Abstract
Hepatitis C virus (HCV) infection continues to disproportionately affect incarcerated populations. New HCV drugs present opportunities and challenges to address HCV in corrections. The goal of this study was to evaluate the impact of the treatment costs for HCV infection in a state correctional population through a budget impact analysis comparing differing treatment strategies. Electronic and paper medical records were reviewed to estimate the prevalence of hepatitis C within the Rhode Island Department of Corrections. Three treatment strategies were evaluated as follows: (1) treating all chronically infected persons, (2) treating only patients with demonstrated fibrosis, and (3) treating only patients with advanced fibrosis. Budget impact was computed as the percentage of pharmacy and overall healthcare expenditures accrued by total drug costs assuming entirely interferon-free therapy. Sensitivity analyses assessed potential variance in costs related to variability in HCV prevalence, genotype, estimated variation in market pricing, length of stay for the sentenced population, and uptake of newly available regimens. Chronic HCV prevalence was estimated at 17 % of the total population. Treating all sentenced inmates with at least 6 months remaining of their sentence would cost about $34 million—13 times the pharmacy budget and almost twice the overall healthcare budget. Treating inmates with advanced fibrosis would cost about $15 million. A hypothetical 50 % reduction in total drug costs for future therapies could cost $17 million to treat all eligible inmates. With immense costs projected with new treatment, it is unlikely that correctional facilities will have the capacity to treat all those afflicted with HCV. Alternative payment strategies in collaboration with outside programs may be necessary to curb this epidemic. In order to improve care and treatment delivery, drug costs also need to be seriously reevaluated to be more accessible and equitable now that HCV is more curable.
Keywords: Hepatitis C, Budget impact analysis, Prisons, Direct-acting antivirals
Introduction
Hepatitis C virus (HCV) infection continues to be a major public health concern, affecting an estimated 2.3 to 5.2 million individuals in the USA.1–4 The Centers for Disease Control and Prevention (CDC) has recommended that individuals born between 1945 and 1965 be uniformly educated, screened, and properly linked to HCV care and treatment, given that this cohort accounts for 75 % of infections.5–9 The economic burden from HCV-related morbidity and mortality in the near term will largely be attributed to this cohort as the HCV-positive population ages and develops cirrhosis.6 Early detection and treatment of infection is necessary prior to development of cirrhosis to avoid these costs.1,5,10,11
While recommendations for uniform screening based on the age cohort are becoming more widely embraced, targeted screening within key high-risk groups remains important.1,4 Incarcerated individuals are at high risk for HCV due to higher rates of transmission risk behaviors among those incarcerated, such as injection drug use.10–20 A 2013 meta-analysis estimated the HCV prevalence in general detainees to be 26 % worldwide and 29 % in North America.16 As HCV testing in corrections is not mandatory in most jurisdictions, the variable prevalence of diagnosed HCV may in part reflect different screening and management practices. Given that many individuals often relapse to high-transmission-risk behaviors upon release and transmit the virus to others, HCV continues to be an important public health concern.
