Abstract
AIM: To investigate whether landiolol, an ultra-short-acting β1-antagonist, can safely and effectively control heart rate in septic patients with supraventricular tachyarrhythmias.
METHODS: We reviewed all patients with sepsis who admitted to our intensive care unit between January 2006 and December 2011. Sixty one septic patients suffered from supraventricular tachyarrhythmias (heart rate ≥ 120 bpm for > 1 h). Among 61 patients, 39 patients were treated with landiolol (landiolol group) and 22 patients were not treated with landiolol (control group). Arterial pressure, heart rate, cardiac rhythm, pulmonary arterial pressure and cardiac output (if a pulmonary arterial catheter was inserted) were compared between the 2 groups at 1, 8 and 24 h after the initiation of tachyarrhythmias.
RESULTS: Mean age and Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores were similar between the 2 groups. Paroxysmal atrial fibrillation/flutter (87%), paroxysmal atrial tachycardia (10%), and paroxysmal supraventricular tachycardia (3%) were observed. The initial landiolol dose administered was 6.3 ± 5.8 g/kg per minute. Rapid and substantial reduction of heart rate was observed in the landiolol group without any deterioration of hemodynamics. Landiolol significantly reduced heart rate (from 145 ± 14 bpm to 90 ± 20 bpm) compared to the control group (from 136 ± 21 bpm to 109 ± 18 bpm, P < 0.05). The conversion to sinus rhythm was observed more frequently in the landiolol group than in the control group at every point (P < 0.01 at 8 h; P < 0.05 at 1 and 24 h).
CONCLUSION: Landiolol safely reduced heart rate and, in part, converted to sinus rhythm in septic patients with supraventricular tachyarrhythmias.
Keywords: Landiolol, Supraventricular tachyarrhythmias, Sepsis, Rate control, Conversion to sinus rhythm
Core tip: The management of tachyarrhythmia is important but it is often difficult because of unstable hemodynamics in septic patients. Landiolol is an ultra-short-acting β1 selective adrenoceptor antagonist. It exerts a more potent negative chronotropic effect and a lesser effect on blood pressure than other β blockers. In fact, landiolol significantly reduced heart rate without any deterioration of hemodynamics in this study. The most impressive finding is high conversion rate to sinus rhythm immediately after landiolol administration. Landiolol could control not only heart rate but also cardiac rhythm in septic patients with supraventricular tachyarrhythmias. Landiolol safely reduced heart rate and, in part, converted to sinus rhythm in septic patients with supraventricular tachyarrhythmia. Landiolol could be a valuable and suitable drug for managing supraventricular tachyarrhythmias in patients with sepsis.
INTRODUCTION
Supraventricular tachyarrhythmias are frequently observed in patients with sepsis. The incidence of paroxysmal atrial fibrillation/flutter (PAF) has been reported to be 31% in critically ill patients with sepsis[1]. Tachyarrhythmias have been identified as a major source of morbidity in critically ill patients[2,3]. Therefore, controlling tachyarrhythmia should be important in such patients.
Measurements of serum catecholamine level and direct measurements of renal sympathetic nerve activity have revealed that severe infection activates the sympathetic nervous system[4-9]. This activation may trigger supraventricular tachyarrhythmias in the presence of severe infection[10]. Therefore, we believed that β blockers can be used to control heart rate (HR) in patients with severe infection. However, it is difficult to use β blocker in patients with severe sepsis because of hemodynamic instability.
Landiolol (ONOACT; Ono Pharmaceutical, Osaka, Japan), a newly developed commercially available agent, is an ultra-short-acting β-adrenoceptor antagonist with a half-life of 4 min in healthy subjects. Landiolol also has high β1 selectivity (β1/β2 = 255) and is 8 times more cardioselective than esmolol[11-14]. Moreover, landiolol exerts a more potent negative chronotropic effect and a lesser effect on blood pressure than esmolol in rabbits[15,16]. In clinical situations, landiolol has been used to treat perioperative tachyarrhythmias in Japan. Landiolol reduced HR significantly without reducing blood pressure and stabilized hemodynamics in postsurgical patients[11,17-20].
Considering these characteristics, landiolol could be valuable and suitable for managing tachyarrhythmias in patients with severe infection. Therefore, we investigated whether landiolol can safely and effectively control heart rate of supraventricular tachyarrhythmias in patients with severe sepsis.
