Abstract
Acute viral bronchiolitis is a leading cause of admission to pediatric intensive care units, but research on the care of these critically ill infants has been limited. Pathology of viral bronchiolitis revealed respiratory obstruction due to intraluminal debris and edema of the airways and vasculature. This and clinical evidence suggest that airway clearance interventions such as hypertonic saline nebulizers and pulmonary toilet devices may be of benefit, particularly in situations of atelectasis associated with bronchiolitis. Research to distinguish an underlying asthma predisposition in wheezing infants with viral bronchiolitis may one day lead to guidance on when to trial bronchodilator therapy. Considering the paucity of critical care research in pediatric viral bronchiolitis, intensive care practitioners must substantially rely on individualization of therapies based on bedside clinical assessments. However, with the introduction of new diagnostic and respiratory technologies, our ability to support critically ill infants with acute viral bronchiolitis will continue to advance.
Keywords: Respiratory syncytial virus, Rhinovirus, Asthma, Hypertonic nebulized saline, Acute viral bronchiolitis
Core tip: Pediatric acute viral bronchiolitis is characterized by small airways obstruction due to inflammatory infiltrates and debris. While this pathology has little or no overlap with asthma, the clinical presentation of wheezing may be similar. Emerging methods to distinguish asthmatics from the general bronchiolitis population, stratify patients according to illness severity, and provide more effective pulmonary clearance and respiratory support may improve outcomes for these patients in the pediatric intensive care unit.
INTRODUCTION AND PURPOSE OF THIS PAPER
Widely recognized as the most common cause of hospitalization for infants, bronchiolitis is responsible for more than 100000 hospitalizations annually and poses a significant risk for respiratory failure requiring mechanical ventilation in infants[1]. Approximately 5% to 30% of infants hospitalized with bronchiolitis require pediatric intensive care[2-4]. To address the needs of this patient population, many institutions have established bronchiolitis order sets and pathways. A number of issues now prompt the need to update and reconsider the implementation of bronchiolitis pathways: institution of new electronic medical systems under the Meaningful Use program[5], the burgeoning pediatric hospitalist movement[6], a national trend toward protocolized and evidence-based hospital care, and the recent publication of an updated AAP bronchiolitis guideline[7].
CLINICAL PRESENTATION AND PATHOLOGY
Bronchiolitis is typically recognized clinically by the presence of wheeze, signs and symptoms of upper and lower respiratory tract infection, and respiratory distress[7]. Apnea can be a major finding, especially in younger infants[8]. Pathological studies of fatal RSV bronchiolitis have revealed multiple contributing factors to obstruction of small to large-sized airways: intraluminal debris, airway wall edema, and compression by edematous bronchial arteries and inflammatory peri-bronchial lymphoid follicular aggregates (Figure 1). The intraluminal debris may be composed of mucus, fibrin, epithelial cells, and inflammatory cells[9].
Figure 1.
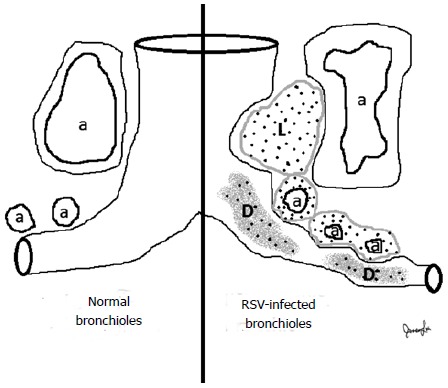
Respiratory syncytial virus infection is associated with vasocentric inflammation affecting bronchioles. Lymphoid aggregates (L), probably developing from bronchiolar-associated lymphoid tissue, are found between pulmonary arteries/arterioles (a) and bronchioles. Congested arterioles surrounding bronchioles contribute to airways obstruction, along with intraluminal debris (D) consisting of mucus, fibrin, epithelial cells, and inflammatory cells. While neutrophils are occasionally obtained from bronchoalveolar lavage, macrophages are the predominant inflammatory cell type in the submucosal infiltrates and intraluminal locations.
MICROBIOLOGY
Etiologic agents of bronchiolitis include most prominently respiratory syncytial virus (RSV) and rhinovirus[7]. Additional viruses implicated in acute bronchiolitis include parainfluenza virus, influenza virus, human metapneumovirus, bocavirus, adenovirus, and coronavirus[7,10].
