Abstract
Thrombocytopenia is often noted in critically ill patients. While there are many reasons for thrombocytopenia, the use of heparin and its derivatives is increasingly noted to be associated with thrombocytopenia. Heparin induced thrombocytopenia syndrome (HITS) is a distinct entity that is characterised by the occurrence of thrombocytopenia in conjunction with thrombotic manifestations after exposure to unfractionated heparin or low molecular weight heparin. HITS is an immunologic disorder mediated by antibodies to heparin-platelet factor 4 (PF4) complex. HITS is an uncommon cause of thrombocytopenia. Reported incidence of HITS in patients exposed to heparin varies from 0.2% to up to 5%. HITS is rare in ICU populations, with estimates varying from 0.39%-0.48%. It is a complex problem which may cause diagnostic dilemmas and management conundrum. The diagnosis of HITS centers around detection of antibodies against PF4-heparin complexes. Immunoassays performed by most pathology laboratories detect the presence of antibodies, but do not reveal whether the antibodies are pathological. Platelet activation assays demonstrate the presence of clinically relevant antibodies, but only a minority of laboratories conduct them. Several anticoagulants are used in management of HITS. In this review we discuss the incidence, pathogenesis, diagnosis and management of HITS.
Keywords: Heparin, Thrombocytopenia, Critically ill, Diagnosis, Management
Core tip: Thrombocytopenia is common in critically ill patients. While there are several causes of thrombocytopenia, heparin induced thrombocytopenia syndrome (HITS) is an uncommon cause often difficult to diagnose and manage. This article summarises the current diagnostic techniques and management options with a focus on critically ill patients with HITS.
INTRODUCTION
It has been over 90 years since the discovery of heparin[1], and by the 1930s heparin was being used clinically as an anticoagulant[2]. Embolic events during heparin therapy were first described in 1957 and were followed by subsequent reports; however thrombocytopenia as a result of heparin therapy was not described until 1969[3-6]. The central features of heparin induced thrombocytopenia (HIT) syndrome (HITS) - thrombocytopenia, thrombosis and its immune pathogenesis - weren’t recognized until the early 1970s[7].
Heparin and its derivatives are used frequently in the critically ill, either as thromboprophylaxis or for anticoagulation in patients with thromboembolic diseases. Thrombocytopenia occurs in 15%-58% of ICU patients[8,9]. Critically ill patients might suffer from a variety of acquired thrombotic risk factors related both to a host of chronic conditions such as obesity, hypertension, and diabetes mellitus, as well as acutely acquired conditions such as the postoperative state, sepsis, trauma, malignancy, other clonal disorders, etc.[10]. Hence, diagnosis of HIT syndrome is one of exclusion in the critically ill.
As HIT syndrome is a highly prothrombotic state, affected patients require ongoing anticoagulation with alternative anticoagulation such as the use of antithrombin anticoagulants or anti factor Xa agents[11,12].
As none of these agents have effective antidotes, management of bleeding associated with these agents is fraught with uncertainty.
HITS has been classified into two subtypes: HITS type 1: Benign non-immune condition occurring in 30%-40% patients exposed to heparin. platelets counts rarely fall below 100000/mcl. Heparin can be safely continued in this scenario and this condition is not discussed any further in the review; HITS type 2: Life threatening condition caused by antibodies against complexes of platelet factor 4 (PF4) and heparin, though occasionally other antigens may be implicated. Further discussion will relate to “type 2 HITS“ only.
In this review, we focus on diagnostic dilemmas and management challenges associated with this complex problem.
PATHOGENESIS
Heparin mediated thrombocytopenia is an immunologic disorder mediated by antibodies to heparin-PF4 complex[13-16]. The Fab fragments from the IgG subclass of antibodies to PF4 bind platelet associated PF4. The Fc fragments of these antibodies bind to FcγIIa receptors on the same or adjacent platelets, resulting in cross linking causing platelet activation. This results in generation of platelet microparticles that have procoagulant activity. This prothrombotic phenomenon is the principal difference between thrombocytopenia induced by heparin and other drugs such as quinine[17]. This marked release of the platelet microparticles is associated with massive thrombin generation, which explains the increased risk of thrombosis associated with HITS[18]. Significantly fewer platelet microparticles are generated in the presence of very high amounts of Heparin or in its absence, suggesting a stoichiometric relationship between HIT-IgG antibodies and heparin[17]. In addition to the increased thrombin generation in HITS, HITS antibodies also bind to endothelium-bound heparin resulting in release of tissue factor (TF) contributing to the overall prothrombotic state[19]. Recently, Monocytes have been found to bind hPF4 onto their surface and form antigenic complexes leading to monocyte activation and ultimately culminating in expression of TF. Both monocyte and endothelial activation may explain recurrence of thrombosis in many patients treated with direct thrombin inhibitors as none of them target these cells[20]. This may also explain the fact that HITS predisposes to both arterial and venous thrombosis even though it is primarily a platelet activation disorder. HIT-IgG antibodies also inhibit the generation of activated protein C by thrombin/thrombomodulin in the presence of PF4, augmenting the thrombotic state[21].
It seems that there might be a crucial period of exposure to heparin in patients who develop HITS. Patients, who suffer from conditions associated with high amounts of PF4 release prior to exposure to heparin, tend to be at a higher risk of developing HITS. For example, amongst elective hip replacement patients who receive preoperative low-molecular weight heparin (LMWH) are at a lower risk of HITS as compared to patients who receive post-operative thromboprophylaxis. This phenomenon of “point immunization” is probably explained by the fact that stoichiometrically optimal concentrations of heparin-PF4 are most likely to occur when PF4 is released prior to exposure to heparin[22]. Free nucleic acids in plasma can induce similar conformational changes in PF-4 as are induced by heparin, mainly because of the highly anionic Phosphate entities on the nucleic acid molecules. This finding may further explain the propensity for certain subgroups of patients (such as those with major tissue damage) to develop pathological antibodies to heparin-PF4 complexes[23].
Two key determinants of antigenicity of a heparin preparation are chain length (approximately 1000 Da) and minimal amount of sulfation per saccharide unit. This explains lower risk of HITS with LMWH preparations as compared to unfractionated heparin (UFH)[24].
Occasionally, HITS can be caused by antibodies to other antigens such as neutrophil activating peptide-2 or interleukin 8.