MATERIALS AND METHODS
Study design and patients selection
This historical cohort, single-center, interventional, and inter-subjective comparison study was approved by the Institutional Review Board of the Kanazawa University Hospital and was registered under ISRCTN number 70831305. Informed consent was obtained from all patients.
Medical records of all patients were screened and followed for sepsis with supraventricular tachyarrhythmia by a single intensivist in the intensive care unit (ICU) of the Kanazawa University Hospital from January 2006 to December 2011, were reviewed. Patients were included in this study if they met the following criteria: (1) systemic inflammatory response syndrome score ≥ 2 with infection; (2) ≥ 18 years of age; (3) supraventricular tachyarrhythmias with HR ≥ 120 bpm for >1 h; (4) no history of chronic supraventricular tachyarrhythmias; and (5) no supraventricular tachyarrhythmias at the time of ICU admission. Patients were divided into 2 groups: those treated with landiolol (landiolol group) and those not treated with landiolol (control group) to control HR of supraventricular tachyarrhythmias.
Measurements
Arterial pressure and HR were compared between the 2 groups at 1, 8, and 24 h after the initiation of tachyarrhythmia. We also investigated heart rhythm and the conversion to sinus rhythm. Pulmonary arterial pressure, central venous pressure (CVP), cardiac output, and cardiac index (CI) were measured if a pulmonary arterial catheter was inserted. Systemic vascular resistance index (SVRI) was calculated as follows: SVRI (dyne·s/cm5 per square meter) = 80 (mean arterial pressure-CVP)/CI.
Endpoints
The primary endpoint was HR reduction of the supraventricular tachyarrhythmias without a decrease in arterial pressure. The secondary endpoint was the frequency of conversion to sinus rhythm.
Statistical analysis
Continuous variables are expressed as mean ± SD. Patient characteristics and hemodynamics of the 2 groups were compared using an independent t test for continuous variables and with either Fisher’s exact test or a chi-square test for categorical variables. Differences of conversion rates were analyzed with Fisher’s exact test or the chi-square test as appropriate. Other data were analyzed by repeated-measures analysis of variance. In all analyses, P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Masayuki Takamura, PhD from Kanazawa University Graduate School of Medicine.
RESULTS
A total of 188 septic patients were admitted to the ICU in this period. Among them, 23 patients were excluded from analysis because of less than 18 years of age. Two patients were excluded because of atrial fibrillation at the time of ICU admission. Supraventricular tachyarrhythmias occurred in 61 patients (37.4%) in leaving 163 septic patients. Among 61 patients, 39 patients were treated with landiolol and 22 patients were not treated with landiolol.
Patient characteristics are indicated in Table 1. There were no significant differences between the 2 groups with respect to age, underlying disease, Acute Physiology and Chronic Health Evaluation II score and Sequential Organ Failure Assessment. Intra-abdominal infection was more (P < 0.05) and urinary tract infection was less (P < 0.05) in landiolol group than in control group. Respiratory tract infection was the most frequent disease in both groups.
Table 1.
Patients’ characteristics
| Landiolol | Control | |
| n | 39 | 22 |
| Age, yr | 70.7 ± 12.3 | 70.8 ± 12.5 |
| Underlying disease | ||
| Cardiovascular disease | 16 (41.0%) | 11 (50.0%) |
| Malignancy | 11 (28.2%) | 3 (13.6%) |
| Immunological disorder | 3 (7.7%) | 2 (9.1%) |
| Others | 9 (23.1%) | 6 (27.2%) |
| Infected site | ||
| Respiratory tract | 17 (43.6%) | 14 (63.6%) |
| Intra-abdominal | 13 (33.3%)a | 2 (9.1%) |
| Blood | 5 (12.8%) | 0 (0%) |
| Skin/soft tissue | 2 (5.1%) | 0 (0%) |
| Urinary tract | 1 (2.6%)a | 4 (18.2%) |
| Others | 1 (2.6%) | 2 (9.1%) |
| APACHE II | 22.8 ± 5.4 | 22.1 ± 7.7 |
| SOFA | 8.8 ± 4.0 | 9.1 ± 3.9 |
P < 0.05 vs control. APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment.
Baseline hemodynamics are summarized in Table 2. Baseline HR was higher in the landiolol group. Systolic arterial pressure and CI were lower in the landiolol group. PAF was the most frequent observation in both groups. Calcium channel blockers and antiarrhythmic agents were used to control HR or cardiac rhythm in the control group.