The US Supreme Court established that incarcerated populations have a constitutional right to adequate healthcare.21 This is typically interpreted to mean that incarcerated individuals should receive care comparable to the standard for persons with access to care in the community.22 Clear treatment standards for hepatitis C have been lacking due to the risks associated with prior therapies and inadequate treatment responses. Prior to 2011, treatment was limited to pegylated-interferon (IFN) and ribavirin (RBV) with cure rates often less than 50 % for patients with the most common genotype, genotype 1, with significant associated toxicities. Protease inhibitors were added in 2011, which improved cure rates for genotype 1 but added additional toxicities.23 Although treatment uptake in correctional settings was limited, successful treatment initiatives have been described.24–27 In November and December of 2013, the US Food and Drug Administration (FDA) approved simeprevir (SMV) and sofosbuvir (SOF) for the treatment of chronic HCV, and combination use of these two drugs introduced a shorter, off-label interferon-free regimen for all genotypes with sustained virologic rates (SVRs) greater than 70 %. SVRs as high as 97 % were achieved in genotype 2 patients with SOF + RBV combination therapy.28,29 In the fall of 2014, SOF and ledipasvir (LDV) together were approved as a fixed-dosed combination and SOF + SMV were approved as combination therapy. More recently, the FDA has approved the co-formulated ombitasvir, paritaprevir, and ritonavir with dasabuvir regimen (OBV/PTV/r + DSV) for genotype 1 patients and those with advanced cirrhosis, also eliminating RBV in some cases.30–32 These therapies, and others anticipated over the next 1–2 years, bring the probability of cure to greater than 90 % for most patients and genotypes and have made interferon-free therapy the standard for HCV treatment.33–35
These all-oral therapies come, however, at a substantial cost, with SOF individually priced as high as $84,000 and SOF/LDV priced at $94,500, both for a 12-week course.36,37 SMV was priced comparably at $66,000 for 12 weeks making the total cost of SOF/SMV combination therapy up to $150,000. The substantial costs that could ensue from rapid uptake to these new treatments have caused insurance payers, particularly Medicaid, to put forth prior authorization requirements and in some cases, refuse or delay coverage to control costs.37,38 Recently, a cost-effectiveness study found that treatment of HCV with SOF for genotype 1 patients was cost-effective for certain incarcerated subpopulations but also acknowledged issues of divided benefits and affordability to the correctional system.39 Thus, long-term cost-effectiveness may still prove to be unfeasible in the context of fixed correctional health budgets. Given the minimal toxicity of these new therapies, correctional health systems may face difficulty rationalizing treatment deferral on medical grounds, creating a potential financial and liability risk.
The goal of this study was to evaluate the clinical burden of hepatitis C within a state department of corrections in order to assess the potential financial impact of these new therapies. We reviewed clinical records to generate a current prevalence estimate for hepatitis C in the correctional population and applied this estimate to inform a budget impact analysis of treatment uptake according to current guidelines. With millions of incarcerated individuals released back into communities annually, evaluating the budgetary implications of new therapeutics in correctional settings is critical to guide treatment policy in this new era of hepatitis C treatment.
Methods
This study was approved by the Institutional Review Boards at Miriam Hospital and Brown University in Providence, RI, and the Medical Research Advisory Group at the Rhode Island Department of Corrections (RIDOC) in Cranston, RI. RIDOC operates all adult correctional facilities in Rhode Island on one campus, with an average daily population of 3160 adults in 2013. Currently, HCV screening is available to inmates at RIDOC, but as with most states, screening is not mandatory.14 Specific to the RIDOC population, studies have reported HCV prevalence rates from 23 to 27 %.12,25,40,41
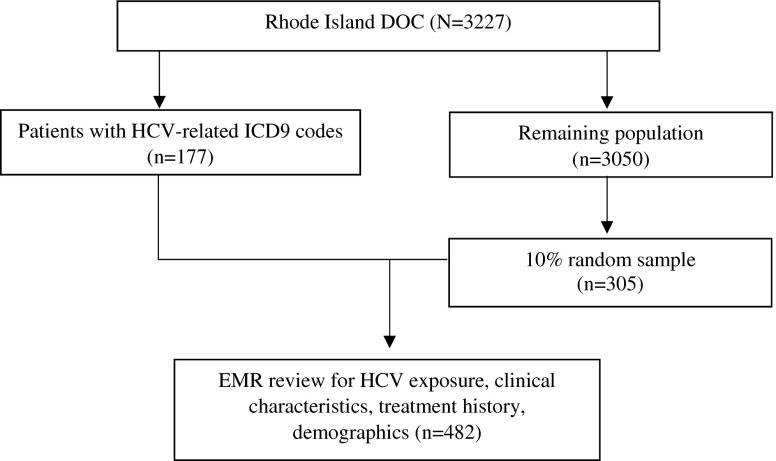
We conducted a cross-sectional survey of all incarcerations at RIDOC as of February 2014 and reviewed the electronic medical records (EMR) for data on screening uptake and clinical characteristics (see Fig. 1). All charts were reviewed for individuals with a hepatitis C ICD9-coded diagnosis or diagnoses potentially consistent with hepatitis C such as cirrhosis (070.44, 070.54, 571.4, 571.5, 573.1, 573.2, 573.3). From the remaining population, we selected a 10 % random sample for validation and additional review in order to obtain sufficient subjects to estimate the prevalence of hepatitis C among those without documentation in the electronic record of their hepatitis C status. Given previous prevalence estimates from studies in Rhode Island, we predicted that a 10 % sample of the population would yield adequate precision in estimating the current prevalence with a confidence interval of 0.1 based on descriptive research methodology for chart reviews.12,25,41,42 Each patient record was reviewed for demographics, HCV antibody status, HCV-RNA, and for those with active hepatitis C, genotype, liver biopsy, and treatment history.