EPIDEMIOLOGY
Reported incidence of HITS in patients exposed to heparin varies from 0.2% to up to 5%[25]. HITS is rare in ICU populations, with estimates varying from 0.39%-0.48%[26]. The incidence of HITS varies widely depending on the preparation of heparin, sex of the patients, and clinical population. The risk of HITS is higher amongst women [Odds ratio (OR) = 2.37]; among surgical patients as compared with medical patients (OR = 3.25); and patients on UFH vs patients receiving LMWH (OR = 5.29)[27]. Amongst surgical patients, although post-cardiac surgery patients tend to have a higher risk of developing HIT-IgG than post-orthopaedic surgical group (20% vs 3.2%), patients are much more likely to develop HITS after Orthopaedic surgery (OR = 21.1)[28]. Patients with major trauma are more likely to be Heparin-PF4 antibody positive and develop HITS as compared to patients with minor trauma[29]. HITS is very rare in obstetric or pediatric patients.
CLINICAL FEATURES
Heparin induced thrombocytopenia is characterized by thrombocytopenia and thrombotic manifestations after exposure to unfractionated heparin or low molecular weight heparin.
Thrombocytopenia
Onset of thrombocytopenia is usually between 5-10 d after the exposure, but it is faster (within a few hours to a day) if the patient has been exposed to heparin within 100 d of current exposure[30].
Platelet count usually drops to 50% or less of the baseline platelet count. Drop in platelet counts 30%-50% of baseline occurs in 10% of the cases[25].
Platelet counts usually do not fall below 20000/mcl. Lower platelet counts may be observed if HITS causes disseminated intravascular coagulation (DIC).
Bleeding is very rare as a complication of thrombocytopenia.
Recovery typically takes 4-14 d after cessation of heparin.
Pattern of thrombocytopenia occurring after the inciting event (such as cardiothoracic surgery) is important as well. A continuous decline after cardiopulmonary bypass is less likely to be due to HITS. A fall in platelet count of at least 40% between 5-10 d post cardiopulmonary bypass is likely to be due to HITS[31].
Thrombotic manifestations
Thrombotic manifestations develop in 20%-50% of the patients. HITS that is not associated with thrombotic phenomena is known as “isolated HITS”.
Thrombosis can affect both arterial and venous beds. However, Venous thromboembolic complications are twice as likely as compared to arterial thrombotic phenomena. About 10%-20% patients suffer DIC.
Risk of thrombosis is higher for days to weeks after heparin is discontinued, even after normalization of the platelet counts[11].
Risk of thrombosis is higher in patients with higher level of antibodies to PF4-heparin complexes[32].
Other clinical manifestations that should raise the suspicion of HITS in appropriate clinical scenario: (1) acute anaphylactoid/anaphylactic reactions after heparin administration: Heparin induced anaphylactoid and anaphylactic reactions are two distinct pathophysiological entities. Heparin induced anaphylactoid reactions are due to activation of platelets and leukocytes in patients harbouring anti heparin-PF4 antibodies, typically administered a heparin bolus after prior exposure to heparin. Heparin induced anaphylactic reaction is due to a contaminant (oversulphated chondroitin sulphate or OSCS) activating the contact system resulting in the clinical manifestations. However, patients exposed to OSCS contaminated heparin are more likely to develop pathological HITS antibodies[33]; (2) heparin induced skin lesions: These painful or pruritic necrotic lesions develop at the site of injection, beginning on day 5 or later after exposure to heparin or LMWH. Non-necrotic lesions at the injection sites are almost always due to delayed hypersensitivity to heparin rather than a manifestation of HITS, especially after exposure to LMWH rather than Unfractionated heparin[34]; (3) heparin induced skin necrosis and venous gangrene: especially in the presence of coumarin, attributed to both macro and micro vascular thrombosis with preserved arterial flow. Inhibition of activated protein C by heparin PF4 antibodies could be a strong contributory factor[21]; and (4) transient global amnesia[35].
These manifestations curiously, tend to occur in the absence of thrombocytopenia[25].
DIAGNOSIS
The diagnosis of HITS centers around detection of antibodies against PF4-Heparin complexes. Immunoassays performed by most pathology laboratories detect the presence of antibodies, but do not reveal whether the antibodies are pathological. Platelet activation assays demonstrate the presence of clinically relevant antibodies, but only a minority of laboratories conduct them. Before elaborating further on the diagnostic assays, it is vital to consider the following facts: (1) heparin-PF4 antibodies can exist naturally in people unexposed to heparin in the past[36]; (2) only IgG subclass of antibodies are pathological. Hence assays which are not IgG specific are likely to yield a higher false positive result; (3) out of the patients who are anti heparin/PF4IgG positive, only a minority will be positive by the “Gold Standard” platelet activation assays; (4) not all patients with platelet activating antibodies develop the clinical syndrome of HITS; and (5) fraction of the patients with heparin/PF4 antibody depends on the patient population and the type of heparin preparation used.
The above phenomena observed have been conceptualized as an “iceberg model” by Warkentin et al[37], and highlight the fact that HITS is a clinicopathological syndrome rather than just a laboratory diagnosis (Figure 1).
Figure 1.
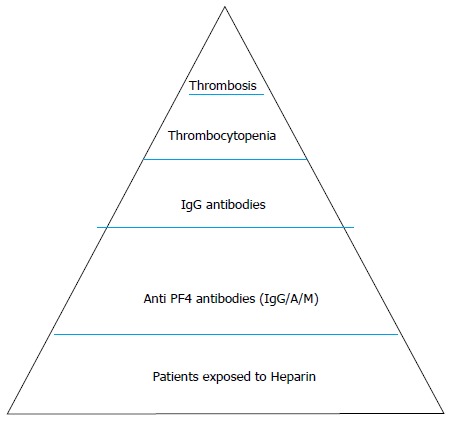
Iceberg model for heparin induced thrombocytopenia syndrome heparin induced thrombocytopenia syndrome as proposed by Warkentin et al[37]. The size of various iceberg sections and the portion seen, can vary in proportion to the other sections depending on the population of patients, preparation of heparin used, etc.
The diagnosis of HITS centers around the pretest probability of HITS being the cause of the drop in platelet count and/or the thrombotic phenomenon observed. In light of this the “4-T’s” scoring system was introduced (Table 1)[38]. Low pretest probability score ruled out HITS in all but one of the 119 patients studied.
Table 1.