What is the significance of viral identification in acute bronchiolitis The triple mission of academic health centers is to deliver leading-edge patient care, conduct research, and educate. In the United States, more than 500 clinical laboratories, many of which are maintained by academic centers, participate in the National Respiratory and Enteric Virus Surveillance System (NREVSS). The Centers for Disease Control and Prevention (CDC) relies on NREVSS to monitor temporal and geographic patterns of relevant virus infections. These viruses significantly include RSV, human metapneumovirus, respiratory adenovirus, and human parainfluenza virus. This surveillance system constitutes an important part of the CDC’s efforts to prevent and control respiratory and enteric viral diseases. For instance, an outbreak of enterovirus D68 in 2014 was identified in the Midwestern United States and subsequently spread throughout the United States. Enterovirus D68 caused unusually severe respiratory illnesses, with almost all confirmed cases confined to children. Although the disease was not reportable nationally, “laboratory detections of enterovirus… are reported voluntarily to (NREVSS)…. (Suspected) clusters or outbreaks should be reported to local or state health department”[11]. Identifying viruses that cause illnesses resembling asthma exacerbations is important for the overall scientific goal of understanding respiratory diseases in general. This leads to the philosophic question of whether microbiological investigation of bronchiolitis-causing viruses at centers participating in NREVSS should be regarded differently from nonparticipating centers. Viral identification is an important part of an academic institution’s broader societal, educational, and research mission. Additionally, for the patient’s family, knowledge that their hospitalized child has RSV or non-RSV virus infection may provide important prognostic information in terms of well-studied risk factors for mortality associated with RSV, potential for longer hospitalization with RSV vs rhinovirus[7], reduced likelihood of bacterial infection in non-critically ill patients with community-acquired pneumonia[12], and potentially increased risk of asthma associated with rhinovirus[13,14]. Finally, a virology-positive diagnosis of RSV or another well-established bronchiolitis-causing agent would possibly help to distinguish cases of bronchiolitis from asthmatics with first-time acute wheeze, although this issue remains under investigation (see section on Asthma below).
What is the risk of RSV reinfection The most recent AAP guidelines suggest that RSV prophylaxis may be discontinued after breakthrough RSV infection[7]. We conducted a literature search to clarify the reinfection risk of RSV in infants receiving palivizumab prophylaxis who experience an acute episode of bronchiolitis. Previous literature has demonstrated that RSV infection is highly prevalent and only partially limited by acquired immunity (Table 1). Risk of reinfection may be related to serum titers of RSV-specific antibody[15,16]. These data suggest that the reinfection risk for RSV in the absence of ongoing passive immunization is high, is correlated with diminished serum titers of RSV-neutralizing antibodies, and can occur within the same RSV season. To our knowledge, neither the effective development of adaptive host immunity to RSV infection in the setting of passive immunization, nor the incidence of RSV reinfection after palivizumab withdrawal has been reported.
Table 1.
Respiratory syncytial virus reinfection risk
| Ref. | Year | n | Reinfection risk |
| Henderson et al[39] | 1979 | 78 | 74% by age 2 if infected in 1st year of age |
| Glezen et al[40] | 1986 | 125 | 76% by 24 mo age if infected before 12 mo age |
| Hall et al[15] | 1991 | 15 | 50% at first challenge with RSV 2 mo after initial infection |
| Kawasaki et al[16] | 2004 | 165 | 25% within a year of first RSV infection |
n: Total cohort of subjects studied; RSV: Respiratory syncytial virus.
SCORING SYSTEM
A scoring system for bronchiolitis would help to standardize care and potentially improve outcomes. Unfortunately, no clinical scoring system has been appropriately validated for reliability, physiologic correlation, and clinical course[17]. The original basis of bronchiolitis scores was physical examination of respiratory distress using commonly assessed clinical variables[18]. Over time, continuing reassessment of bronchiolitis scoring has made apparent that the most common scoring system for bronchiolitis, the RDAI, has poor construct validity for overall respiratory status and limited discriminative ability to determine major clinical outcomes like length of stay[19]. Recent efforts have focused on modeling clinical indicators associated with worse clinical outcomes. A secondary analysis of a randomized, controlled multicenter trial in 20 emergency departments related to bronchiolitis concluded that oxygen saturation was the best predictor of hospitalization and length of stay[20]. Among previously healthy infants with RSV bronchiolitis who were admitted to a single academic center, risk factors for respiratory failure that were identified in the emergency department included lethargy, grunting, and PaCO2 ≥ 65 mmHg[4]. Prodhan and colleagues also noted that among RSV-infected infants admitted to the intensive care unit with respiratory failure, the major radiologic predictor of prolonged mechanical ventilation was atelectasis, not hyperinflation[21]. Walsh et al[22] validated a model to predict admission from the emergency department based on age, dehydration, work of breathing, and initial heart rate. Weisgerber et al[23] developed a model to predict prolonged length of stay based on need for supplemental oxygen, respiratory rate, gestation, and caloric intake. The topic of predictive modeling for bronchiolitis has recently been reviewed systematically[24]. Development of a clinical score for bronchiolitis that accurately reflects relevant indicators of bronchiolitis outcomes could potentially enable research on earlier interventions to ameliorate or prevent critical bronchiolitis disease.