Table 2.
Hemodynamics
| Landiolol | Control | |
| Heart rate, bpm | 145 ± 14a | 136 ± 21 |
| Systolic arterial pressure, mmHg | 113 ± 34a | 137 ± 39 |
| Diastolic arterial pressure, mmHg | 60 ± 17 | 66 ± 13 |
| Mean arterial pressure, mmHg | 78 ± 21 | 86 ± 28 |
| Diastolic pulmonary arterial pressure, mmHg | 19 ± 6 | 20 ± 7 |
| Cardiac output, L/min | 3.9 ± 1.7 | 5.8 ± 1.5 |
| Cardiac index, L/min per square meter | 2.5 ± 1.1a | 4.0 ± 1.3 |
| SVRI, dyne∙s/m5 per square meter | 2068 ± 795 | 1615 ± 399 |
| Arrhythmia | ||
| Paroxysmal atrial fibrillation/flutter | 34 (87%) | 13 (60%) |
| Paroxysmal atrial tachycardia | 4 (10%) | 8 (36%) |
| Paroxysmal supraventricular tachycardia | 1 (3%) | 1 (5%) |
| Concomitant drugs to control arrhythmia | ||
| Calcium-channel blocker | 3 (8%) | 5 (22%) |
| Other β blockers | 0 (0%) | 3 (14%) |
| Disopyramid phosphate | 0 (0%) | 1 (5%) |
| Amiodarone | 0 (0%) | 1 (5%) |
P < 0.05 vs control. SVRI: Systemic vascular resistance index.
The initial dose of landiolol was 6.3 ± 3.3 g/kg per minute. Landiolol significantly reduced HR from 145 ± 14 bpm to 119 ± 28 bpm (P < 0.01) without reducing arterial pressure at 1 h after the initiation of tachyarrhythmia (Figures 1 and 2). At that time, HR did not change significantly in the control group (from 136 ± 21 bpm to 135 ± 21 bpm) (Figure 1). The conversion rate to sinus rhythm was 25.6% in the landiolol group but 0% in the control group (Figure 1, P < 0.05).
Figure 1.
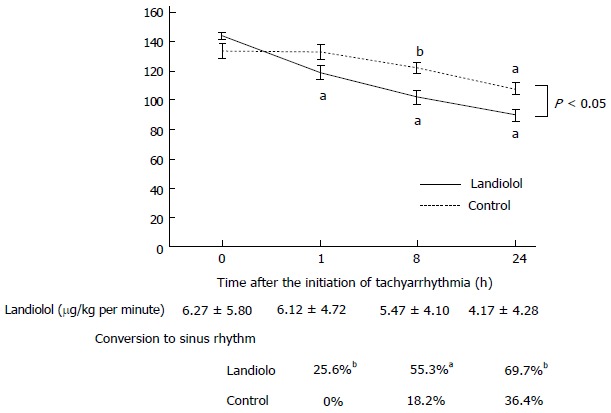
Heart rate and sinus rhythm conversion rate. Rapid and substantial reduction of heart rate (HR) was observed in the landiolol group. Reduction in HR was observed in the landiolol group than the control. In addition, the conversion from supraventricular arrhythmia to sinus rhythm was observed more frequently in the landiolol group than in the control group at every point. Results are expressed as mean ± SE. aP < 0.01, bP < 0.05 vs time 0 h.
Figure 2.
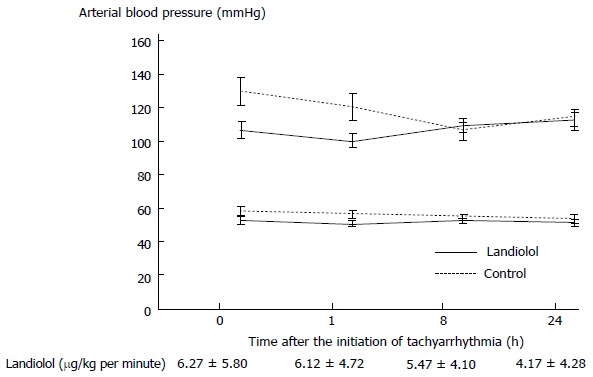
Arterial pressure. Landiolol did not change atrial blood pressure. Results are expressed as mean ± SE.