FIG. 1.
Data extraction process and analytic sample.
Each test or procedure was confirmed by lab results or provider notation in the patient’s EMR. Patients were considered to have HCV exposure if their record contained a positive result for a positive HCV antibody test, a positive HCV-RNA test, or a confirmed HCV genotype test. If lab results were unavailable, provider notation of HCV positivity was taken as confirmation of HCV exposure. Patients were considered to have chronic infection if the most recent lab results indicated detectable HCV-RNA. The most recently recorded HCV genotype result was recorded as the patient’s genotype. Staging was recorded as METAVIR scores (F0-F4) based either on a provider notation of a previous liver biopsy and result or pathology report from a liver biopsy.
Prior HCV treatment was recorded either based on provider notation of prior treatment or recorded treatments in patients’ medication logs. Patients were considered treatment naïve if there was no record or if a provider had noted no prior treatment. Demographic variables—age as of February 2014, sex, and race—were obtained from patients’ EMRs. Age was stratified into four categories—“18 to 28,” “29 to 38,” “39 to 48,” and “49 and older”—for confidentiality to prevent reverse identification. For the sample of individuals who were HCV-positive, we obtained anticipated release dates and calculated the remaining length of stay as of February 2014, when data was collected.
Within the 10 % validation sample, we used chi-square tests to compare population demographics between those with known and unknown HCV status. HCV prevalence in the total population was then estimated assuming that those with unknown HCV status were similar to those with known HCV status.
The estimated HCV prevalence and distribution by genotype and fibrosis staging, along with treatment history for certain genotypes, was used to inform the budget impact analysis. To determine the estimated prevalence of infected persons, patients identified based on ICD9 coding were weighted as one individual and patients from the 10 % validation sample were weighted as ten individuals. We then calculated the proportion of sentenced HCV-positive inmates with at least 6 months remaining and applied this distribution to those with active infection for a practical estimate of those who would be treatment eligible. To inform treatment course and duration, we generated the genotype distribution and the distribution of fibrosis staging by whether a patient was treatment naïve or experienced. For cost projection purposes, we assumed that treatment history, genotype, and staging in our analytic sample are representative of the overall population of persons with active hepatitis C.
The RIDOC 2014 budget is publicly available from the Rhode Island State Budget Office.43 Total costs of HCV medication guidelines by the American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) as of January 2015 with cure rates from clinical trials is summarized in Table 1.28,29,44,31,32,45 For the purposes of calculating total medication costs, we assumed entirely interferon-free regimens with the highest SVRs from clinical trials across all genotypes, given the current community standard of care. For genotypes 1 and 4, we calculated total drug costs of a SOF/LDV regimen, with varying duration of 8 to 24 weeks based on fibrosis and treatment history for genotype 1 patients. For genotypes 2 and 3, we calculated costs of a SOF + RBV regimen for 12 to 24 weeks according to current guidelines. We did not include costs for personnel, follow-up visits, or other laboratory work. Total costs were computed assuming that all patients would complete treatment while incarcerated at RIDOC.
TABLE 1.