4T score as studied by Lo et al[38]
| Points (0, 1, or 2 for each of 4 categories: maximum possible score = 8) | |||
| 2 | 1 | 0 | |
| Thrombocytopenia | > 50% fall or platelet nadir ≥ 20 × 109/L | 30%-50% fall or platelet count 10-19 × 109/L | Fall < 30% or platelet nadir < 10 × 109/L |
| Timing of fall in platelet count | Clear onset between day 5-101; or less than 1 d (if history of heparin exposure within 30 d) | Consistent with d 5-10 fall, but not clear (e.g., missing platelet counts) or onset of thrombocytopenia after d10 or fall ≤ 1 d (prior heparin exposure 30-100 d ago) | Platelet count fall < 4 d without recent heparin exposure |
| Thrombosis or other sequelae (e.g., Skin lesions) | New thrombosis; skin necrosis; acute systemic reaction post unfractionated heparin bolus | Progressive or recurrent thrombosis; erythematous skin lesions; suspected thrombosis not yet proven | None |
| Other cause of thrombocytopenia | None apparent | Possible other cause is evident | Definite |
| 4T score: 6-8 = High; 4-5 = Intermediate; 0-3 = Low | |||
5-10 d after exposure to heparin or low molecular weight heparin.
Patients with intermediate or high pre-test probability of HITS should be investigated further with enzyme linked immunosorbent assay (ELISA) based methods[12].
These assays use heparin-PF4 or polyvinyl sulfate-PF4 immobilised onto microtiter plates as antigens. Antibodies in the patient’s plasma bind to these antigens and is detected using goat anti human IgG/A/M bound to alkaline phosphatase. Substrate, subsequently added, changes colour in presence of the enzyme. The intensity of the colour change is measured as optical density (OD) and is directly proportional to the concentration of the antibodies[39]. Even though these tests are very sensitive (negative predictive values of close to 100%), they tend to yield a high number of false positive results for HITS, depending on the manufacturer of the assay kit and the clinical population. Higher rates of false positive ELISAs are noted in patients post cardiac surgery and those with antiphospholipid antibody (APLA) syndrome. Anti PF4 antibodies rather than anti PF4/heparin antibodies are responsible for false positive ELISA in sera with APLA syndrome[40].
Following measures may be taken to increase the specificity of ELISA based assays (Table 2): (1) using IgG specific assays: As IgG antibodies are pathogenic, using specific assays targeting IgG antibodies rather than non-specific assays improves the specificity of the test without sacrificing the sensitivity of the assay; (2) using higher OD cut offs: As higher titers of antibodies are associated with a greater probability of HITS, using higher cutoff values (for example 1.0 instead of 0.4) might increase the specificity of the assay. However, this comes at cost of sacrificing sensitivity of the assay; and (3) confirmatory step using high concentration of heparin: As heparin and anti heparin-PF4 antibodies have a stoichiometric relationship, re-performing the ELISA test with higher concentrations of heparin may confirm the presence of anti heparin-PF4 antibodies. However, this approach requires the test to be performed twice, increasing the cost and the turn around time. It can also be falsely negative if the titre of the antibodies is very high.
Table 2.
Characteristics of various assays for heparin induced thrombocytopenia syndrome
| 4T score ≤ 3[73-76] | ELISA[77] | IgG specific ELISA[77] | OD cut off ≥ 1.0[78] | Heparin confirmation step for IgG specific ELISA[79] | Serotonin release assay[80] | Whole blood impedence Aggregometry[44] | |
| Sensitivity | - | 100% | 100% | 80% | 94% | 100% | 90.3%-93.6% |
| Specificity | - | 81% | 89% | 85% | 90%-93% | 95%-97% | 89%-96% |
| PPV | - | 28% | 40% | 42% | 45% | NA | 84.4%-94.8% |
| NPV | 100% | 100% | 100% | 84% | 99.50% | NA | - |
PPV: Positve predictive value; NPV: Negative predictive value; NA: Not applicable as serotonin release assay is the gold standard assay for diagnosis of heparin induced thrombocytopenia syndrome.
Diagnosis of HITS should be confirmed with functional platelet assays in patients with intermediate pretest probability and positive ELISA or in patients with high pretest probability with negative ELISA (Figure 2).
Figure 2.
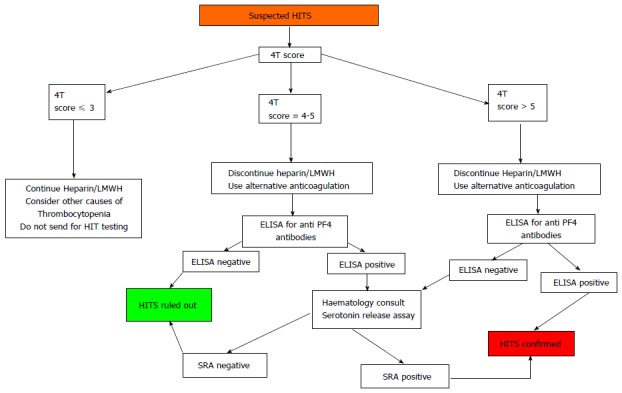
Diagnostic algorithm for Heparin induced thrombocytopenia syndrome. Note that Confirmatory assays for HITS should be considered on the basis of pre test probability rather than ELISA alone. HITS: Heparin induced thrombocytopenia syndrome; SRA : Serotonin release assay. ELISA : Enzyme linked immunosorbent assay.
Selection of platelet donors can be potentially critical for these assays as certain polymorphisms on the FcγRIIa receptors affects the response of platelets to the activating monoclonal antibodies[41].
Serotonin release assay (SRA) is the gold standard test for diagnosis of HITS. It utilizes washed donor platelets incubated with 14C-labelled serotonin. It is considered positive when more than 20% serotonin is released at therapeutic heparin concentrations (0.1-0.3 IU/mL), but not at supra-therapeutic heparin levels (10-100 IU/mL)[42]. In Australia, out of 675 SRAs requested to the only centre performing this assay between 2010-2012, around 19% were positive for HITS. Interestingly, amongst cases in which 4T score was available, almost 96% had intermediate or high probability 4T score[43].
Whole blood impedance aggregometry (WBIA) is emerging as a useful alternative to SRA with faster turnaround time (around 15 min), does not use washed platelets and no radioactive waste products. The laboratories running this assay still need access to high reactive platelet donors as using less responsive platelet donors might result in false negative WBIA. The agreement of WBIA with SRA improves if a higher cut off of 50% instead of 20%, and if a high dose heparin confirmatory step is used[44].