After that, a substantial reduction in HR was observed in the landiolol group without any deterioration of hemodynamics. At 24 h after the initiation of tachyarrhythmia, landiolol reduced HR dramatically from 145 ± 14 bpm to 90 ± 20 bpm (Figure 1). A lesser degree of HR reduction was seen in the control group (from 136 ± 21 bpm to 109 ± 18 bpm) than in the landiolol group (Figure 1). The conversion from to sinus rhythm was observed more frequently in the landiolol group than in the control group at every point (Figure 1, P < 0.01 at 8 h; P < 0.05 at 24 h).
Baseline diastolic pulmonary arterial pressures were similar between groups and did not change (Figure 3). In the landiolol group, baseline CI was lower and did not decrease compared to the control group (Figure 3).
Figure 3.
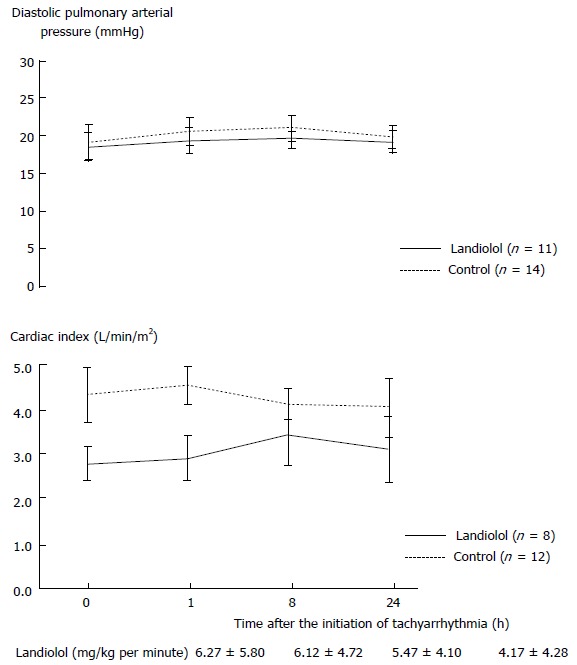
Pulmonary arterial catheter parameters. Landiolol did not affect diastolic pulmonary arterial pressure or cardiac index. Results are expressed as mean ± SE.
Finally, the duration of landiolol administration was 80.7 ± 78.5 h and the significant bradycardia have never been observed in any treated patients.
DISCUSSION
This is the first report to investigate the clinical use of landiolol for treating supraventricular tachyarrhythmia in patients with severe sepsis. Its major findings are as follows: (1) low-dose landiolol rapidly and substantially reduced HR in septic patients with supraventricular tachyarrhythmia; (2) low-dose landiolol did not reduce arterial pressure and cardiac output; and (3) low-dose landiolol immediately and significantly converted supraventricular tachyarrhythmias to sinus rhythm in septic patients.
Severe infection or sepsis generally activates sympathetic nervous system. Plasma norepinephrine and epinephrine plasma levels have been reported to be approximately 6 times and 60 times higher in conscious rats with endotoxicosis than in control rats, respectively[5]. In 1 human study, the serum levels of both norepinephrine and epinephrine were significantly higher in postoperative patients with sepsis than in those without sepsis[4]. Moreover, by direct measurement of sympathetic nerve activity, renal sympathetic nerve activity was also increased approximately 3.5 fold by the systemic administration of lipopolysaccharide in rats[6,21].
There is a close association between autonomic nervous system activity and supraventricular tachyarrhythmia. Sepsis-induced activation of the sympathetic nervous system is partially associated with supraventricular tachyarrhythmia in patients with severe sepsis[10]. Sympathetic activation of the heart facilitates arrhythmogenesis by increasing calcium entry and the spontaneous release of calcium from the sarcoplasmic reticulum[22,23]. Therefore, β blockers are the reasonable drug for controlling HR in the presence of supraventricular tachyarrhythmia in septic patients. The landiolol infusion at the dose of 5-10 μg/kg per minute much lower than described dose in the package insert, significantly decreased HR in 82% of postoperative patients with PAF[19]. Consistent with these previous studies, low-dose landiolol rapidly and substantially reduced HR in our septic patients with supraventricular tachyarrhythmia. Therefore, the low dose (6.3 ± 3.3 g/kg per minute) of landiolol administered was enough to inhibit excessive activation of sympathetic nerve activity and to significantly reduce HR in septic patients with tachyarrhythmia.