ASTHMA EVALUATION IN EARLY CHILDHOOD
The key differential diagnosis when evaluating a wheezing infant with viral respiratory disease is asthma exacerbation. Viral respiratory infections - especially RSV, parainfluenza, and rhinovirus - are identified by the Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma to be “one of the most important causes of asthma exacerbation and may also contribute to the development of asthma”[25]. Approximately 40% of infants hospitalized with RSV may continue to wheeze or have asthma even into young adulthood[26]. Importantly, children who develop asthma symptoms before the age of 3 years are more likely to experience declines in lung function growth than those who develop asthma symptoms after 3 years of age[25]. Thus, efforts to predict development of childhood asthma are ongoing. While a thorough review of asthma prediction is beyond the scope of this editorial, we present as examples 3 asthma prediction tools in Table 2[27-29]. The wide range of predictive values is notable, which may be attributable to the different age ranges and clinical baseline of the analyzed patient cohorts. As efforts continue to determine whether early anti-inflammatory therapies can alter the decline in lung function growth[30] associated with early childhood asthma, it seems likely that research will return to focus on wheezing associated with preschool viral illness. The proscription against a bronchodilator trial in the latest AAP bronchiolitis guideline - regardless of history of recurrent wheeze, atopy, or family history of asthma - will need to be reconciled with both asthma biology and more long-term efforts to modify the natural history of childhood asthma.
Table 2.
Selected asthma prediction tools
| Ref. | Clough et al[29] | Castro-Rodríguez et al[27] | Zhang et al[28] |
| Year | 1999 | 2000 | 2014 |
| n | 107 | 1246 | 128 |
| Cohort | Age 3 mo to 3 yr Wheeze onset < 12 wk prior Parental history of asthma or eczema Parental positive allergen skin prick test | Longitudinal healthy birth cohort | Age 2-20 mo 1st wheeze |
| Outcome prediction | Ongoing wheeze requiring treatment 1 yr after presentation | Active asthma during the school years 6-13 | Multi-trigger wheezing after 2 yr |
| Prediction results | 71% accuracy overall, 57% sensitivity, 84% specificity, 76% PPV, 68% NPV | 42% sensitivity, 85% specificity, 59% PPV, 73% NPV | 95% sensitivity, 74% specificity, 59% PPV, 94% NPV |
| Components of tool | Age at presentation Serum soluble interleukin-2 receptor concentration | Wheezing by parent report Major criteria: parental MD asthma, MD eczema Minor criteria: MD allergic rhinitis, Wheezing apart from colds, eosinophilia ≥ 4% | Wheezing severity score Family or personal history of atopic disease Number of exfoliated airway epithelial cells in sputum |
n: Number of subjects; NPV: Negative predictive value; PPV: Positive predictive value; ROC: Receiver operator characteristic.
BETA2-AGONISTS FOR ACUTE PEDIATRIC BRONCHIOLITIS
The relevant Cochrane review for bronchodilator therapy in bronchiolitis is directed at first-time wheezing infants receiving beta2-agonists. Exclusion criteria for studies on first-time wheezing infants generally included prior history of wheeze, previous bronchodilator or steroid use, and underlying lung or cardiac disease (including asthma). Additionally, most of the studies excluded patients requiring intensive care. Regarding bronchodilators, the AAP bronchiolitis guideline noted “variable study designs” and “inclusion of infants who had a history of previous wheezing in some studies”. This is an unreferenced statement and requires clarification. Of the 33 studies included in the most recent relevant Cochrane analysis, only 4 studies included infants with prior history of wheeze. Two of those studies included only 3 or fewer infants with prior wheeze in each study arm[17]. In other words, the inclusion of infants with any prior wheeze in bronchodilator trials for bronchiolitis was extremely limited. The AAP guideline makes reference to this: “Those studies showing benefit (of bronchodilators)… include older children with recurrent wheezing…. Although it is true that a small subset of children with bronchiolitis may have reversible airway obstruction…, attempts to define a subgroup of responders have not been successful to date”. Furthermore, the AAP guideline goes on to state, “Children with severe disease or with respiratory failure were generally excluded from these trials, and this evidence cannot be generalized to these situations”[7]. Therefore, significant question remains as to whether to trial a beta2-agonist in infants with prior history of wheezing, atopy, or more severe clinical presentations of acute viral bronchiolitis.