TREATMENT
Once a presumptive diagnosis of HITS is made on the basis of pretest probability and anti PF4 antibody assays, therapeutic dosing of alternative anticoagulant is needed along with cessation of the offending agent. Patients are hypercoagulable for days to even weeks despite normalization of platelet counts[11]. Hence, patients may need to be transitioned to oral anticoagulants once platelet counts have normalized.
Ideal anticoagulant for treatment of HITS should have following characteristics: (1) should have no risk of generating HITS antibodies; (2) should have robust evidence supporting its use in HITS; (3) should be able to provide predictable anticoagulation and be able to be monitored by an widely available assay; (4) should have short half life; (5) should be easily reversible by an antidote which is readily available; (6) should have a low risk of bleeding and other adverse effects; (7) metabolism and elimination should be reliable and independent of renal or hepatic dysfunction; (8) should be safe to use in special subgroup of patients such as those who are pregnant or need to go on to cardiopulmonary bypass; and (9) should be easily available in both oral and intravenous preparations for easy transition between short and longer term anticoagulation.
Unfortunately, such an anticoagulant doesn’t exist. Most of the problems from anticoagulation in HITS arise because of the lack of familiarity with non-heparin anticoagulants.
Following are the different categories of anticoagulants which can be used for HITS, based on the clinical scenario (Tables 3 and 4): (1) direct thrombin Inhibitors: Univalent direct thrombin inhibitors (argatroban; dabigatran), bivalent direct thrombin inhibitors [recombinant hirudins (lepirudin, desirudin); synthetic hirudin ( bivalirudin )]; and (2) factor Xa antagonists: danaparoid, fondaparinux, rivaroxaban, apixaban.
Table 3.
Characteristics of alternative anticoagulants
| Drug | Route of elimination | Plasma half life | Monitoring | Interaction of antibodies with HITS antibodies | Antidote |
| Lepirudin | Renal | 60 min, up to 200 h in anuric patients[81,82] | aPTT (1.5-2 times baseline) ACT on CPB ECT (Not affected by presence of VKAs or UFH) | None | None ?Haemofiltration[47] |
| Desirudin | Renal | 2-3 h | None | None | None |
| Danaparoid | Renal | 24 h | Anti-Xa activity (0.5-0.8 U/mL) | Possible, but very rare | None |
| Argatroban | Hepatic | 40-50 min | aPTT (1.5-3 times baseline) ACT on CPB | None | None |
| Bivalirudin | Enzymatic 80% (Thrombin), renal 20% | 25 min | aPTT (1.5-2.5 times baseline) ACT on CPB | None | None ?Haemofiltration[52] |
| Fondaparinux | Renal | 17-20 h | None, Anti Xa levels with renal impairment | Case reports only[45,61,62] | None |
aPTT: Activated partial thromboplastin time; ACT: Activated clotting time; ECT: Ecarin clotting time; CPB: Cardiopulmonary bypass; HITS: Heparin induced thrombocytopenia syndrome; UFH: Unfractionated heparin.
Table 4.
Dosage and availability of anticoagulation agents for heparin induced thrombocytopenia syndrome
| Drug | Bolus | Dosage | Dosage in renal impairment | Dosage in hepatic impairment | Availability in Australia |
| Lepirudin | Only if life or limb threatening thrombosis. 0.4 mg/kg iv | 0.1-0.15 mg/kg per hour | Cr. Cl. 45-60: 50% of original infusion rate. Cr. Cl. 30-44: 30% of original infusion rate. Cr. Cl. 15-29: 15% of original infusion rate according to body weight. Avoid if Cr. Cl. Lower or use 0.005 mg/kg per hour if on haemofiltration | No change | Discontinued |
| Desirudin | None | 15-30 mg sc bd. Limited data | Not recommended given paucity of data | No change | Not available |
| Danaparoid | IV according to body weight. < 60 kg: 1500 U; 60-75 kg: 2250 U; 75-90 kg: 3000 U; > 90 kg: 3750 U | 400 U/h IV × 4 h followed by 300 U/h IV × 4 h followed by 200 U/h iv | Reduce dose by 30% and monitor antiXa activity | No change | Available |
| Bivalirudin | None | 0.15-0.2 mg/kg per minute | Cr. Cl 10-29: 0.06 mg/kg per minute; Cr. Cl < 10: 0.015 mg/kg per minute iv | No change | Available |
| Fondaparinux | None | < 50 kg: 5 mg sc; 50-100 kg: 7.5 mg sc; > 100 kg: 10 mg sc | Cr. Cl 30-50: monitor closely. Cr. Cl < 30: Contraindicated | No change | Available |
| Argatroban | None | 2 mcg/kg per minute iv | No change | 0.5 mcg/kg per minute | Not available |
Cr. Cl.: Creatinine clearance in mL/min; sc: Subcutaneous; iv: Intravenous.
DIRECT THROMBIN INHIBITORS
Lepirudin
A recombinant hirudin derived from yeast cells, Lepirudin was the first drug approved by United States Food and Drug Administration, for the treatment of HITS in 1998. Even though it reduced new thromboembolic manifestations, it increased the risk of major bleeding in a combined analysis of 3 prospective trials with historical controls[45].
Retreatment with lepirudin can increase the risk of anaphylaxis almost half the patients will develop antibodies to lepirudin on initial use. The risk of antibody formation can be reduced by avoiding the bolus and reducing the duration of infusion as much as possible[46].
Although no antidote is available, use of activated Factor VII to control bleeding and haemofiltration has been described[47,48].
Argatroban
Argatroban is a synthetic L-arginine derivative, which is tolerated well by patients with moderate renal dysfunction[49].
Even though the half life is short and use in mild to moderate renal dysfunction is safe, rebound hypercoagulability after cessation of infusion, and spurious prolongation of Prothrombin time when given with warfarin are significant issues, especially when transitioning to longer term oral anticoagulation.
A severity of illness based dosing regime for continuous renal replacement therapy in critically ill is available but not validated[50].
Bivalirudin
Bivalirudin is a hirudin based synthetic direct thrombin inhibitor, which binds to free as well as bound thrombin reversibly. It has the shortest half-life amongst the direct thrombin inhibitors and has higher reversibility as up to 80% is eliminated by enzymatic proteolysis[51]. Due to better pharmacokinetic profile, this is the agent used most widely in patients needing cardiopulmonary bypass. hemodialysis, haemofiltration or plasmapheresis may be used to reverse its effect, even though the data available for efficacy of these therapies is limited[52].