Landiolol reduced HR significantly without reducing arterial pressure and stabilized hemodynamics in postsurgical patients[11,17-20]. Consistent with these studies, landiolol neither reduces arterial pressure nor deteriorates hemodynamics in our septic patients. Recent prospective, multicenter, single-blind, randomized, parallel-group study showed that low-dose landiolol rapidly decreased HR of atrial fibrillation/flutter without an increase in the incidence of adverse events in patients with LV dysfunction[24]. Landiolol may have more negative chronotropic effect than negative inotropic effect, especially at a low dose. Landiolol has a higher β1-selectivity (β1/β2 = 255) and 8 times more cardioselective than esmolol, which is also short-acting β1-selective β adrenergic receptor blocker [11-14]. Landiolol exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol in rabbits[15,16]. Consistent with these reports, in our study, landiolol did not decrease CI despite HR reduction. Another reason is that HR reduction by landiolol causes better hemodynamics. The landiolol-induced HR reduction in patients with tachyarrhythmia allows sufficient left ventricular filling time, which subsequently allows more stroke volume. Moreover, the conversion to sinus rhythm, in part, results in sufficient atrial kick, which also creates more stroke volume. Therefore, landiolol did not decrease arterial pressure and stabilized hemodynamics.
The most impressive findings in our study is high conversion rate to sinus rhythm immediately after landiolol administration. Surprisingly, within one hour after landiolol administration, conversion to sinus rhythm from supraventricular tachyarrhythmias were observed in more than a quarter of patients treated with landiolol, but in none without landiolol. A few case studies have reported landiolol-induced conversion to sinus rhythm in patients with atrial fibrillation or flutter[25,26]. Recently, landiolol has been reported to be more effective and safer than diltiazem for conversion to normal sinus rhythm in patients with postoperative atrial fibrillation after open heart surgery[20]. Landiolol-induced reduction of HR improves hemodynamics and converts supraventricular tachyarrhythmias to sinus rhythm. However, landiolol may function as an antiarrhythmic agent and directly affects the restoration to sinus rhythm. The use of β blockers has recently been reported to have an anti-oxidative and anti-inflammatory effect. However, no study has reported the antiarrhythmic effect of landiolol in supraventricular tachyarrhythmia. As the excessive sympathetic nervous activation caused by sepsis may be associated with maintaining supraventricular arrhythmia, landiolol that has more direct suppressive effect of sympathetic activity than other drugs may cause the conversion to sinus rhythm.
Our study has several potential limitations. First, as this is the historical cohort study, the drug selection for managing tachyarrhythmia was mainly dependent on intensivists or primary doctors examining the patient then. These selection biases might have affected the results observed. However landiolol was administrated in more hemodynamically unstable patients, such as lower systolic blood pressure and lower CI, than control group. Therefore we believe that these selection biases may not overestimate the benefit of landiolol that we observed in results. Second, baseline arterial pressure was relatively high, and diastolic pulmonary arterial pressure was not very low. Because sufficient volume resuscitation was first conducted in our study, few patients with intravascular hypovolemia were observed. Third, the number of patients performed a pulmonary arterial catheter analysis was relatively a few in present study. Therefore, the power of the statistical analysis may be weak. However, we did not need to perform a pulmonary arterial catheter analysis because the patients’ hemodynamics did not worsen. Therefore, we are convinced that landiolol did not cause hemodynamic deterioration. Finally, we did not evaluate prognosis such as ICU stay length or mortality. Although ICU stay length was similar between the 2 groups, mortality was higher in the control group than in the landiolol group. We did not perform multivariate analysis of mortality; therefore, this requires further investigation.
Landiolol safely reduced heart rate and, in part, converted to sinus rhythm in septic patients with supraventricular tachyarrhythmia. Landiolol could be a valuable and suitable drug for managing tachyarrhythmias in patients with sepsis.
ACKNOWLEDGMENTS
We thank patients and staff at Kanazawa University Hospital who participated in this project.
COMMENTS
Background
Supraventricular tachyarrhythmias are frequently observed in patients with sepsis. The management of tachyarrhythmia is important as tachyarrhythmias have been identified as a major source of morbidity in critically ill patients. However it is often difficult because of unstable hemodynamics in septic patients.