CHEST RADIOGRAPHY, ATELECTASIS AND AIRWAY CLEARANCE THERAPY
The AAP bronchiolitis guideline recommends against routine chest radiography in children with bronchiolitis, except for “cases in which respiratory effort is severe enough to warrant ICU admission or where signs of an airway complication (such as pneumothorax) are present”[7].
While mild-to-moderate presentations of bronchiolitis are unlikely to benefit from chest radiography, detection of radiographic atelectasis in more severe disease may be clinically important. In a study of 46 children with RSV-related respiratory failure, a multiple logistic regression model was developed by Prodhan et al[4,21] to predict length of mechanical ventilation. After excluding hyperinflation due to lack of association, the model included only age and radiologic atelectasis. On days 1 and 2 of mechanical ventilation this model correctly classified patients requiring > 8 d of mechanical ventilation in 84% of cases, and had an area under the ROC curve of 0.92[21]. This suggests that development of atelectasis in severe bronchiolitis is highly correlated with worse clinical outcome.
The cumulative literature on severe bronchiolitis and our own clinical experience in pediatric intensive care support the idea that the ability to clear obstructed airways and prevent or reverse atelectasis is directly related to an improved clinical course. That atelectasis predicts clinical outcome substantially explains why the literature on chest physiotherapy in acute bronchiolitis in infants has been uniformly negative. As reported in the relevant Cochrane review[31], patient selection for these trials did not specifically test whether patients with evidence of impaired mucus clearance would fare better with chest physiotherapy. Atelectasis, when reported at all, was in the range of 10%-25% of subjects. In one of the trials, a patient in the control arm who developed atelectasis was withdrawn from the study in order to receive chest physiotherapy[32]. This suggests that randomized trials of chest physiotherapy may be limited by clinicians who would not allow their patients to participate if the patients were clinically likely to benefit from chest physiotherapy. Most of the chest physiotherapy trials were conducted on small numbers of subjects. The data could not be pooled because of major differences between studies in both study design and chest physiotherapy technique. To our knowledge, none of the chest physiotherapy trials in bronchiolitis tested recent pulmonary toilet devices like The Vest (pneumovest), intrapulmonary percussive ventilator, MetaNeb, or Cough Assist. A randomized trial on the use of cough assist in acute bronchiolitis is currently underway.
Currently the literature on chest physiotherapy in acute bronchiolitis should be regarded as limited to non-critically ill bronchiolitis and inadequate to make any conclusions regarding patients with suspected or radiologic atelectasis. We believe that clinicians should make individualized decisions on chest radiography and chest physiotherapy in bronchiolitis, particularly to evaluate and treat atelectasis. Although this may seem to be in contrast to the AAP guideline: “Clinicians should not use chest physiotherapy for infants and children with a diagnosis of bronchiolitis”[7], the nature of the guidelines is as “an evidence-based shared baseline…. (not to) tell you what to do in the case of every patient”[33].
THREE PERCENT OF HYPERTONIC SALINE NEBULIZER THERAPY
Nebulized hypertonic saline potentially addresses the pathophysiology of airways obstruction in acute viral bronchiolitis by reducing pulmonary edema and loosening intraluminal debris to facilitate mobilization. The most recent Cochrane review on hypertonic saline therapy for acute bronchiolitis was undertaken on 11 inpatient and outpatient studies, all of which were randomized, double-blind, parallel-group, controlled trials (RCTs) using 0.9% saline as a control. All of the trials excluded patients with prior wheeze or severe bronchiolitis (requiring mechanical ventilation or intensive care, or oxygen saturation < 85% on room air). The concentration of hypertonic saline was 3% in all but one trial, which included both 3% and 5% concentrations. The 6 inpatient trials involving 500 participants revealed a pooled reduction in length of hospital stay by 1.15 d (95%CI: -1.49 to -0.82, P < 0.0001) for children treated with hypertonic saline, with average stays ranging 3.5 to 7.4 d. All 6 inpatient trials demonstrated a benefit in reducing duration of hospitalization[34]. Subsequent to this Cochrane review, randomized trials of inhaled hypertonic saline have revealed mixed results[35-38]. Resolving the differences between the many RCTs on nebulized hypertonic saline will likely require either a meta-analysis approach or an updated Cochrane review. In the meantime, evidence in support of 3% hypertonic saline therapy for hospitalized pediatric bronchiolitis includes clinical and biologic plausibility, numerous well-designed RCT’s, substantial benefit in a number of trials, and virtually no observed harm, including a notable absence of bronchospasm[34]. While the AAP guideline on hypertonic saline nebulizer for inpatient bronchiolitis appropriately balances the mostly if not uniformly positive evidence, the development of an institutional protocol could reasonably implement hypertonic saline for every admitted patient with acute bronchiolitis, especially if the institutional average length of stay for bronchiolitis exceeds three days.