Desirudin
Desirudin is a recombinant hirudin and is a bivalent, irreversible direct thrombin inhibitor. There is very limited data about use of Desirudin for HITS. An open label randomized pilot trial comparing Desirudin with Argatroban for HITS (PREVENT-HIT) was closed because of poor accrual[53,54].
FACTOR XA INHIBITORS
Fondaparinux
Fondaparinux is a sulfated pentasaccharide derivative of heparin which binds to antithrombin, inhibiting factor X[55]. Even though the frequency of heparin - PF4 antibody is similar to Low molecular weight heparins, Fondaparinux induced antibodies are seldom pathogenic[56,57].
The risk of bleeding while treating HITS is around 5%. Even though some cases of HITS caused by Fondaparinux have been described in literature, benefits such as ease of administration, predictable pharmacokinetics in patients with normal renal function and lack of effect on aPTT makes it an attractive option in patients in whom benefits outweigh low risk of exacerbation of HITS[45,58-62].
Danaparoid
Danaparoid, a mixture of low molecular sulphated glycosaminoglycans, Heparan, dermatan and chondroitin sulphate is a factor Xa inhibitor. The requirement for monitoring anti Xa levels in most of the critically ill patients developing HITS and risk of cross reaction with heparin-PF4 antibody (< 10% patients) are the factors which need to be considered before using Danaparoid for HITS[63].
TRANSITION TO ORAL VITAMIN K ANTAGONISTS
For isolated HITS, alternative anticoagulation with or without transition to oral vitamin K antagonists is recommended for up to 4-6 wk. For HITS associated with thrombosis, switching to warfarin followed by continuation of warfarin therapy for 3 mo is recommended.
Transition to warfarin should only be made once platelet count is > 150000/mcl. Warfarin needs to be overlapped with alternative anticoagulation for at least 5 d and until the INR is in the therapeutic range.
Fondaparinux or Danaparoid may be required for transition of Argatroban to oral vitamin K antagonists due to effect of Argatroban on INR.
SPECIAL PATIENT POPULATIONS
Pregnancy
HITS is extremely rare during pregnancy and all the data regarding diagnosis and management is anecdotal. Thrombocytopenia occurs in 7%-8% pregnancies. Most common reason for thrombocytopenia is gestational (haemodilution, increased platelet aggregation due to raised thromboxane A2, increased platelet consumption) followed by other causes such as HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, Preeclampsia/eclampsia, acute fatty liver disease of pregnancy, Idiopathic thrombocytopenic purpura, etc.[64].
In a review of 2777 pregnancies involving exposure to LMWH, none of the patients developed thrombocytopenia attributable to HITS[65].
Ease of administration, safety in longer term use, and transplacental transfer are special considerations in this group of patients. Despite case reports of the use of argatroban, fondaparinux and danaparoid are preferred because of their subcutaneous administration and availability of large data on longer term use during pregnancy[66-68].
Cardiopulmonary bypass
Patients with history of HITS that require cardiopulmonary bypass can be managed based on their HITS antibody status (Figure 3).
Figure 3.
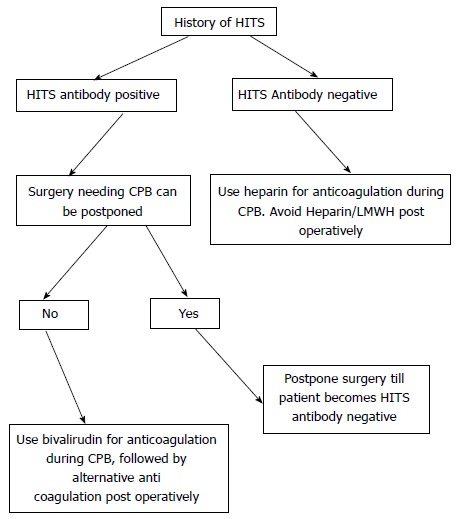
Suggested approach for patients with heparin induced thrombocytopenia syndrome needing cardiopulmonary bypass. HITS: Heparin induced thrombocytopenia syndrome; CPB: Cardiopulmonary bypass.
Bivalirudin is the alternative anticoagulant most well described in literature, however lepirudin and argatroban have also been described[69-72].
We recommend Bivalirudin for anticoagulation while on cardiopulmonary bypass, as it has been compared directly with heparin in an open label randomized control trial with heparin and protamine reversal. As Bivalirudin is metabolized by thrombin in the blood, care must be taken to avoid any stasis in the venous circuit, surgical field, and the vein grafts. Citrate phosphate dextrose acetate is used for anticoagulation in the cell saver.
CONCLUSION
The diagnosis of HITs in critically ill patients requires early recognition for successful management. Exclusion of other causes for thrombocytopenia and or thrombosis with special consideration to the temporal relationship of onset of thrombocytopenia with exposure to UFH/LMWH is vital. Use of clinical pretest probability scores such as 4T score in conjunction with more specific assays such as anti-IgG heparin PF4 antibody may reduce over-diagnosis of the disease. Confirmatory tests such as the SRA should be considered for equivocal cases; new tests such as WBIA based assay show promise. Attention to risk of bleeding with invasive interventions, presence and degree of renal/hepatic dysfunction, and availability and cost of alternative anticoagulation agents is important. Finally, patients requiring cardiopulmonary bypass and pregnant patients present rare and challenging scenarios.
