Abstract
Uruguay is a middle-income country and the smallest in South America. Its population is under 3.3 million. The demographic and epidemiological characteristics are similar to those of developed countries, with a high burden associated with congenital anomalies. Infant mortality rate (IMR) decreased from 37/1000 live births, in 1980, to 8.8/1000, in 2013. This is largely explained by medical and social policies. IMR related to congenital anomalies, however, remained unchanged for the last 30 years. Therefore, programmes for prevention of congenital disorders were developed, such as the National Newborn Screening Programme. Mandatory, universal, free infant screening was implemented two decades ago. The Ministry of Public Health created the Comprehensive Plan on Birth Defects and Rare Diseases (PIDCER), to develop a strategic public policy tool enabling comprehensive, universal, quality care during their entire lifetime. Recent national legislation created provisions for newborn and infant screening, including for congenital hypothyroidism, phenylketonuria, congenital adrenal hyperplasia, cystic fibrosis and medium-chain acyl-CoA dehydrogenase, via blood spot test, otoacoustic emissions, systematic physical examination and hip ultrasound. We discuss how this programme was implemented, the current situation of rare diseases, the institution managing disability in Uruguay and the development of new laws based on the MPH’s PIDCER. It illustrates how Uruguay is developing public policies in the genomic era, based both on science and bioethics.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-015-0236-2) contains supplementary material, which is available to authorized users.
Keywords: Newborn screening, Bioethics, Genetics, Rare diseases, Public policy, Congenital disorders
Introduction
Uruguay is a middle-income country and the smallest in South America, with a population less than 3,300,000, and approximately 48,000 annual births. The infant mortality rate (IMR) declined from 37/1000 live births, in 1980, to 8.8/1000, in 2013. This is largely explained by policies introduced by the Ministry of Public Health (MPH) and other social policies. The rate of infant mortality due to malformations, deformations and chromosomal anomalies (MDCA) CIE-10 has shown an almost static trend over the last 30 years, at around 2.4–3.0/1000 and accounting for 1 in 4 deaths. In 2012, MDCA corresponded to 28 % of infant mortality (124/447) (Larrandaburu and Noble 2013). According to the Latin-American Collaborative Study of Congenital Malformations (ECLAMC), 1000 to 1900 children are born each year with congenital anomalies (Castilla and Orioli 2004; Larrandaburu and Giachetto 2012).
This work discusses the current situation on rare diseases (RD), the institution dealing with disability, the development of new laws based on the MPH’s Comprehensive Plan on Birth Defects and Rare Diseases (PIDCER) and the relevant ethical issues (Larrandaburu et al. 2013).
Congenital anomalies, a public health problem
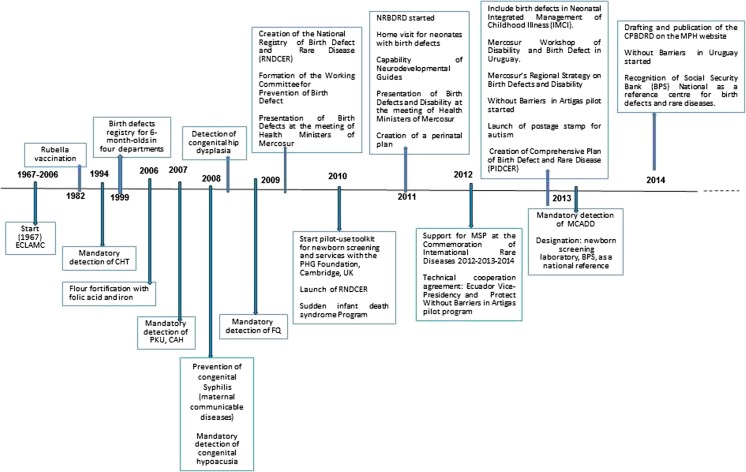
Congenital anomalies are considered, worldwide, a public health problem. They are associated with significant morbidity and frequently cause disability and represent a significant cost to the health system and society as a whole (Christianson et al 2006; Howson et al 2008). Some actions driven by the MPH in our country pertaining to congenital disease have been in place for several years, while others were recently initiated or are still in the planning phase. These include prevention, detection, registration, treatment, monitoring and research into the aetiology of congenital anomalies (CA) (Fig. 1). The MPH placed these disorders on the Public Agenda, in accordance with the resolution of the 63rd World Health Assembly, in 2010 (WHO 2010). This called on member states to prevent CA and made a series of recommendations: (1) to develop and implement newborn screening programmes and (2) to provide support to those affected and their families. One of the priorities was to evaluate and organise the National Newborn Screening Programme, in conjunction with the UK’s Public Health Genomics Foundation, using their Toolkit for Health Needs Assessment in Congenital Disorders (Nacul et al. 2014).
Fig. 1.