ADDRESSING THE ACADEMIC MISSION OF ADVANCING HEALTH CARE
In conclusion, we applaud the 2014 revision of the AAP guideline on bronchiolitis and suggest further research to: (1) develop and validate severity scores to help guide clinical therapies; (2) incorporate early identification of childhood asthma; (3) study methods to identify and address atelectasis; and (4) consolidate the available data on inhaled hypertonic saline. Most importantly for the bedside practitioner, the pragmatic clinical setting and individualized assessment continue to guide medical care. With the development of new medical technologies and informatics, we are beginning to investigate bronchiolitis using a different set of tools and in a different way from those in the past, although constrained by the same limitations on resources and funds. In this way, academic centers can continue to fulfill our mission to educate, study, and provide the best health care to each of our patients.
Footnotes
Conflict-of-interest statement: The authors have disclosed that they have no potential conflicts of interest (including but not limited to commercial, personal, political, intellectual, or religious interests) that are related to the work submitted for consideration of publication. This is an unfunded work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 5, 2015
First decision: April 27, 2015
Article in press: July 14, 2015
P- Reviewer: Belliato M, Kelesidis T, Zhang YJ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papenburg J, Hamelin MÈ, Ouhoummane N, Carbonneau J, Ouakki M, Raymond F, Robitaille L, Corbeil J, Caouette G, Frenette L, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prais D, Schonfeld T, Amir J. Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics. 2003;112:548–552. doi: 10.1542/peds.112.3.548. [DOI] [PubMed] [Google Scholar]
- 4.Prodhan P, Sharoor-Karni S, Lin J, Noviski N. Predictors of respiratory failure among previously healthy children with respiratory syncytial virus infection. Am J Emerg Med. 2011;29:168–173. doi: 10.1016/j.ajem.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363:501–504. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 6.Mussman GM, Conway PH. Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7:350–357. doi: 10.1002/jhm.951. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder AR, Mansbach JM, Stevenson M, Macias CG, Fisher ES, Barcega B, Sullivan AF, Espinola JA, Piedra PA, Camargo CA. Apnea in children hospitalized with bronchiolitis. Pediatrics. 2013;132:e1194–e1201. doi: 10.1542/peds.2013-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 10.Everard ML. Acute bronchiolitis and croup. Pediatr Clin North Am. 2009;56:119–133, x-xi. doi: 10.1016/j.pcl.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Midgley CM, Jackson MA, Selvarangan R, Turabelidze G, Obringer E, Johnson D, Giles BL, Patel A, Echols F, Oberste MS, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Moore MR, et al. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:617–630. doi: 10.1093/cid/cir625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calışkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G, Bisgaard H, Jackson DJ, Gern JE, Lemanske RF, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma. J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki Y, Hosoya M, Katayose M, Suzuki H. Role of serum neutralizing antibody in reinfection of respiratory syncytial virus. Pediatr Int. 2004;46:126–129. doi: 10.1046/j.1442-200x.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 17.Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;6:CD001266. doi: 10.1002/14651858.CD001266.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowell DI, Lister G, Von Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics. 1987;79:939–945. [PubMed] [Google Scholar]
- 19.Destino L, Weisgerber MC, Soung P, Bakalarski D, Yan K, Rehborg R, Wagner DR, Gorelick MH, Simpson P. Validity of respiratory scores in bronchiolitis. Hosp Pediatr. 2012;2:202–209. doi: 10.1542/hpeds.2012-0013. [DOI] [PubMed] [Google Scholar]
- 20.Corneli HM, Zorc JJ, Holubkov R, Bregstein JS, Brown KM, Mahajan P, Kuppermann N. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28:99–103. doi: 10.1097/PEC.0b013e3182440b9b. [DOI] [PubMed] [Google Scholar]