Footnotes
Conflict-of-interest statement: None of the authors have any conflicts of interests (including but not limited to commercial, personal, political. Intellectual, or religious interests) in relation to this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 3, 2014
First decision: December 26, 2014
Article in press: May 14, 2015
P- Reviewer: De Cristofaro R, Kadusevicius E, Puddu PE S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Howell W, Holt , E Two new factors in blood coagulation: heparin and pro-antithrombin. Am J Physiol. 1918;47:328–341. [Google Scholar]
- 2.Crafoord C. Preliminary Report on post operative treatment with heparin as a preventative of thrombosis. Acta Chirurgica Scand. 1936;79:407–426. [Google Scholar]
- 3.Weismann RE, Tobin RW. Arterial embolism occurring during systemic heparin therapy. AMA Arch Surg. 1958;76:219–225; discussion 225-227. doi: 10.1001/archsurg.1958.01280200041005. [DOI] [PubMed] [Google Scholar]
- 4.Roberts B, Rosato FE, Rosato EF. Heparin--a cause of arterial emboli? Surgery. 1964;55:803–808. [PubMed] [Google Scholar]
- 5.Natelson EA, Lynch EC, Alfrey CP, Gross JB. Heparin-induced thrombocytopenia. An unexpected response to treatment of consumption coagulopathy. Ann Intern Med. 1969;71:1121–1125. doi: 10.7326/0003-4819-71-6-1121. [DOI] [PubMed] [Google Scholar]
- 6.Kelton JG, Warkentin TE. Heparin-induced thrombocytopenia: a historical perspective. Blood. 2008;112:2607–2616. doi: 10.1182/blood-2008-02-078014. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes GR, Dixon RH, Silver D. Heparin induced thrombocytopenia with thrombotic and hemorrhagic manifestations. Surg Gynecol Obstet. 1973;136:409–416. [PubMed] [Google Scholar]
- 8.Priziola JL, Smythe MA, Dager WE. Drug-induced thrombocytopenia in critically ill patients. Crit Care Med. 2010;38:S145–S154. doi: 10.1097/CCM.0b013e3181de0b88. [DOI] [PubMed] [Google Scholar]
- 9.Moreau D, Timsit JF, Vesin A, Garrouste-Orgeas M, de Lassence A, Zahar JR, Adrie C, Vincent F, Cohen Y, Schlemmer B, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 10.Ortel TL. Acquired thrombotic risk factors in the critical care setting. Crit Care Med. 2010;38:S43–S50. doi: 10.1097/CCM.0b013e3181c9ccc8. [DOI] [PubMed] [Google Scholar]
- 11.Girolami B, Prandoni P, Stefani PM, Tanduo C, Sabbion P, Eichler P, Ramon R, Baggio G, Fabris F, Girolami A. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: a prospective cohort study. Blood. 2003;101:2955–2959. doi: 10.1182/blood-2002-07-2201. [DOI] [PubMed] [Google Scholar]
- 12.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 13.Kelton JG, Sheridan D, Santos A, Smith J, Steeves K, Smith C, Brown C, Murphy WG. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72:925–930. [PubMed] [Google Scholar]
- 14.Newman PM, Chong BH. Heparin-induced thrombocytopenia: new evidence for the dynamic binding of purified anti-PF4-heparin antibodies to platelets and the resultant platelet activation. Blood. 2000;96:182–187. [PubMed] [Google Scholar]
- 15.Horsewood P, Hayward CP, Warkentin TE, Kelton JG. Investigation of the mechanisms of monoclonal antibody-induced platelet activation. Blood. 1991;78:1019–1026. [PubMed] [Google Scholar]
- 16.Chong BH, Fawaz I, Chesterman CN, Berndt MC. Heparin-induced thrombocytopenia: mechanism of interaction of the heparin-dependent antibody with platelets. Br J Haematol. 1989;73:235–240. doi: 10.1111/j.1365-2141.1989.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–3699. [PubMed] [Google Scholar]
- 18.Warkentin TE, Sheppard JI. Generation of platelet-derived microparticles and procoagulant activity by heparin-induced thrombocytopenia IgG/serum and other IgG platelet agonists: a comparison with standard platelet agonists. Platelets. 1999;10:319–326. doi: 10.1080/09537109975960. [DOI] [PubMed] [Google Scholar]
- 19.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Engl J Med. 1987;316:581–589. doi: 10.1056/NEJM198703053161004. [DOI] [PubMed] [Google Scholar]
- 20.Rauova L, Hirsch JD, Greene TK, Zhai L, Hayes VM, Kowalska MA, Cines DB, Poncz M. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116:5021–5031. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalska MA, Krishnaswamy S, Rauova L, Zhai L, Hayes V, Amirikian K, Esko JD, Bougie DW, Aster RH, Cines DB, et al. Antibodies associated with heparin-induced thrombocytopenia (HIT) inhibit activated protein C generation: new insights into the prothrombotic nature of HIT. Blood. 2011;118:2882–2888. doi: 10.1182/blood-2011-02-335208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warkentin TE. HIT paradigms and paradoxes. J Thromb Haemost. 2011;9 Suppl 1:105–117. doi: 10.1111/j.1538-7836.2011.04322.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaax ME, Krauel K, Marschall T, Brandt S, Gansler J, Fürll B, Appel B, Fischer S, Block S, Helm CA, et al. Complex formation with nucleic acids and aptamers alters the antigenic properties of platelet factor 4. Blood. 2013;122:272–281. doi: 10.1182/blood-2013-01-478966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, Kelton JG. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330–1335. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 25.Cuker A. Recent advances in heparin-induced thrombocytopenia. Curr Opin Hematol. 2011;18:315–322. doi: 10.1097/MOH.0b013e3283497ef2. [DOI] [PubMed] [Google Scholar]
- 26.Selleng K, Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35:1165–1176. doi: 10.1097/01.CCM.0000259538.02375.A5. [DOI] [PubMed] [Google Scholar]
- 27.Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108:2937–2941. doi: 10.1182/blood-2005-11-012450. [DOI] [PubMed] [Google Scholar]
- 28.Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–1708. [PubMed] [Google Scholar]
- 29.Lubenow N, Hinz P, Thomaschewski S, Lietz T, Vogler M, Ladwig A, Jünger M, Nauck M, Schellong S, Wander K, et al. The severity of trauma determines the immune response to PF4/heparin and the frequency of heparin-induced thrombocytopenia. Blood. 2010;115:1797–1803. doi: 10.1182/blood-2009-07-231506. [DOI] [PubMed] [Google Scholar]