Actions taken by the Ministry of Public Health of Uruguay in relation to birth defects and Disability, time line
History and results from newborn screening in Uruguay
National programmes are intended to reduce mortality and possible disabilities associated with endocrine, metabolic or genetic CA (Borrajo 2007). They have been applied systematically on a large scale. In the early 1960s, the first newborn screening programme, aimed at the detection of phenylketonuria (PKU), was established in the USA. The development of filter paper for collection of blood spots by Robert Guthrie enabled mass screening (Ross 2008). Canada and the USA were the first countries to create a pilot screening programme for congenital hypothyroidism (American Academy of Pediatrics 2006). Public health is the science and art of preventing disease, prolonging life and promoting health through organised measures (Winslow 1920). Newborn screening (NBS) fits perfectly with this concept, by including early detection and pre-symptomatic treatment. In Uruguay, newborn screening started in the early 1990s, with the measurement of thyroid stimulating hormone (TSH) levels in the umbilical cord blood. Since 1994, this became mandatory through the screening programme to detect congenital hypothyroidism (CH) (Queiruga 1994), which has expanded progressively to include other diseases. Table 1 summarises those included in the NBS, the corresponding Orphanet number and the estimated incidence rate according to the National Registry of Congenital Defects and Rare Diseases 2010–2013 (RNDCER 2014). Total births in this period were 191,008.
Table 1.
Incidence of disease included in the mandatory National Newborn Screening Programme detected by blood and reported to National Registry of Congenital Defects and Rare Diseases (RNDCER) 2010–2013
| Disease | Orphanet No. | Year started | Absolute cases | Incidence |
|---|---|---|---|---|
| Congenital hypothyroidism—CHa | ORPHA442 | 1994 | 48 | 1:3900 |
| Phenylketonuria—PKUb | ORPHA716 | 2008 | 10 | 1:19,000 |
| Congenital adrenal hyperplasia—CAHb | ORPHA418 | 2008 | 12 | 1:15,800 |
| Cystic fibrosis—CFc | ORPHA586 | 2010 | 18 | 1:10,500 |
| Medium-chain acyl CoA dehydrogenase deficiency—MCADDd | ORPHA42 | 2008 | 3 | 1:63,300 |
The National Newborn Screening Programme in Uruguay (PNPNL) is mandatory, universal and free. It was founded through the coordinated efforts of different state institutions. Systematic detection of congenital deafness through otoacoustic emissions (OEA) has been mandatory since 2009 (Uruguay 2008) and is conducted by licensed speech therapists.
The introduction of tandem mass spectrometry (MS/MS) in NBS produced a technological revolution worldwide, as it allows the detection of multiple congenital metabolic anomalies in a single test using one blood spot (Wilcken et al. 2003). The use of MS/MS in Uruguay began as a pilot study in 2008 and has not yet been completed. More 20 metabolic diseases are currently included in the screening (Queiruga et al. 2010); however, only PKU and medium-chain acyl-CoA dehydrogenase deficiency (MCADD) screening are mandatory.
Haemoglobinopathies were included in the pilot study in 2012, as a result of technological availability combined with the special interest by the community of African descent and the Health Initiative Group (Iniciativas Sanitarias y Anemia Falciforme 2014). The last census conducted in Uruguay in 2011 showed that 4.8 % of the population reports that their main ancestry is African (Cabella et al. 2013). Studies in Montevideo (where about half of the population lives), based on blood groups, serum proteins and red cell polymorphisms, showed a contribution of 7 % of African genes, 92 % European and 1 % Amerindian. In the northeast of the country (Tacuarembó Department), the contribution from each of these ethnic groups was 15, 65 and 20 %, respectively (Sans et al. 1997). Hidalgo et al. (2005) also demonstrated a ‘tri-hybrid’ model of the Uruguayan population, with 5.6 % of African genes. Since the start of the pilot plan, four cases of haemoglobinopathy were reported to RNDCER: two of sickle-cell anaemia and two double heterozygotes for haemoglobin C/beta-thalassemia and haemoglobin S/beta-thalassemia.
The last presidential decree concerning NBS in Uruguay 2013 (Table 1) standardises, regulates and establishes several concepts: (1) it defines the PNPNL as a set of medical practices involving compulsory NBS for genetic, endocrine and other diseases thorough (a) blood spot, (b) otoacoustic emission and (c) strengthening the systematic newborn physical examination to detect minor or major congenital anomalies and hip dysplasia by ultrasound; (2) centralises the country’s newborn screening to a single laboratory, Social Insurance Bank (BPS) Screening Laboratory, which guarantees uniformity and rapid diagnostic confirmation, treatment and follow-up of those cases recognised through screening; (3) evaluates the health needs of the population, based on bioethical principles and scientific evidence related to the pathologies included or excluded in the PNPNL. It is important to highlight that the bioethical principles included in the law relate to beneficence, non-maleficence, justice, autonomy and the preservation of confidentiality.
The PNPNL dynamics in Uruguay is as follows: All newborns, either in public or private hospitals, are entered in the programme. If the diagnosis is confirmed, they are entitled to receive adequate, lifelong treatment and follow-up (medical, pharmacological, nutritional, surgical and speech therapy), as well as genetic counselling. The coverage is virtually 100 % of births in the country (Lemes et al. 2012). Obtaining a blood spot from the heel is routinely performed 40 h after birth (which makes it likely that the infant has begun to consume but has not yet left hospital). If a new sample is required, the parents are contacted immediately (Table 2).
Table 2.
