, death, or last contact. Patients who were alive at last contact and who had not progressed were treated as censored for EFS analysis. The log-rank test was used to compare survival distributions. All statistical tests were 2-sided and p-values of less than 0.05 were considered to be statistically significant. The data analysis was conducted using SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics
The median age of the patients at the time of AHSCT was 56 years (range, 17 – 75 years), and 42 patients (68%) were male (Table 1). Insert Table 1. Forty-nine patients (79%) received R-CHOP as the initial therapy and thirteen patients (21%) received only CHOP. Group 1 included 39 patients, of which 17 were in initial CR at the time of AHSCT. Group 2 included 12 patients, and Group 3 included 11 patients, seven of whom had PIF and four who relapsed post-CR with disease resistant to salvage therapy. There were no significant differences in the clinical characteristic between these groups. However, the patients in Group 3 tended to have more adverse prognostic factors and had a significantly higher two-year progression rate compared to Groups 1 and 2 (Table 1). We identified only five cases with immunoblastic morphology (5/62, 8.1%) including three cases in group 3, none in group 2, and two in group 1.
Table 1.
Patient characteristics by clinical groups.
| Total (n=62) | Group 1 (n=39) | Group 2 (n=12) | Group 3 (n=11) | P= | |
|---|---|---|---|---|---|
| Age at AHSCT (years) | |||||
| Median (range) | 56 (17-75) | 58 (17-75) | 49 (38-58) | 57 (30-67) | 0.35 |
| Gender | |||||
| Male | 42 (68%) | 24 (62%) | 10 (83%) | 8 (73%) | 0.40 |
| Female | 20 (32%) | 15 (38%) | 2 (17%) | 3 (27%) | |
| Receiving rituximab | 49 (79%) | 29 (74%) | 9 (75%) | 11 (100%) | 0.19 |
| Number of prior chemotherapy regimens | |||||
| 1 | 11 (20%) | 8 (24%) | 3(25%) | 0 ( 0%) | 0.023 |
| 2 | 27 (50%) | 20 (61%) | 4(33%) | 3 (33%) | |
| ≥3 | 16 (30%) | 5 (15%) | 5(42%) | 6 (67%) | |
| LDH at AHSCT | |||||
| Normal | 36 (62%) | 25 (69%) | 6 (55%) | 5(45%) | 0.28 |
| Elevated | 22 (38%) | 11 (31%) | 5 (45%) | 6(55%) | |
| Extranodal involvement at AHSCT | |||||
| No | 37 (69%) | 25 (74%) | 6 (50%) | 6 (75%) | 0.30 |
| Yes | 17 (31%) | 9 (26%) | 6 (50%) | 2 (25%) | |
| Stage at AHSCT | |||||
| CR | 12 (23%) | 11 (33%) | 0 (0%) | 1 (13%) | |
| I/II | 22 (41%) | 13 (39%) | 6 (50%) | 3(38%) | 0.15 |
| III/IV | 19 (36%) | 9 (27%) | 6 (50%) | 4(50%) | |
| 2-year progression | 33% | 21% | 42% | 64% | 0.0072 |
Table 1: Note: Clinical data were not available in some cases and, therefore, addition of the numbers may not equal the total number at the top. AHSCT: autologous hematopoietic stem cell transplantation, LDH: serum lactate dehydrogenase, CR: complete remission.
The clinical characteristics of the patients according to the cell-of-origin algorithms and LMO2 expression are given in Table 2. Insert Table 2. There were no significant differences in any of these characteristics in any of the groups, and no significant differences in the two-year progression rates were found. There was no enrichment of GCB cases in Groups 1 or 3, but there was an increased number of GCB cases in Group 2 (Table 2).
Table 2.