Research frontiers
Landiolol, an ultra-short-acting β1 selective adrenoceptor antagonist, exerts a more potent negative chronotropic effect and a lesser effect on blood pressure than other β blockers. The current research hotspots is whether landiolol can safely and effectively control heart rate of supraventricular tachyarrhythmias in septic patients.
Innovations and breakthroughs
Landiolol significantly reduced heart rate without any deterioration of hemodynamics. The most impressive finding in the study is high conversion rate to sinus rhythm immediately after landiolol administration. Surprisingly, within one hour after landiolol administration, conversion to sinus rhythm from supraventricular tachyarrhythmias were observed in more than a quarter of patients treated with landiolol, but in none without landiolol. Landiolol could control not only heart rate but also cardiac rhythm in septic patients with supraventricular tachyarrhythmias.
Applications
Landiolol safely reduced heart rate and, in part, converted to sinus rhythm in septic patients with supraventricular tachyarrhythmia. Landiolol could be a valuable and suitable drug for managing supraventricular tachyarrhythmias in patients with sepsis.
Terminology
Landiolol, a newly developed commercially available agent, is an ultra-short-acting β-adrenoceptor antagonist (a half-life of 4 min), has high β1 selectivity (β1/β2 = 255) and exerts a more potent negative chronotropic effect and a lesser effect on blood pressure than esmolol.
Peer-review
The paper is interesting and well written.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Kanazawa University Hospital Institutional Review Board.
Clinical trial registration: This study is registered at http://www.controlled-trials.com/isrctn/. The registration identification number is ISRCTN 70831305.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors state that they have no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at masaki46228@m-kanazawa.jp.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 22, 2014
First decision: September 28, 2014
Article in press: April 29, 2015
P- Reviewer: Quesada A, Willms D, Yousef A S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23:178–183. doi: 10.1177/0885066608315838. [DOI] [PubMed] [Google Scholar]
- 2.Leibovici L, Gafter-Gvili A, Paul M, Almanasreh N, Tacconelli E, Andreassen S, Nielsen AD, Frank U, Cauda R. Relative tachycardia in patients with sepsis: an independent risk factor for mortality. QJM. 2007;100:629–634. doi: 10.1093/qjmed/hcm074. [DOI] [PubMed] [Google Scholar]
- 3.Christian SA, Schorr C, Ferchau L, Jarbrink ME, Parrillo JE, Gerber DR. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008;23:532–536. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Groves AC, Griffiths J, Leung F, Meek RN. Plasma catecholamines in patients with serious postoperative infection. Ann Surg. 1973;178:102–107. doi: 10.1097/00000658-197307000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SB, Romano FD. Plasma catecholamines in the conscious rat during endotoxicosis. Circ Shock. 1984;14:189–201. [PubMed] [Google Scholar]
- 6.Cumming AD, Kline R, Linton AL. Association between renal and sympathetic responses to nonhypotensive systemic sepsis. Crit Care Med. 1988;16:1132–1137. doi: 10.1097/00003246-198811000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Waddell SC, Davison JS, Befus AD, Mathison RD. Role for the cervical sympathetic trunk in regulating anaphylactic and endotoxic shock. J Manipulative Physiol Ther. 1992;15:10–15. [PubMed] [Google Scholar]
- 8.Saito M, Akiyoshi M, Shimizu Y. Possible role of the sympathetic nervous system in responses to interleukin-1. Brain Res Bull. 1991;27:305–308. doi: 10.1016/0361-9230(91)90116-2. [DOI] [PubMed] [Google Scholar]
- 9.Green PG, Luo J, Heller PH, Levine JD. Further substantiation of a significant role for the sympathetic nervous system in inflammation. Neuroscience. 1993;55:1037–1043. doi: 10.1016/0306-4522(93)90317-9. [DOI] [PubMed] [Google Scholar]
- 10.Otake H, Suzuki H, Honda T, Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50:627–641. doi: 10.1536/ihj.50.627. [DOI] [PubMed] [Google Scholar]