The National Newborn Screening Programme in Uruguay, blood spot dynamics
| Phases | Pre analytical | Analytical | Post analytical |
|---|---|---|---|
| Actions | •Blood collection in all maternity units in Uruguay •Timely submission of samples for analysis |
•Storage, processing and analysis of samples at: •Social Insurance Bank (BPS)a National Screening Laboratory •Laboratories that perform umbilical cord blood TSH measurements |
•Normal •Pathological: repeat test of the first sample. •If abnormal: a second sample is requested. •Continued abnormal result: confirmatory testing. •If positive for disease: RNDCERb is notified, and treatment and follow-up begins. •Make contact by telephone or actively search for patient in suspected or confirmed cases. |
| Responsible | •Public and private health providers •National Post Office (sample transportation) |
•National Screening Laboratory Social Insurance Bank (BPS) •22 laboratories from public and private providers |
•Social Insurance Bank (BPS) •Public and private providers •Customs Programmec •CHLAEPd |
The customs plan is a programme in operation for over 30 years in the public health system, which aims to ensure that newborns and breastfeeding women can be traced following discharge from the Maternity in Montevideo: public health sector users residing in the capital are thus contacted and taken to a health facility for a repeat sample collection (ASSE 2014). Patients from the countryside are contacted through the Honorary Commission to Fight Tuberculosis and Prevalent Diseases (CHLAEP 2014). Both patients and their accompanying family members are entitled to transportation from their place of origin and housing during their stay at the capital (BPS 2014). In cases where cystic fibrosis (CF), PKU or MCADD are detected, patients both in the public and private sectors are referred to the Department of Medical and Surgical Specialties (DEMEQUI) of BPS located in Montevideo where treatment, genetic counselling and disease follow-up are centralised.
From January 2010 to December 2013 (Table 1), the following were reported: 10 cases of PKU, 18 cases of CF, three cases of MCADD, 48 cases of CH and 12 cases of congenital adrenal hyperplasia (CAH). For CH and CAH, however, there is no systematic monitoring and decisions are left to the judgement of the physician they voluntarily selected; however, if the provider does not offer genetic counselling, patients have the right to request it from BPS. Much concern exists at the ministerial level about compliance with the timeframe set by PNPNL, which should not exceed 30–45 days between diagnosis and the start of treatment.
At present, if a non-mandatory disease is diagnosed, the BPS (BPS 2014) assumes comprehensive care of the patient, including consultation with a multidisciplinary group, nutritional and pharmacological treatment, rehabilitation and genetic counselling, independently of the health provider they are assigned.
Mandatory otoacoustic emissions are performed on all newborns prior to discharge. If hearing loss is detected, children are entitled to hearing aids and/or cochlear implants. Since the programme’s inception in 2009, fewer than 10 children have had cochlear implants. There are no published data regarding coverage or sensitivity of the screening programme for congenital hearing loss.
All of these actions by the MPH, in connection with the PNPNL, are relatively new, and their impact in terms of public health is not yet known. Therefore, we investigated its impact on the knowledge concerning RD and current policies promulgated by the MPH among users of the system (i.e. those affected by RD, their families and members of the National Honorary Commission of the Disabled, CNHD (2014).
Survey about public awareness of rare diseases, congenital anomalies and current policies
A 10-question survey was developed. This was anonymous, completed via the internet (SurveyMonkey®) and available for 10 days in October 2014. The questionnaire was given to both members and substitute members of the Associations of Families and Individuals Affected by Rare Diseases and Other Disabilities and members of the CNHD (Uruguay 2014); 58 invitations were sent via email (to 43 members of the CNHD, which includes four disability federations, and to 15 individuals or members of the associations of families and individuals affected).
Those directly involved in NBS have not taken part in this survey (health professionals, associations of people suffering from the diseases included as part of the NBS and their parents). Those groups are expected to have a better understanding of the subject, so that the data presented here are biased in that we anticipate a lower level of knowledge among the actual participants.
The questionnaire employed is provided as electronic supplementary material. Briefly, the questions were designed to determine the individual’s knowledge about RD, their impact on the population and the new governmental policies related to RD. It also included some open questions about the survey participants’ views on the efficacy of these policies, the need for improvement and the role of respondents in relation to those needs; 33 responses (57 %) were obtained, 22 (67 % of these) being from affected individuals or members of associations.
Only 25 % of respondents answered that RD are conditions that affect fewer than 1:2000 people born (European Commission Public Health Position Statement on Rare Diseases and Orphan Drugs 2014); 38 % suggested an incidence of 1:100,000 and 28 % 1:10,000. The majority felt that there is a lot of misinformation regarding these diseases, both among professionals (81 %) and the general population (87 %). Only 42 % reported having knowledge of the health policies in respect to these diseases. A similar proportion knew that most disorders identified by the PNPNL are defined as RD.
More than half believed that Uruguay is still deficient in terms of health care, medical knowledge, educational and professional integration in society and laws concerning RD. In spite of this, almost half (48 %) acknowledged that there is specific, new legislation for NBS implemented as of 2013 and 76 % believe that this approach can better prevent a large number of CA.