Patient characteristics according to the cell-of-origin algorithms and LMO2 expression.
| Total | Hans Algorithm | P= | Choi Algorithm | P= | LMO2 | LMO2 | P= | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=62) | GCB (n=31) | non-GCB (n=31) | GCB (n=36) | non-GCB (n=26) | positive (n=43) | negative (n=19) | ||||
| Age at AHSCT (years) | ||||||||||
| Median (range) | 56 (17-75) | 57 (22-75) | 54 (16-70) | 0.13 | 56 (22-75) | 55 (17-70) | 0.67 | 56 (22-75) | 55 (17-67) | 0.56 |
| Gender | ||||||||||
| Male | 42 (68%) | 23 (74%) | 19 (61%) | 0 28 | 26 (72%) | 16 (62%) | 0.37 | 30 (70%) | 12 (63%) | 0.61 |
| Female | 20 (32%) | 8 (36%) | 12 (39%) | 10 (28%) | 10 (38%) | 13 (30%) | 7 (37%) | |||
| Receiving rituximab | 49 (79%) | 23 (74%) | 26 (84%) | 0.35 | 28 (78%) | 21 (81%) | 0.78 | 33 (77%) | 16 (84%) | 0.51 |
| Numbers of prior chemotherapy regimens | ||||||||||
| 1 | 11 (20%) | 6 (21%) | 5 (20%) | 0.96 | 6 (19%) | 5 (23%) | 0.53 | 7 (18%) | 4 (25%) | 0.50 |
| 2 | 27 (50%) | 14 (48%) | 13 (52%) | 18 (56%) | 9 (41%) | 21 (55%) | 6 (38%) | |||
| ≥3 | 16 (30%) | 9 (31%) | 7 (28%) | 8 (25%) | 8 (36%) | 10 (26%) | 6 (38%) | |||
| Clinical groups | ||||||||||
| Group 1 | 39 (63%) | 17 (55%) | 22 (71%) | 21 (58%) | 18 (69%) | 27 (62%) | 12 (63%) | |||
| Group 2 | 12 (19%) | 9 (29%) | 3 (10%) | 0.15 | 10 (28%) | 2 ( 8%) | 0.13 | 8 (19%) | 4 (21%) | 1.0 |
| Group 3 | 11 (18%) | 5 (16%) | 6 (19%) | 5 (14%) | 6 (23%) | 8 (19%) | 3 (16%) | |||
| LDH at AHSCT | ||||||||||
| Normal | 36 (62%) | 20 (69%) | 16 (55%) | 0.28 | 23 (68%) | 13 (54%) | 0.30 | 24 (60%) | 12 (67%) | 0.63 |
| Elevated | 22 (38%) | 9 (31%) | 13 (45%) | 11(32%) | 11 (46%) | 16 (40%) | 6 (33%) | |||
| Extranodal involvement at AHSCT | ||||||||||
| No | 37 (69%) | 17 (63%) | 20 (74%) | 0.38 | 20 (65%) | 17 (74%) | 0.46 | 25 (66%) | 12 (75%) | 0.51 |
| Yes | 17 (31%) | 10 (37%) | 7 (26%) | 11 (35%) | 6 (26%) | 13 (34%) | 4 (25%) | |||
| Stage at AHSCT | ||||||||||
| CR | 12 (23%) | 6 (21%) | 6 (24%) | 6 (20%) | 6 (26%) | 9 (24%) | 3 (19%) | |||
| I/II | 22 (41%) | 12 (43%) | 10 (40%) | 0.97 | 12 (40%) | 10 (43%) | 0.75 | 14 (38%) | 8 (50%) | 0.71 |
| III/IV | 19 (36%) | 10 (36%) | 9 (36%) | 12 (40%) | 7 (30%) | 14 (38%) | 5 (31%) | |||
| 2-year progression | 33% | 39% | 26% | 0.12 | 36% | 28% | 0.30 | 28% | 43% | 0.52 |
Table 2: Note: Clinical data were not available in some cases and, therefore, addition of the numbers may not equal the total number at the top. AHSCT: autologous hematopoietic stem cell transplantation; LDH: serum lactate dehydrogenase; CR: complete remission; GCB: germinal center B-cell.