Estimated total costs and cure rates per treatment course of HCV treatment guidelines
| Treatment regimen | Clinical recommendationa | Total estimated costb | Estimated cure ratec | |
|---|---|---|---|---|
| SOF + IFN + RBV | $93,400 | 85–90 % | ||
| 12 weeks | GT3; GT4 | |||
| SMV + IFN + RBV | 93 % | |||
| 12 weeks | GT 4 | $75,800 | ||
| SOF + RBV | 85–95 % | |||
| 12 weeks | GT2 non-cirrhotic | $84,200 | ||
| 16 weeks | GT2 cirrhotic | $112,000 | ||
| 24 weeks | GT3, GT4 | $168,300 | ||
| SOF + SMV | 92 % | |||
| 12 weeks | GT1 non-cirrhotic; GT4 | $150,400 | ||
| 24 weeks | GT1 cirrhotic | $300,700 | ||
| SOF + LDV | 95–99 % | |||
| 8 weeks | GT1 non-cirrhotic | $63,000 | ||
| 12 weeks | GT1 treatment naïve cirrhotic, treatment experienced non-cirrhotic; GT4 | $94,500 | ||
| 24 weeks | GT1 treatment experienced cirrhotic | $189,000 | ||
| OBV/PTV/r + DSV + RBV | 95–98 % | |||
| 12 weeks | GT1 treatment naïve; GT1 treatment experienced non-cirrhotic; GT1b; GT4 | $83,500 | ||
| 24 weeks | GT1a cirrhotic | $167,000 | ||
| OBV/PTV/r + DSV | 12 weeks | GT1b non-cirrhotic | $83,300 | 90–99 % |
aClinical recommendations based on January 2015 update of AASLD/IDSA/IAS guidelines for initiation and retreatment of hepatitis C
bTotal estimated costs calculated based on full treatment course of individual regimens and unit costs, which were provided by RIDOC
cEstimated cure rates and ranges obtained from clinical trials data
We analyzed total drug costs of three treatment strategies: (1) treating all those eligible with chronic infection, (2) treating patients with any demonstrated fibrosis, and (3) treating only patients with advanced fibrosis. For each strategy, we computed the total drug costs sustained by RIDOC and the cost per patient cured with data from clinical trials. We calculated budget impact as the proportion of the correctional pharmacy budget and overall healthcare budget accrued by total drug costs.
Sensitivity analyses were performed to evaluate the impact of varying parameters for each strategy on the budget. We selected the total drug costs of treating all sentenced HCV-positive inmates with at least 6 months remaining as a reference to assess relative deviations of the calculated changes. For varying genotype distributions, we used data from two published studies in Rhode Island.24,25 To capture hypothetical market pricing of newly available and upcoming therapies, we projected costs given either 25 or 50 % reduction or an increase by 25 or 50 % based on current total medication costs. To further account for incarceration periods, we assessed treating those with a remaining length of stay of at least 3 months, at least 9 and 12 months or more. Given the recent approval of co-formulated OBV/PTV/r + DSV, we analyzed the total costs of treatment with a 12 week regimen for genotype 1 non-cirrhotic and genotype 4 patients and a 24-week regimen for genotype 1 cirrhotic patients. Due to unavailable data, we did not differentiate between genotypes 1a and 1b for this portion of the analysis.
SAS 9.3 was used for all statistical analyses.
Results
The RIDOC population at the time of sampling was 3227 individuals. Out of 177 individuals with potentially related ICD9 diagnoses, 160 patients were confirmed to be HCV positive, one patient was negative, and 16 patients had no record of exposure (see Fig. 2). Out of 305 patients sampled from the remaining population, 10 (3 %) patients were identified as HCV positive, 36 (12 %) patients were negative, and 259 (85 %) patients had no record of screening.
FIG. 2.
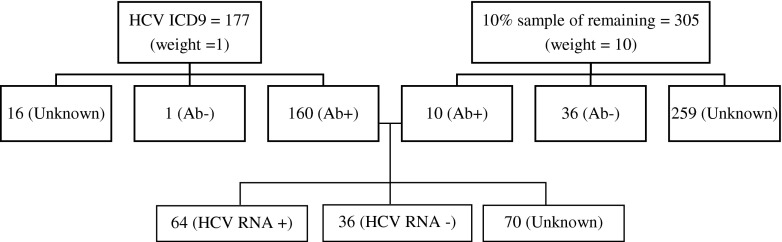
HCV analytic sample.