Interestingly, when answering the question regarding the reasons why these disorders were selected by PNPNL, the responses were disease prevalence (42 %), availability of a screening test and diagnostic confirmation (48 %), existence of treatment (33 %) and the lobbying activities of pressure groups (36 %). When asked what should follow confirmation of the diagnosis of a RD, the answers were ensuring lifelong treatment (91 %), monitoring (87 %) and genetic counselling (76 %); the majority (91 %) thought that recovery from these diseases could not be guaranteed although treatment may have positive benefits. Regarding the role of the participants in improving the current situation, the responses were to disseminate knowledge (84 %), increase awareness (81 %) and education (69 %). Of the association representatives, 46 % thought their disease could be included in the PNPNL in the near future, while 36 % considered it not to be feasible.
Economic impact
There is no current information published on individual screening costs for the detection of the mandatory diseases or those included in the pilot plan in Uruguay. In 2007, Costa Rica achieved one of the most complete coverages of NBS in the world (98.9 %) and spent US$18 per newborn for the detection of 24 diseases (Manuel Saborio, personal communication). The current technological capability to identify genetic anomalies at the population screening level are virtually unlimited and can challenge the health system, in respect to both science and economics (Pàmpols-Ros et al. 2010). The global trend is for laboratories to reduce the costs of these tests; however, the main challenge for newborn and prenatal screening is ensuring access to confirmatory tests, treatment, follow-up and genetic counselling. Services must thus be readily available, as failing to meet the needs revealed by screening will generate ethical problems in healthcare. Although difficult to assess, costs must also be taken into consideration. These elements were already considered by the WHO Expert Panel, in 2006, which made three basic recommendations for developing countries, prior to initiating a NBS programme: (a) carefully weigh the priorities in general healthcare, particularly in genetics; (b) take into account the necessary infrastructure and services; and (c) analyse cost-effectiveness. According to 91 % of respondents, they believe that lifelong treatment must be guaranteed for diseases detected through screening, whereas 87 % said that monitoring was also a priority and 76 % believe that genetic counselling must be available. Less than 10 % considered a cure to be a necessity. Other relevant points included were to encourage community involvement and comprehensive support for those affected and their families.
The second principle of Wilson and Jungner (1968), which states that there should be a known and accepted treatment for diseases included in a NBS programme, has already been achieved for sickle cell anaemia and other haemoglobinopathies, CF and some congenital errors of metabolism. The criteria used for these additions have been based on the fact that early detection and prevention, coupled with therapeutic advances, increase survival of children who would otherwise not reach adulthood (Forman et al. 2013; Leão and Aguiar 2008). For many inborn errors of metabolism, early diagnosis allows supportive treatments and genetic counselling for parents and other relatives. While this could justify testing, the lack of the need for written informed consent when screening for such diseases is controversial.
It is interesting to point out that only 33 % of those surveyed considered this second principle applicable for disease selection in the NBS programme in Uruguay and that lobbyists were somewhat more important in the selection of the disease (36 %). Other responses pointed to the increasing biotechnological capabilities of the country.
In the 1990s, the use of MS/MS to detect or exclude congenital metabolic defects changed the scientific and political landscape of NBS. Reports from the Evaluation of Healthcare Technologies suggested using MS/MS only for MCADD and PKU; however, several programmes around the world have expanded the number of diseases identified, and this was often done without the support of a pilot study (Ross 2008). In Uruguay, the pilot plan has included expanded studies. These techniques have brought forth the ethical problem of false positives, as they have a psychological impact on the family in cases where the test needs to be repeated. This situation generates anxiety and stress in parents and a possible misperception of the health of the newborn. These problems are minimised when proper information is given to the family prior to testing, as well as the education of parents and the dissemination of information in the general population regarding these public health actions (Gurian et al. 2006; Tu et al. 2012). At present, however, there are no national data available regarding the impact on the population of Uruguay of the use of MS/MS.
Ethical aspects
NBS is an extension of genetic screening programmes that have been applied on a massive scale for more than half a century (WHO 2006). These programmes are represented on all continents and have transformed the lives of many families around the world. Its implementation has produced an enormous amount of accumulated experience that has been crystallised into countless publications. One of the topics always addressed are the ethical and genetic questions produced by large-scale public health actions for the early detection of disease, timely treatment and the prevention or lessening of serious and irreversible health damage (Galán-Rodas et al. 2013). Uruguay has over two decades of experience in NBS for CH, having diagnosed 350 cases of this condition (Queiruga et al. 2010). There is no documented evidence regarding the acceptance of NBS by the general population or health professionals. There is also no available information on the rejection by parents or relatives of tests performed on newborns or infants. The results of our survey showed that 76 % support the new laws in Uruguay and that these approaches may help prevent CA.