- 21.Prodhan P, Westra SJ, Lin J, Karni-Sharoor S, Regan S, Noviski N. Chest radiological patterns predict the duration of mechanical ventilation in children with RSV infection. Pediatr Radiol. 2009;39:117–123. doi: 10.1007/s00247-008-1042-3. [DOI] [PubMed] [Google Scholar]
- 22.Walsh P, Rothenberg SJ, O’Doherty S, Hoey H, Healy R. A validated clinical model to predict the need for admission and length of stay in children with acute bronchiolitis. Eur J Emerg Med. 2004;11:265–272. doi: 10.1097/00063110-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Weisgerber MC, Lye PS, Li SH, Bakalarski D, Gedeit R, Simpson P, Gorelick MH. Factors predicting prolonged hospital stay for infants with bronchiolitis. J Hosp Med. 2011;6:264–270. doi: 10.1002/jhm.903. [DOI] [PubMed] [Google Scholar]
- 24.Luo G, Nkoy FL, Gesteland PH, Glasgow TS, Stone BL. A systematic review of predictive modeling for bronchiolitis. Int J Med Inform. 2014;83:691–714. doi: 10.1016/j.ijmedinf.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 25.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma: Full Report 2007, in National Asthma Education and Prevention Program 2007, National Institutes of Health. National Heart Lung and Blood Institute: Bethesda; 2007. p. 415. [DOI] [PubMed] [Google Scholar]
- 26.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 27.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhou C, Liu J, Yang H, Zhao S. A new index to identify risk of multi-trigger wheezing in infants with first episode of wheezing. J Asthma. 2014;51:1043–1048. doi: 10.3109/02770903.2014.936449. [DOI] [PubMed] [Google Scholar]
- 29.Clough JB, Keeping KA, Edwards LC, Freeman WM, Warner JA, Warner JO. Can we predict which wheezy infants will continue to wheeze. Am J Respir Crit Care Med. 1999;160:1473–1480. doi: 10.1164/ajrccm.160.5.9807019. [DOI] [PubMed] [Google Scholar]
- 30.Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koëter GH, Rijcken B, Postma DS. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years. A 30-year follow-up study. Am J Respir Crit Care Med. 1999;160:1830–1837. doi: 10.1164/ajrccm.160.6.9812100. [DOI] [PubMed] [Google Scholar]
- 31.Roqué i Figuls M, Giné-Garriga M, Granados Rugeles C, Perrotta C. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev. 2012;2:CD004873. doi: 10.1002/14651858.CD004873.pub4. [DOI] [PubMed] [Google Scholar]
- 32.Bohé L, Ferrero ME, Cuestas E, Polliotto L, Genoff M. Indications of conventional chest physiotherapy in acute bronchiolitis. Medicina (B Aires) 2004;64:198–200. [PubMed] [Google Scholar]
- 33.Quinonez RA, Ralston SL. Bronchiolitis: the rationale behind the new AAP guideline. Medscape 2014, WebMD LLC: New York, NY; 2014. Available from: http://www.medscape.com/viewarticle/834677_5. [Google Scholar]
- 34.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2013;7:CD006458. doi: 10.1002/14651858.CD006458.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Everard ML, Hind D, Ugonna K, Freeman J, Bradburn M, Cooper CL, Cross E, Maguire C, Cantrill H, Alexander J, et al. SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis. Thorax. 2014;69:1105–1112. doi: 10.1136/thoraxjnl-2014-205953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Baker C, Lang ME, Schrager SM, Liley FF, Papa C, Mira V, Balkian A, Mason WH. Nebulized hypertonic saline for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2014;168:657–663. doi: 10.1001/jamapediatrics.2014.301. [DOI] [PubMed] [Google Scholar]
- 37.Florin TA, Shaw KN, Kittick M, Yakscoe S, Zorc JJ. Nebulized hypertonic saline for bronchiolitis in the emergency department: a randomized clinical trial. JAMA Pediatr. 2014;168:664–670. doi: 10.1001/jamapediatrics.2013.5306. [DOI] [PubMed] [Google Scholar]
- 38.Sharma BS, Gupta MK, Rafik SP. Hypertonic (3%) saline vs 0.93% saline nebulization for acute viral bronchiolitis: a randomized controlled trial. Indian Pediatr. 2013;50:743–747. doi: 10.1007/s13312-013-0216-8. [DOI] [PubMed] [Google Scholar]
- 39.Henderson FW, Collier AM, Clyde WA, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 40.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]