Survival by clinical groups
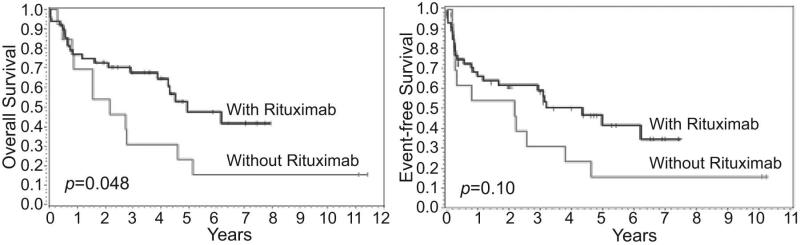
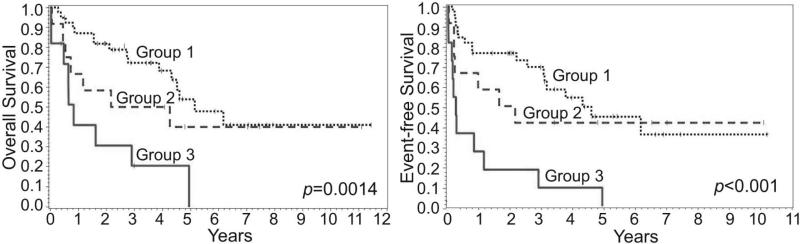
The survival after AHSCT of patients receiving either R-CHOP or CHOP as initial therapy was compared, and those treated with rituximab appeared to have a better OS (p=0.048) and in EFS (p=0.10) (Figure 2). Insert Figure 2. The survival after AHSCT among Groups 1-3 was also analyzed, and those in Group 3 with chemotherapy-resistant disease had the worst OS and EFS (Figure 3). Insert Figure 3.
Figure 2.
Overall and event-free survival of patients after autologous hematopoietic stem cell transplantation according to the initialuse of rituximab
Figure 3.
Overall and event-free survival of patients after autologous hematopoietic stem cell transplantation by clinical groups
Survival by immunophenotype groups
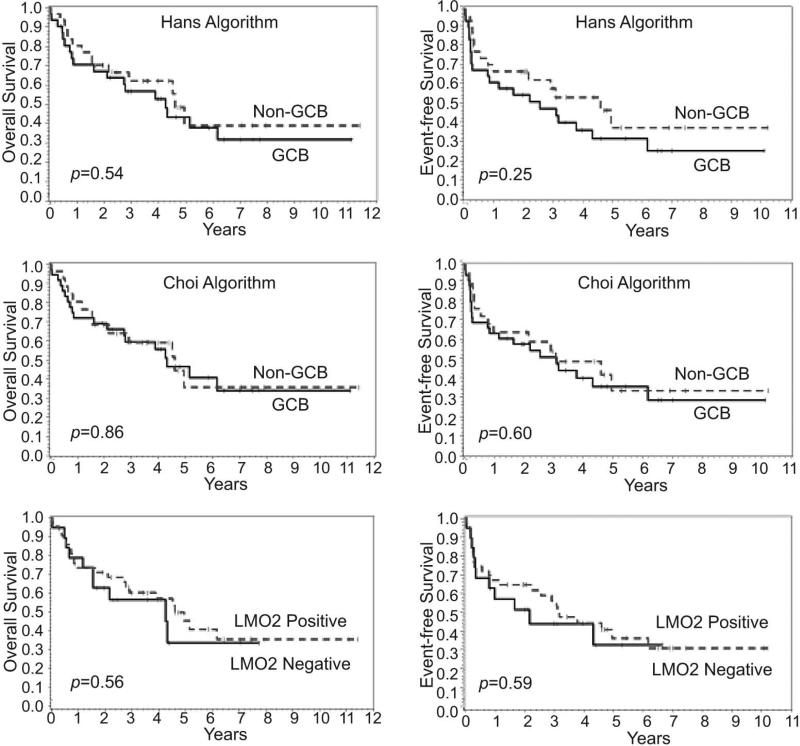
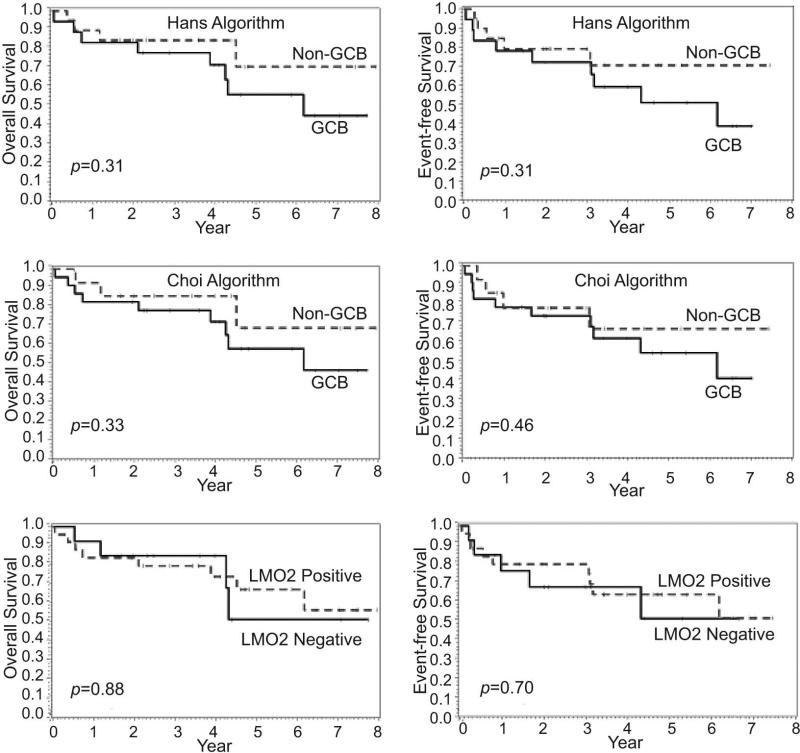
Using the Hans algorithm, 50% of the cases were classified as GCB type and 50% as non-GCB type, whereas the Choi algorithm classified 58% of the cases as GCB type and only 42% as non-GCB type (Table 2). However, no significant differences were found in the survival of patients after AHSCT using either of these cell-of-origin algorithms (Figure 4). Insert Figure 4. LMO2 was positive in 69% of the cases, but no significant difference was found in the survival of patients after AHSCT based on LMO2 expression (Figure 4).
Figure 4.
Overall and event-free survival of patients after autologous hematopoietic stem cell transplantation according to the cell-of-origin algorithms and LMO2 expression