It is not a common practice to ask for written informed consent for mandatory or optional screening. Those tests are mandatory and performed before discharge from hospital, thus ensuring compliance. There is some consensus that the positive aspects of mandatory NBS make up for the invasion of privacy and loss of parental autonomy (Pelias and Markward 2001). As reported by Pàmpols-Ros et al. (2010), the ethical justification given for mandatory NBS is that societies should promote the welfare of children, through early detection and treatment of screened diseases, in spite of parental objection. This means that, in many cases, the affected families are forced to deal with a situation without being properly informed, consulted or prepared. On the other hand, some authors like Tuja Takala (1999) discuss the right of people to know and not to know the results of diagnostic tests in genetic diseases. This position is based on recognising the autonomy of the subjects, which gives them the right to remain in ignorance. This right can be invoked in different situations for adults, but in NBS, problems intensify once parents make decisions on behalf of their children. We must recognise, however, that NBS not only implies knowledge of a specific diagnosis but will also usually translate into preventative and therapeutic measures that will significantly impact on the child through the prevention of disability. In countries where health information is deficient, NBS programmes can be used as an opportunity to educate. It would be ideal for people to choose freely to accept NBS, to see it as a right, to empower themselves and set it as a priority. This right is based on individual choice, after the parent has obtained all information necessary to make a responsible decision. This needs to be disseminated through healthcare authorities. Education and training are the main components of NBS. Although the collection of blood samples is mandatory at hospitals, parents can still opt out when a repeat sample is needed. This model thus preserves the autonomy of parents, while ensuring that the majority of newborns are tested (Schneider 2009).
Comprehensive plan of birth defects and rare diseases (PIDCER)
There are important ethical, social and legal issues that apply to medical genetic services and NBS. We will analyse these issues separately and from the perspective of Uruguay’s PIDCER (Ordinance 767/2013) (Fig. 1), which includes the PNPNL as one of its key elements. This public policy tool, recently established by the MPH in Uruguay, has a seven-point plan for action directed towards the general population and to groups with special needs: (1) development and updating of regulatory and legal framework; (2) promotion of healthcare; (3) surveillance and monitoring services; (4) organisation of healthcare services; (5) establishment of human health resource services; (6) strengthening national, regional, and international cooperation; and (7) investigation.
Distributive justice
Uruguay is moving towards regulation of all services in medical genetics and the creation of medical genetics services that guarantee equal access to the entire population, independently of their economic and social status.
Non-discrimination or stigmatisation
PIDCER is based on the Convention on the Rights of Persons with Disabilities (United Nations 2006). One of its primary objectives is to raise population awareness and thereby avoid discrimination against minority groups and carriers of congenital and non-congenital conditions. The CNHD (2014) and the National Disability Programme (PRONADIS 2014) hold political responsibility in terms of disability within the state structure and through the 18.651 law, which point to the comprehensive protection of the rights of people with disabilities (Uruguay 2010). The health chapter specifies that the state should implement strategies to support and contribute towards the prevention of impairment and disability, through different actions, such as genetic counselling and research on metabolic and other disorders, to prevent genetic diseases and congenital anomalies. The CNHD is composed of governmental institutions and members of civil society (e.g. the associations of those affected by genetic disorders).
Beneficence and non-maleficence
This is linked to the National Integrated Health System-SNIS (Uruguay 2008). Its aims include the integration of public and private sub-systems, promoting services and defining responsibilities common to both, with a single funding system: the National Health Fund (FONASA), which ensures universal coverage and to which the Resources Fund (FNR 2014) is linked, aiming at the provision of highly specialised medicine. Integration of medical genetics in SNIS is still evolving, since the health services still lack formal medical genetic services. The BPS has two geneticists who see patients diagnosed through NBS. Some health provider services have geneticists and others hire outsourced professionals. This law makes it clear that the prevention of disability and impairment is an essential aspect of SNIS (González et al. 2009). The rights that users have in terms of primary, secondary and tertiary prevention of birth defects are promoted from key elements set forth by PIDCER.
Protection of privacy and confidentiality
Regulation of privacy and confidentiality are part of the rights of patients and are established within the laws of our country in the SNIS and, hence, in PIDCER. Compliance is a safeguard against any action that may discriminate against or stigmatise patients with CA, whether or not of genetic origin. Since 2012, the Sexual and Reproductive Health Law and regulatory decree (Uruguay 2012a, b) have moved towards the improvement of sexual and reproductive health services, in terms of gender rights and equality, reduction of risks from unsafe abortion and reduction in mortality and morbidity of women. Unlike other countries in the region, where abortion is illegal, voluntary interruption of pregnancy is allowed in Uruguay, in safe conditions, up to week 12, and at any gestational stage once a disease incompatible with life is diagnosed. In case of termination on account of a severe foetal anomaly, permission needs to be sought from the Committee of Pregnancy Interruption of the Ministry of Public Health (Uruguay 2012b). This legislation specifies that voluntary termination of pregnancy (TOP) requires signed informed consent, with protection of personal data. The law determines that no woman expressing the desire to terminate a pregnancy and meeting the requirements of the law will be denied this (Mautone and Rodríguez 2013). Therefore, access to TOP is a universal right for women, free or with a minimal cost (MSP 2014).
It is important to highlight that those families with a history of having a child diagnosed with a metabolic disorder through NBS are entitled to know their reproductive risk. Legislation grants a certain amount of flexibility regarding the decision to abort outside the legal timeframe, if there is a risk to the life or bio-psycho-social health of the woman.