- 30.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 31.Gruel Y, Pouplard C. Post-operative platelet count profile: the most reliable tool for identifying patients with true heparin-induced thrombocypenia after cardiac surgery. J Thromb Haemost. 2010;8:27–29. doi: 10.1111/j.1538-7836.2009.03646.x. [DOI] [PubMed] [Google Scholar]
- 32.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2:2133–2137. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE, Greinacher A. Heparin-induced anaphylactic and anaphylactoid reactions: two distinct but overlapping syndromes. Expert Opin Drug Saf. 2009;8:129–144. doi: 10.1517/14740330902778180. [DOI] [PubMed] [Google Scholar]
- 34.Schindewolf M, Kroll H, Ackermann H, Garbaraviciene J, Kaufmann R, Boehncke WH, Ludwig RJ, Lindhoff-Last E. Heparin-induced non-necrotizing skin lesions: rarely associated with heparin-induced thrombocytopenia. J Thromb Haemost. 2010;8:1486–1491. doi: 10.1111/j.1538-7836.2010.03795.x. [DOI] [PubMed] [Google Scholar]
- 35.Teh CH, Robertson MN, Warkentin TE, Henriksen PA, Brackenbury ET, Anderson JA. Transient global amnesia as the presenting feature of heparin-induced thrombocytopenia. J Card Surg. 2010;25:300–302. doi: 10.1111/j.1540-8191.2010.01007.x. [DOI] [PubMed] [Google Scholar]
- 36.Greinacher A, Holtfreter B, Krauel K, Gätke D, Weber C, Ittermann T, Hammerschmidt S, Kocher T. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood. 2011;118:1395–1401. doi: 10.1182/blood-2011-03-342857. [DOI] [PubMed] [Google Scholar]
- 37.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535–555. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 38.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 39.Otis SA, Zehnder JL. Heparin-induced thrombocytopenia: current status and diagnostic challenges. Am J Hematol. 2010;85:700–706. doi: 10.1002/ajh.21770. [DOI] [PubMed] [Google Scholar]
- 40.Pauzner R, Greinacher A, Selleng K, Althaus K, Shenkman B, Seligsohn U. False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Thromb Haemost. 2009;7:1070–1074. doi: 10.1111/j.1538-7836.2009.03335.x. [DOI] [PubMed] [Google Scholar]
- 41.Tan CW, Ward CM, Morel-Kopp MC. Evaluating heparin-induced thrombocytopenia: the old and the new. Semin Thromb Hemost. 2012;38:135–143. doi: 10.1055/s-0032-1301411. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67:27–30. [PubMed] [Google Scholar]
- 43.Just S, Brighton , T Review of SRA results for confirmation of Heparin Induced Thrombotic Thrombocytopenia (HITT) HAA. 2013:Abstract. [Google Scholar]
- 44.Morel-Kopp MC, Tan CW, Brighton TA, McRae S, Baker R, Tran H, Mollee P, Kershaw G, Joseph J, Ward C. Validation of whole blood impedance aggregometry as a new diagnostic tool for HIT: results of a large Australian study. Thromb Haemost. 2012;107:575–583. doi: 10.1160/TH11-09-0631. [DOI] [PubMed] [Google Scholar]
- 45.Pistulli R, Oberle V, Figulla HR, Yilmaz A, Pfeifer R. Fondaparinux cross-reacts with heparin antibodies in vitro in a patient with fondaparinux-related thrombocytopenia. Blood Coagul Fibrinolysis. 2011;22:76–78. doi: 10.1097/MBC.0b013e328340ff24. [DOI] [PubMed] [Google Scholar]
- 46.Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003;108:2062–2065. doi: 10.1161/01.CIR.0000096056.37269.14. [DOI] [PubMed] [Google Scholar]
- 47.Mon C, Moreno G, Ortiz M, Diaz R, Herrero JC, Oliet A, Rodriguez I, Ortega O, Gallar P, Vigil A. Treatment of hirudin overdosage in a dialysis patient with heparin-induced thrombocytopenia with mixed hemodialysis and hemofiltration treatment. Clin Nephrol. 2006;66:302–305. doi: 10.5414/cnp66302. [DOI] [PubMed] [Google Scholar]
- 48.Oh JJ, Akers WS, Lewis D, Ramaiah C, Flynn JD. Recombinant factor VIIa for refractory bleeding after cardiac surgery secondary to anticoagulation with the direct thrombin inhibitor lepirudin. Pharmacotherapy. 2006;26:569–577. doi: 10.1592/phco.26.4.576. [DOI] [PubMed] [Google Scholar]
- 49.Hursting MJ, Jang IK. Impact of renal function on argatroban therapy during percutaneous coronary intervention. J Thromb Thrombolysis. 2010;29:1–7. doi: 10.1007/s11239-009-0357-8. [DOI] [PubMed] [Google Scholar]
- 50.Link A, Girndt M, Selejan S, Mathes A, Böhm M, Rensing H. Argatroban for anticoagulation in continuous renal replacement therapy. Crit Care Med. 2009;37:105–110. doi: 10.1097/CCM.0b013e3181932394. [DOI] [PubMed] [Google Scholar]
- 51.Sakr Y. Heparin-induced thrombocytopenia in the ICU: an overview. Crit Care. 2011;15:211. doi: 10.1186/cc9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mann MJ, Tseng E, Ratcliffe M, Strattman G, De Silva A, Demarco T, Achorn N, Moskalik W, Hoopes C. Use of bivalirudin, a direct thrombin inhibitor, and its reversal with modified ultrafiltration during heart transplantation in a patient with heparin-induced thrombocytopenia. J Heart Lung Transplant. 2005;24:222–225. doi: 10.1016/j.healun.2003.11.401. [DOI] [PubMed] [Google Scholar]
- 53.Frame JN, Rice L, Bartholomew JR, Whelton A. Rationale and design of the PREVENT-HIT study: a randomized, open-label pilot study to compare desirudin and argatroban in patients with suspected heparin-induced thrombocytopenia with or without thrombosis. Clin Ther. 2010;32:626–636. doi: 10.1016/j.clinthera.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Boyce SW, Bandyk DF, Bartholomew JR, Frame JN, Rice L. A randomized, open-label pilot study comparing desirudin and argatroban in patients with suspected heparin-induced thrombocytopenia with or without thrombosis: PREVENT-HIT Study. Am J Ther. 2011;18:14–22. doi: 10.1097/MJT.0b013e3181f65503. [DOI] [PubMed] [Google Scholar]
- 55.Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med. 2013;368:737–744. doi: 10.1056/NEJMct1206642. [DOI] [PubMed] [Google Scholar]