Since significant survival differences were found for patients receiving R-CHOP versus CHOP (Figure 2), and for Groups 1 and 2 versus Group 3 (Figure 3), we performed additional survival analysis of the patients in Groups 1 and 2 who received R-CHOP as first-line treatment (38 patients). However, no significant differences were found in these cases based on the cell-of-origin algorithms or LMO2 expression (Figure 5). Insert Figure 5.
Figure 5.
Overall and event-free survival of patients after autologous hematopoietic stem cell transplantation according to the cell-of-origin algorithms and LMO2 expression for patients in Groups 1 and 2 receiving rituximab as initial treatment (38 patients)
Discussion
The IPI and GEP or cell-of-origin algorithms using immunostaining are currently the most important tools available to predict the response to chemotherapy and survival of newly-diagnosed patients with DLBCL. In DLBCL patients with chemosensitive disease at the time of progression or relapse, high-dose therapy (HDT) followed by AHSCT is considered the standard of care [21]. Also, for patients with poor-risk DLBCL in first CR or partial remission (PR), HDT and AHSCT is a feasible and effective treatment option [23]. It has been our policy at Nebraska to do HDT and AHSCT for patients with DLBCL in first CR who were intermediate/high or high risk by the IPI. However, few biological markers to predict outcome have been evaluated in DLBCL patients receiving AHSCT.