The focus of NBS in Latin America, translated into the language of law and politics, usually refers to tests performed through dried blood spots and, in some cases, detection of congenital deafness (Borrajo 2007; Giugliani 2010) and does not consider physical examination of the newborn under the same programme. The PNPNL, in Uruguay, considers this to be the first part of the NBS programme, which is low cost, of high performance and does not generate apparent ethical issues, unlike practice in the UK, which has a specific programme for physical examination of the newborn (NHS Newborn and Infant Physical Examination Programme 2014). In Uruguay, 99 % of births take place in hospital and this exam is performed routinely at birth and before discharge from hospital.
Final thoughts
There are still many challenges ahead. Namely, a national debate regarding screening of haemoglobinopathies should be promoted immediately, in order to determine whether or not to incorporate them into the mandatory screening, as well as defining the position on expanded screening and whether making it mandatory would be of benefit. Improved training of health personnel for the identification of congenital anomalies is another priority. In the meantime, applying the new whole-genome or whole-exome sequencing (WGS/WES) to newborn and prenatal screening must be discussed. This is already the focus of debate in some countries, like Canada and the UK (Bombard et al. 2014). There is an urgent need to translate the results of scientific research into policy, in a responsible way. Developed countries are already obtaining successful results; the focus should now be directed towards middle- and low-income countries (Kancherla et al. 2014). Political will and commitment of these nations are indispensable for developing plans on a global scale to prevent birth defects and disability. Some worldwide and regional initiatives are already underway, such as the Global Disability Action Plan (WHO 2014), the Regional Plan of Action on Disability and Rehabilitation (PAHO 2014) and Mercosur’s Regional Strategy on Birth Defects and Disability (Ministry of Public Health of Uruguay 2013).
The results of the present work provide a baseline for the situation on congenital anomalies and RD in Uruguay. This is based on the opinions of those surveyed in relation to the state of medical knowledge, the availability of medical care, the arguments for and against including additional disorders in the screening programme, the role of disease support associations, the views of those affected by these disorders and national health policies. It also shows that the smallest country in South America is moving along this path, by incorporating sustainable legislation that is based on human rights, ethical principles, scientific evidence, public debate and the participation of patients and families in public healthcare policies.
Finally, we believe that the current PNPNL answers the major current needs of Uruguay, placing it at the forefront in the region with respect to addressing these issues, which certainly should have a comprehensive focus based on rights and inclusion. This ongoing project raises major challenges but might already be changing the current landscape and will eventually benefit the current and future citizens of this country.
Electronic supplementary material
(PDF 1045 kb)
Acknowledgments
Dr. Larrandaburu has a PhD scholarship from CAPES (Brazilian Ministry of Education).
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethical standards
This article does not contain any studies on human or animal subjects.
Footnotes
This article is part of the special issue on “Genetics and Ethics in Latin America”
Contributor Information
Mariela Larrandaburu, Phone: 59824091200, Email: marielalarrandaburu@gmail.com.
Lavinia Schuler-Faccini, Phone: 555199756770, Email: lavinia.faccini@ufrgs.br.
References
- American Academy of Pediatrics (2006) Susan Rose, and the Section on Endocrinology and Committee on Genetics, American Thyroid Association, Rosalind Brown, and the Public Health Committee and Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 117:2290-2303 [DOI] [PubMed]
- ASSE (2014) Administración de los Servicios de Salud del Estado. Available at: www.asse.com.uy/uc_7414_1.html. Accessed 25 Jan 2015
- Bombard Y, Miller FA, Hayeems R, et al. Public views on participating in newborn screening using genome sequencing. Eur J Hum Genet. 2014;22:1248–1254. doi: 10.1038/ejhg.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrajo GJ. Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis. 2007;30:466–481. doi: 10.1007/s10545-007-0669-9. [DOI] [PubMed] [Google Scholar]
- BPS (2014) Banco de Previsión Social. Available at: www.bps.gub.uy/3555/ayudas_especiales.html. Accessed 25 Jan 2015
- Cabella W, Nathan M, Tenenbaum M (2013) La población afrouruguaya en el censo 2011. En Atlas Sociodemográfico y de la Desigualdad en Uruguay. Programa de Población. Facultad de Ciencias Sociales, p.15. Ediciones TRILCE. Available at: www.ine.gub.uy/biblioteca/Atlas_Sociodemografico/Atlas_fasciculo_2_Afrouruguayos.pdf. Accessed 25 Jan 2015