- 11.Atarashi H, Kuruma A, Yashima M, Saitoh H, Ino T, Endoh Y, Hayakawa H. Pharmacokinetics of landiolol hydrochloride, a new ultra-short-acting beta-blocker, in patients with cardiac arrhythmias. Clin Pharmacol Ther. 2000;68:143–150. doi: 10.1067/mcp.2000.108733. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama A, Takahara A, Hashimoto K. Electrophysiologic, cardiohemodynamic and beta-blocking actions of a new ultra-short-acting beta-blocker, ONO-1101, assessed by the in vivo canine model in comparison with esmolol. J Cardiovasc Pharmacol. 1999;34:70–77. doi: 10.1097/00005344-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Motomura S, Hagihara A, Narumi Y, Hashimoto K. Time course of a new ultrashort-acting beta-adrenoceptor-blocking drug, ONO-1101: comparison with those of esmolol and propranolol by using the canine isolated, blood-perfused heart preparations. J Cardiovasc Pharmacol. 1998;31:431–440. doi: 10.1097/00005344-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Iguchi S, Iwamura H, Nishizaki M, Hayashi A, Senokuchi K, Kobayashi K, Sakaki K, Hachiya K, Ichioka Y, Kawamura M. Development of a highly cardioselective ultra short-acting beta-blocker, ONO-1101. Chem Pharm Bull (Tokyo) 1992;40:1462–1469. doi: 10.1248/cpb.40.1462. [DOI] [PubMed] [Google Scholar]
- 15.Sasao J, Tarver SD, Kindscher JD, Taneyama C, Benson KT, Goto H. In rabbits, landiolol, a new ultra-short-acting beta-blocker, exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol. Can J Anaesth. 2001;48:985–989. doi: 10.1007/BF03016588. [DOI] [PubMed] [Google Scholar]
- 16.Ikeshita K, Nishikawa K, Toriyama S, Yamashita T, Tani Y, Yamada T, Asada A. Landiolol has a less potent negative inotropic effect than esmolol in isolated rabbit hearts. J Anesth. 2008;22:361–366. doi: 10.1007/s00540-008-0640-4. [DOI] [PubMed] [Google Scholar]
- 17.Konishi R, Maeda R, Endo I, Inoue N, Seo N. Successful control of rapid heart rate in a patient with atrial fibrillation by continuous intravenous administration of landiolol hydrochloride. Masui. 2003;52:515–518. [PubMed] [Google Scholar]
- 18.Ogata J, Okamoto T, Minami K. Landiolol for the treatment of tachyarrhythmia associated with atrial fibrillation. Can J Anaesth. 2003;50:753. doi: 10.1007/BF03018726. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Terajima K, Sato C, Akada S, Miyagi Y, Hongo T, Takeda S, Tanaka K, Sakamoto A. Clinical role and efficacy of landiolol in the intensive care unit. J Anesth. 2008;22:64–69. doi: 10.1007/s00540-007-0573-3. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto A, Kitakaze M, Takamoto S, Namiki A, Kasanuki H, Hosoda S. Landiolol, an ultra-short-acting β₁-blocker, more effectively terminates atrial fibrillation than diltiazem after open heart surgery: prospective, multicenter, randomized, open-label study (JL-KNIGHT study) Circ J. 2012;76:1097–1101. doi: 10.1253/circj.cj-11-1332. [DOI] [PubMed] [Google Scholar]
- 21.Pålsson J, Ricksten SE, Delle M, Lundin S. Changes in renal sympathetic nerve activity during experimental septic and endotoxin shock in conscious rats. Circ Shock. 1988;24:133–141. [PubMed] [Google Scholar]
- 22.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 23.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai R, Kinugawa K, Inoue H, Atarashi H, Seino Y, Yamashita T, Shimizu W, Aiba T, Kitakaze M, Sakamoto A, et al. Urgent management of rapid heart rate in patients with atrial fibrillation/flutter and left ventricular dysfunction: comparison of the ultra-short-acting β1-selective blocker landiolol with digoxin (J-Land Study) Circ J. 2013;77:908–916. doi: 10.1253/circj.cj-12-1618. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto N, Aomori T, Kanamoto M, Usui T, Shiga T, Yamamoto K, Saito S. Influence of hemodynamic variations on the pharmacokinetics of landiolol in patients undergoing cardiovascular surgery. Biol Pharm Bull. 2012;35:1655–1660. doi: 10.1248/bpb.b110727. [DOI] [PubMed] [Google Scholar]
- 26.Mayahara T, Goto M, Sato M, Kanazawa T, Isomine S, Nakajima H, Sakaida K. Conversion of atrial fibrillation to sinus rhythm during landiolol infusion. J Anesth. 2004;18:304–306. doi: 10.1007/s00540-004-0258-0. [DOI] [PubMed] [Google Scholar]