- 56.Pappalardo F, Scandroglio A, Maj G, Zangrillo A, D’Angelo A. Treatment of heparin-induced thrombocytopenia after cardiac surgery: preliminary experience with fondaparinux. J Thorac Cardiovasc Surg. 2010;139:790–792. doi: 10.1016/j.jtcvs.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 57.Grouzi E, Kyriakou E, Panagou I, Spiliotopoulou I. Fondaparinux for the treatment of acute heparin-induced thrombocytopenia: a single-center experience. Clin Appl Thromb Hemost. 2010;16:663–667. doi: 10.1177/1076029609347900. [DOI] [PubMed] [Google Scholar]
- 58.Warkentin TE. Fondaparinux: does it cause HIT? Can it treat HIT? Expert Rev Hematol. 2010;3:567–581. doi: 10.1586/ehm.10.54. [DOI] [PubMed] [Google Scholar]
- 59.Warkentin TE, Davidson BL, Büller HR, Gallus A, Gent M, Lensing AW, Piovella F, Prins MH, Segers AE, Kelton JG. Prevalence and risk of preexisting heparin-induced thrombocytopenia antibodies in patients with acute VTE. Chest. 2011;140:366–373. doi: 10.1378/chest.10-1599. [DOI] [PubMed] [Google Scholar]
- 60.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356:2653–2655; discussion 2653-2655. doi: 10.1056/NEJMc070346. [DOI] [PubMed] [Google Scholar]
- 61.Rota E, Bazzan M, Fantino G. Fondaparinux-related thrombocytopenia in a previous low-molecular-weight heparin (LMWH)-induced heparin-induced thrombocytopenia (HIT) Thromb Haemost. 2008;99:779–781. doi: 10.1160/TH07-09-0573. [DOI] [PubMed] [Google Scholar]
- 62.Salem M, Elrefai S, Shrit MA, Warkentin TE. Fondaparinux thromboprophylaxis-associated heparin-induced thrombocytopenia syndrome complicated by arterial thrombotic stroke. Thromb Haemost. 2010;104:1071–1072. doi: 10.1160/TH10-05-0284. [DOI] [PubMed] [Google Scholar]
- 63.Tardy-Poncet B, Wolf M, Lasne D, Bauters A, Ffrench P, Elalamy I, Tardy B. Danaparoid cross-reactivity with heparin-induced thrombocytopenia antibodies: report of 12 cases. Intensive Care Med. 2009;35:1449–1453. doi: 10.1007/s00134-009-1464-x. [DOI] [PubMed] [Google Scholar]
- 64.Berkley E, Kilpatrick , SJ Thrombocytopenia in preganacy: making the differential diagnosis. Contempary OB/GYN. 2009;54:36–38. [Google Scholar]
- 65.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401–407. doi: 10.1182/blood-2005-02-0626. [DOI] [PubMed] [Google Scholar]
- 66.Knol HM, Schultinge L, Erwich JJ, Meijer K. Fondaparinux as an alternative anticoagulant therapy during pregnancy. J Thromb Haemost. 2010;8:1876–1879. doi: 10.1111/j.1538-7836.2010.03926.x. [DOI] [PubMed] [Google Scholar]
- 67.Nagler M, Haslauer M, Wuillemin WA. Fondaparinux - data on efficacy and safety in special situations. Thromb Res. 2012;129:407–417. doi: 10.1016/j.thromres.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 68.Lindhoff-Last E, Kreutzenbeck HJ, Magnani HN. Treatment of 51 pregnancies with danaparoid because of heparin intolerance. Thromb Haemost. 2005;93:63–69. doi: 10.1160/TH04-06-0345. [DOI] [PubMed] [Google Scholar]
- 69.Dyke CM, Koster A, Veale JJ, Maier GW, McNiff T, Levy JH. Preemptive use of bivalirudin for urgent on-pump coronary artery bypass grafting in patients with potential heparin-induced thrombocytopenia. Ann Thorac Surg. 2005;80:299–303. doi: 10.1016/j.athoracsur.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 70.Dyke CM, Smedira NG, Koster A, Aronson S, McCarthy HL, Kirshner R, Lincoff AM, Spiess BD. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: the EVOLUTION-ON study. J Thorac Cardiovasc Surg. 2006;131:533–539. doi: 10.1016/j.jtcvs.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 71.Koster A, Hansen R, Kuppe H, Hetzer R, Crystal GJ, Mertzlufft F. Recombinant hirudin as an alternative for anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II: a 1-year experience in 57 patients. J Cardiothorac Vasc Anesth. 2000;14:243–248. [PubMed] [Google Scholar]
- 72.Martin ME, Kloecker GH, Laber DA. Argatroban for anticoagulation during cardiac surgery. Eur J Haematol. 2007;78:161–166. doi: 10.1111/j.1600-0609.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 73.Pouplard C, Gueret P, Fouassier M, Ternisien C, Trossaert M, Régina S, Gruel Y. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific to heparin/PF4 for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2007;5:1373–1379. doi: 10.1111/j.1538-7836.2007.02524.x. [DOI] [PubMed] [Google Scholar]
- 74.Crowther MA, Cook DJ, Albert M, Williamson D, Meade M, Granton J, Skrobik Y, Langevin S, Mehta S, Hebert P, et al. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. J Crit Care. 2010;25:287–293. doi: 10.1016/j.jcrc.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T’s score and particle gel immunoassay. Br J Haematol. 2008;143:721–726. doi: 10.1111/j.1365-2141.2008.07401.x. [DOI] [PubMed] [Google Scholar]
- 76.Demma LJ, Winkler AM, Levy JH. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesth Analg. 2011;113:697–702. doi: 10.1213/ANE.0b013e3182297031. [DOI] [PubMed] [Google Scholar]
- 77.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7:1260–1265. doi: 10.1111/j.1538-7836.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 78.Ruf KM, Bensadoun ES, Davis GA, Flynn JD, Lewis DA. A clinical-laboratory algorithm incorporating optical density value to predict heparin-induced thrombocytopenia. Thromb Haemost. 2011;105:553–559. doi: 10.1160/TH10-09-0610. [DOI] [PubMed] [Google Scholar]
- 79.Bakchoul T, Giptner A, Bein G, Santoso S, Sachs UJ. Performance characteristics of two commercially available IgG-specific immunoassays in the assessment of heparin-induced thrombocytopenia (HIT) Thromb Res. 2011;127:345–348. doi: 10.1016/j.thromres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Warkentin TE, Sheppard JA, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146:341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Vanholder R, Camez A, Veys N, Van Loo A, Dhondt AM, Ringoir S. Pharmacokinetics of recombinant hirudin in hemodialyzed end-stage renal failure patients. Thromb Haemost. 1997;77:650–655. [PubMed] [Google Scholar]
- 82.Eichler P, Friesen HJ, Lubenow N, Jaeger B, Greinacher A. Antihirudin antibodies in patients with heparin-induced thrombocytopenia treated with lepirudin: incidence, effects on aPTT, and clinical relevance. Blood. 2000;96:2373–2378. [PubMed] [Google Scholar]