- Castilla EE, Orioli IM. ECLAMC: the Latin-American collaborative study of congenital malformations. Community Genet. 2004;7:76–94. doi: 10.1159/000080776. [DOI] [PubMed] [Google Scholar]
- Christianson, A, Howson CP, Modell B (2006) Global report on birth defects. The Hidden Toll of dying and disabled children. March of Dimes Birth Defects Foundation. White Plains. Available at: www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-full-report.pdf. Accessed 25 Sept 2014
- CHLAEP (2014) Comisión Honoraria de la Lucha Antituberculosa y Enfermedades Prevalentes. Available at: www.chlaep.org.uy. Accessed 20 Sept 2014
- CNHD (2014) Comisión Nacional Honoraria de la Discapacidad. Available at: www.cnhd.org/intgracion.htm; Accessed 20 Sept 2014
- European Commission Public Health Position Statement on Rare Diseases and Orphan Drugs (2014) EC regulation on orphan medicinal products. Available at: http://ec.europa.eu/health/rare_diseases/policy/index_en.htm. Accessed 20 Sept 2014
- FNR (2014) Fondo Nacional de Recursos. Available at: www.fnr.gub.uy. Accessed 25 Sept 2014
- Forman J, Coyle F, Levy-Fisch J, Roberts, Terry SH, Legge M. Screening criteria: the need to deal with new developments and ethical issues in newborn metabolic screening. J Community Genet. 2013;4:59–67. doi: 10.1007/s12687-012-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán-Rodas E, Dueñas M, Obando S, Saborio M. Tamizaje neonatal en el Perú: ¿hacia dónde vamos? Rev Peru Med Exp Salud Publica. 2013;30:714–728. [PubMed] [Google Scholar]
- Giugliani R. Inborn errors of metabolism in Latin America: challenges and opportunities. J Inherit Metab Dis. 2010;33(Suppl 2):S315–S320. doi: 10.1007/s10545-010-9112-8. [DOI] [PubMed] [Google Scholar]
- González T, Olesker D, Oreggioni I et al. (2009) La Construcción del Sistema Nacional Integrado de Salud 2005–2009. Available at: www.psico.edu.uy/sites/default/files/cursos/nas_la_construccion.pdf. Accessed 25 Sept 2014
- Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- Hidalgo P, Bengochea M, Abilleira D, Cabrera A, Alvarez I. Genetic admixture estimate in the Uruguayan population based on the Loci LDLR, GYPA, HBGG, GC and D7S8. Int J Hum Genet. 2005;5:217–222. [Google Scholar]
- Howson CP, Christianson A, Modell B (2008) Controlling birth defects: reducing the hidden toll of dying and disabled children in low-income countries, disease control priorities project. Available at: www.marchofdimes.org/materials/partner-controlling-birth-defects-reducing-hidden-toll-of-dying-children-low-income-countries.pdf. Accessed 30 Sept 2014
- Iniciativas Sanitarias y Anemia Falciforme (2014) Available at: www.iniciativas.org.uy/interculturalidad. Accessed 25 Sept 2014
- Kancherla V, Oakley GP, Jr, Brent RL. Urgent global opportunities to prevent birth defects. Semin Fetal Neonatal Med. 2014;19:153–160. doi: 10.1016/j.siny.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Larrandaburu M, Giachetto G. Importancia de los defectos congénitos y enfermedades raras. In: Pérez M, Acosta C, Callorda C, editors. Seguimiento domiciliario del binomio madre-hijo en situación de vulnerabilidad. Montevideo: Facultad de Enfermería, Comisión Sectorial de Educación Permanente; 2012. pp. 93–104. [Google Scholar]
- Larrandaburu M, Noble A. Los defectos congénitos. Síndrome de las tres D. Rev Méd Urug. 2013;29:250–252. [Google Scholar]
- Larrandaburu M, Noble A, Olivera Z (2013) Ministerio de Salud Pública, Plan Integral de Defectos Congénitos y Enfermedades Raras-PIDCER. Available at: www.msp.gub.uy/sites/default/files/archivos_adjuntos/DOCUMENTO%20MARCO%20PIDCER.pdf. Accessed 30 Oct 2014
- Leão L, Aguiar MJ. Newborn screening: what pediatricians should know. Triagem neonatal: o que os pediatras deveriam saber. J Pediatr (Rio J) 2008;84(4 Supl):S80–S90. doi: 10.1590/S0021-75572008000500012. [DOI] [PubMed] [Google Scholar]
- Lemes A, Queijo C, Garlo P, Machado M, Queiruga G. Pesquisa Neonatal. Arch Pediatr Urug. 2012;83:40–44. [Google Scholar]
- Mautone M, Rodríguez H. Objeción de conciencia en el ámbito de la salud. Rev Méd Urug. 2013;29:40–42. [Google Scholar]
- MSP (2014) Ministerio de Salud Pública. Available at: http://www.msp.gub.uy/noticia/interrupci%C3%B3n-voluntaria-del-embarazo-tasas-moderadoras. Accessed 25 Jan 2015
- Nacul LC, Stewart A, Alberg C, et al. A toolkit to assess health needs for congenital disorders in low- and middle-income countries: an instrument for public health action. J Public Health. 2014;36:243–250. doi: 10.1093/pubmed/fdt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Newborn and Infant Physical Examination Programme (2014). Available at: http://newbornphysical.screening.nhs.uk. Accessed 30 Oct 2014
- PAHO (2014) Panamerican Health Organization. Plan of action on disabilities and rehabilitation. 53rd Directing Council 66th Session of the Regional Committee of WHO for the Americas. Available at: www.paho.org/hq/index.php?option=com_content&view=article&id=9774:53rd-directing-council&Itemid=41062&lang=es. Accessed 30 Oct 2014