A few studies have used cell-of-origin algorithms in an attempt to predict the outcome of patients with relapsed or refractory DLBCL following AHSCT. Using the Hans algorithm [7], Moskowitz et al [24] found no difference in OS by cell-of-origin for patients with relapsed or primary refractory chemosensitive disease following AHSCT. Similarly, Costa et al [25] found no difference in the risk of progression or in OS for patients with relapsed or primary refractory chemosensitive disease following AHSCT using the same algorithm. However, in a series of poor-risk DLBCL patients treated with AHSCT as first line therapy, van Imhoff et al [26] found that the GCB type had a favorable impact on OS using the Hans algorithm. In contrast, Nyman et al [27] failed to confirm this finding in a similar group of high-risk DLBCL patients treated with AHSCT as first-line therapy, and concluded that dose intensification seems to eliminate the adverse prognostic impact of the non-GCB type in high-risk DLBCL.
To address this issue, we used two cell-of-origin algorithms, Hans et al [7] and Choi et al [9], to investigate the outcome of DLBCL patients who underwent AHSCT. Since the phenotype (GCB or non-GCB) present at initial diagnosis appears to be unchanged at the time of progression [24,25], we included 12 cases with only tissue obtained during progression. However, neither of these algorithms or LMO2 expression predicted the survival of our patients, confirming other similar studies using the Hans' algorithm [24,25]. We are not able to address this issue in patients receiving AHSCT as part of first-line therapy due to the small number of patients in our series. However, Hallack Neto et al [28] have suggested that AHSCT in first CR improves survival regardless of cell of origin. Further studies are needed to clarify the findings for AHSCT as first-line therapy in DLBCL.
As expected, we found that patients with PIF and those who relapsed post-CR and were resistant to standard salvage therapy (Group 3) derived no benefit from AHSCT. In this group, all 11 patients had received rituximab as part of their initial treatment. Therefore, it appears that AHSCT will not improve the survival outcome of such patients, and other innovative salvage therapies should be attempted.
A recent study found that AHSCT was equally effective in DLBCL patients in relapse after first-line therapy whether they had received rituximab or not, and should remain the standard of care for relapsed DLBCL [29]. However, we found that a major predictive factor for patients receiving AHSCT for DLBCL was the use of rituximab as part of initial therapy (Figure 2). This finding is in keeping with the results of a large study which concluded that pre-transplant rituximab is associated with a lower rate of progression and improved survival following AHSCT [30]. Therefore, we also performed a survival analysis of our patients in Groups 1 and 2 who received R-CHOP as first-line treatment (38 patients, Figure 5). However, neither of the cell-of-origin algorithms or LMO2 expression was predictive of outcome in this favorable subgroup.
In the recent CORAL (Collaborative Trial in Relapsed Aggressive Lymphoma) study, Gisselbrecht et al [31] found that patients with DLBCL who were initially treated with a CHOP-like regimen and rituximab before AHSCT had a significantly worse response rate and survival than those who had not received rituximab initially. All of the patients received rituximab as part of HDT prior to AHSCT. However, this adverse effect was only seen in the patients who relapsed early (<12 months) after initial therapy with rituximab, and not in those who relapsed later. In our study, the small number and heterogeneous nature of the patients precluded such a detailed analysis.
LMO2 expression has been reported to be a useful tool in predicting the survival in DLBCL patients who receive CHOP or R-CHOP therapy [10,17]. The present study is the first, to our knowledge, to explore the use of LMO2 in predicting the outcome of patients with DLBCL receiving AHSCT. However, our data indicate that LMO2 expression does not predict the survival of the patients with DLBCL receiving AHSCT.
In summary, neither the cell-of-origin algorithms nor LMO2 expression predicted the survival of patients with DLBCL receiving AHSCT in this patient cohort. However, the number of patients in this study was small and our results should be confirmed by larger studies.