- Pàmpols-Ros T, Terracini B, Abajo-Iglesias FJ, et al. Recomendaciones sobre los aspectos éticos de los programas de cribado de población para enfermedades raras. Rev Esp Salud Pública. 2010;84:121–136. doi: 10.1590/S1135-57272010000200002. [DOI] [PubMed] [Google Scholar]
- Pelias M, Markward N. Newborn screening, informed consent, and future use of archived tissue samples. Genet Test. 2001;5:179–185. doi: 10.1089/10906570152742218. [DOI] [PubMed] [Google Scholar]
- PRONADIS (2014) Ministerio de Desarrollo Social, Programa Nacional de la Discapacidad. Available at: http://pronadis.mides.gub.uy. Accessed 30 Oct 2014
- Queiruga G. Detección sistemática de Hipotiroidismo Congénito a todos los recién nacidos de Uruguay. Asociación de Química y Farmacia del Uruguay. Rev Institucional. 1994;11:7–11. [Google Scholar]
- Queiruga G, Lemes A, Ferolla C, Machado M, Queijo C, Garlo P, Parallada G (2010) Pesquisa Neonatal: Lo que una Gota de Sangre Puede Prevenir. Instituto de Seguridad Social. Primera Edición. Available at: www.bps.gub.uy/documentos/folletos/pesquisa%20neonatal.pdf. Accessed 25 Oct 2014
- RNDCER (2014) Registro Nacional de Defectos Congénitos y Enfermedades Raras. Available at: www2.msp.gub.uy/uc_4896_1.html. Accessed 28 Oct 2014
- Ross LF. Ethical and policy issues in pediatric genetics. Am J Med Genet C Semin Med Genet. 2008;148C:1–7. doi: 10.1002/ajmg.c.30162. [DOI] [PubMed] [Google Scholar]
- Sans M, Salzano FM, Chakraborty R. Historical genetics in Uruguay: estimates of biological origins and their problems. Hum Biol. 1997;69:161–170. [PubMed] [Google Scholar]
- Schneider CE (2009) Thou Good and Faithful Servant. Hastings Center Report. Available at: www.thehastingscenter.org/Search/Results.aspx?searchtext=Thou%20Good%20and%20Faithful%20Servant. Accessed 28 Oct 2014 [DOI] [PubMed]
- Takala T. The right to genetic ignorance confirmed. Bioethics. 1999;13:288–293. doi: 10.1111/1467-8519.00157. [DOI] [PubMed] [Google Scholar]
- Tu W-J, He J, Chen H, Shi X-D, Li Y. Psychological effects of false-positive results in expanded newborn screening in China. PLoS ONE. 2012;7:e36235. doi: 10.1371/journal.pone.0036235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2006) Convention on the Rights of Persons with Disabilities (CRPD). Nueva York: NU, 006. Available at: www.un.org/disabilities/default.asp?id=199. Accessed 20 Oct 2014
- Uruguay (1994) Presidential Decree N°430/1994. Available at http://www.impo.com.uy/bases/decretos/430-1994/1. Accessed 25 Oct 2014
- Uruguay (2007) Presidential Decree N°416/2007. Available at: www.impo.com.uy/bases/decretos/416-2007. Accessed 25 Oct 2014
- Uruguay (2008) Ley del Sistema Nacional Integrado de Salud. Available at: http://www.parlamento.gub.uy/leyes/AccesoTextoLey.asp?Ley=18065&Anchor=. Accessed 25 Oct 2014
- Uruguay (2009) Presidential Decree N°577/2009. Available at: www.impo.com.uy/bases/decretos/577-2009. Accessed 25 Oct 2014
- Uruguay (2010) Ley de Protección Integral a las Personas con Discapacidad. Available at: www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---ilo_aids/documents/legaldocument/wcms_132657.pdf. Accessed 25 Oct 2014
- Uruguay (2012a) Ley de Salud Sexual y Reproductiva. Available at www.impo.com.uy/bases/leyes/18987-2012. Accessed 25 Oct 2014
- Uruguay (2012b) Presidential Decree N°375/2012. Available at: www.impo.com.uy/bases/decretos/375-2012. Accessed 25 Oct 2014
- Uruguay (2013) Presidential Decree N°325/2013. Available at: www.impo.com.uy/bases/decretos/325-2013. Accessed 25 Oct 2014
- Uruguay (2014) Presidential Decree N° 351/2014. Seguimiento de la Convención de los Derechos de las Personas con Discapacidad (CNHD). Available at: www.cnhd.org/downloads/documents/201407280208.pdf. Accessed 25 Oct 2014
- WHO (2006) World Health Organization. Expert Review Panel Medical genetic services in developing countries. The Ethical, Legal and Social Implications of genetic testing and screening Human Genetics Chronic Diseases and Health Promotion. World Health Organization. Available at: www.who.int/genomics/publications/GTS-MedicalGeneticServices-oct06.pdf. Accessed 25 Oct 2014
- WHO (2010) World Health Organization. Sixty-third world health assembly: Birth Defects. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf. Accessed 20 Oct 2014
- WHO (2014) World Health Organization. Sixty-seven world health assembly: Global disability action plan 2014–2021. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf. Accessed 20 Oct 2014
- Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborn for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- Wilson J, Jungner G. Principles of screening for disease. Geneva: World Health Organization; 1968. p. 34. [Google Scholar]
- Winslow CEA. The untilled fields of public health. Science. 1920;51:23–33. doi: 10.1126/science.51.1306.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1045 kb)