Acknowledgements
This work was supported in part by an NCI grant (5U01/CA 114778)
Footnotes
Conflict of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9(7):717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 2.Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues Lyon. IARC Press; 2008. pp. 233–237. [Google Scholar]
- 3.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 6.Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 8.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26(28):4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 9.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15(17):5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350(18):1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 11.Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A. 1991;88(10):4367–4371. doi: 10.1073/pnas.88.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren AJ, Colledge WH, Carlton MB, et al. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78(1):45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 13.Royer-Pokora B, Loos U, Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene. 1991;6(10):1887–1893. [PubMed] [Google Scholar]
- 14.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15(10):3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 15.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188–2195. [PubMed] [Google Scholar]
- 16.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 17.Natkunam Y, Farinha P, Hsi ED, et al. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol. 2008;26(3):447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- 18.Cabanillas F, Hagemeister FB, McLaughlin P, et al. Results of MIME salvage regimen for recurrent or refractory lymphoma. J Clin Oncol. 1987;5(3):407–412. doi: 10.1200/JCO.1987.5.3.407. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez M, Chabner BA, Pearson D, et al. Role of a doxorubicin-containing regimen in relapsed and resistant lymphomas: an 8-year follow-up study of EPOCH. J Clin Oncol. 2000;18(21):3633–3642. doi: 10.1200/JCO.2000.18.21.3633. [DOI] [PubMed] [Google Scholar]
- 20.Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988;71(1):117–122. [PubMed] [Google Scholar]
- 21.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal J, Greiner TC, Patel K, et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. 2007;21(11):2332–2343. doi: 10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papajik T, Raida L, Faber E, et al. High-dose therapy and autologous stem cell transplantation in patients with diffuse large B-cell lymphoma in first complete or partial remission. Neoplasma. 2008;55(3):215–221. [PubMed] [Google Scholar]
- 24.Moskowitz CH, Zelenetz AD, Kewalramani T, et al. Cell of origin, germinal center versus nongerminal center, determined by immunohistochemistry on tissue microarray, does not correlate with outcome in patients with relapsed and refractory DLBCL. Blood. 2005;106(10):3383–3385. doi: 10.1182/blood-2005-04-1603. [DOI] [PubMed] [Google Scholar]
- 25.Costa LJ, Feldman AL, Micallef IN, et al. Germinal center B (GCB) and non-GCB cell-like diffuse large B cell lymphomas have similar outcomes following autologous haematopoietic stem cell transplantation. Br J Haematol. 2008;142(3):404–412. doi: 10.1111/j.1365-2141.2008.07207.x. [DOI] [PubMed] [Google Scholar]
- 26.van Imhoff GW, Boerma EJ, van der Holt B, et al. Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(25):4135–4142. doi: 10.1200/JCO.2006.05.5897. [DOI] [PubMed] [Google Scholar]
- 27.Nyman H, Jantunen E, Juvonen E, et al. Impact of germinal center and non-germinal center phenotypes on overall and failure-free survival after high-dose chemotherapy and auto-SCT in primary diffuse large B-cell lymphoma. Bone Marrow Transplant. 2008;42(2):93–98. doi: 10.1038/bmt.2008.92. [DOI] [PubMed] [Google Scholar]
- 28.Hallack Neto AE, Dulley FL, Coelho Siqueira SA, et al. Prognostic impact of diffuse large B-cell lymphoma subgroups in patients undergoing autologous SCT. Bone Marrow Transplant. 2009;43(4):323–325. doi: 10.1038/bmt.2008.330. [DOI] [PubMed] [Google Scholar]
- 29.Smith SD, Bolwell BJ, Rybicki LA, et al. Comparison of outcomes after auto-SCT for patients with relapsed diffuse large B-cell lymphoma according to previous therapy with rituximab. Bone Marrow Transplant. 2010:1–5. doi: 10.1038/bmt.2010.95. [DOI] [PubMed] [Google Scholar]
- 30.Fenske TS, Hari PN, Carreras J, et al. Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2009;15(11):1455–1464. doi: 10.1016/j.bbmt.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]