Demographic characteristics of the analytic sample are depicted in Table 2. The majority of the HCV-positive population were 49 years and older (47 %), white (75 %), and male (95 %). With respect to age group, sex, and race, there were no significant differences between those with known and unknown HCV status (p value = 0.10, 0.66, 0.53, respectively). The 16 individuals with provider documented HCV infection but no documented laboratory results were assumed to be chronically infected for the budget impact analysis giving a total of 176 HCV positives and one negative in the ICD9 group. When the known prevalence in the sample group (22 %) is applied to the 259 unknowns, this results in 56 additional positives and 203 negatives. After appropriate weighting, we calculated an estimated prevalence of 26 % or 836 HCV-antibody-positive individuals at RIDOC.
TABLE 2.
Demographic characteristics of study sample by HCV screening status (n, %)
| Overall analytic sample (n = 482) | 10 % Validation sample (n = 305) | |||||||
|---|---|---|---|---|---|---|---|---|
| HCV positive (n = 170) | HCV negative (n = 37) | Unknown (n = 275) | p valuea | HCV positive (n = 10) | HCV negative (n = 36) | Unknown (n = 259) | p valuea,b | |
| Age | <0.001 | 0.1 | ||||||
| 18 to 28 | 3 (1) | 9 (25) | 93 (36) | 0 (0) | 9 (25) | 93 (36) | ||
| 29 to 38 | 29 (25) | 17 (45) | 73 (28) | 4 (40) | 16 (44) | 72 (28) | ||
| 39 to 48 | 43 (27) | 7 (19) | 62 (22) | 3 (30) | 7 (19) | 57 (22) | ||
| 49 and older | 95 (47) | 4 (11) | 47 (15) | 3 (30) | 4 (11) | 37 (14) | ||
| Sex | 0.53 | 0.66 | ||||||
| Male | 156 (95) | 35 (95) | 265 (97) | 10 (100) | 34 (94) | 251 (97) | ||
| Female | 14 (5) | 2 (6) | 10 (3) | 0 (0) | 2 (6) | 8 (3) | ||
| Race | 0.02 | 0.53 | ||||||
| White | 103 (75) | 17 (46) | 114 (45) | 8 (80) | 16 (46) | 105 (45) | ||
| Black | 27 (12) | 11 (31) | 70 (30) | 0 (0) | 11 (31) | 69 (30) | ||
| Hispanic | 23 (14) | 8 (23) | 59 (25) | 1 (10) | 8 (23) | 58 (25) | ||
a p values based on χ 2 test
bDue to non-positivity, χ 2 tests were computed between those with known screening (HCV positive and negative, n = 46) and unknown screening (n = 259). Data based on raw frequencies and weighted percentages. Frequencies may not sum to totals due to missing data. Column percentages may not sum to 100 % due to rounding
Clinical characteristics of HCV-positive individuals are summarized in Table 3 with estimations for our budget impact analysis setup. Among HCV-antibody positives, 71 % were treatment naïve and 67 % had chronic infection, lending to a 17 % HCV prevalence rate in the entire RIDOC population. Among those with confirmed genotype (n = 71, 62 %), 69 % were genotype 1, 8 % were genotype 2, 18 % were genotype 3, and 6 % were genotype 4. For patients with available biopsy results (n = 33, 19 %), 9 % had no fibrosis, 55 % were stages 1–2, and 36 % were stages 3–4. Given these clinical characteristics, we project that 327 individuals (10 % of the total RIDOC population) would be eligible for treatment based on a length of stay of at least 6 months remaining, with a majority being genotype 1 (n = 225) and stage 1–2 fibrosis (n = 178) patients.
TABLE 3.
Extrapolating distribution of clinical characteristics data for estimate of budget impact
| Percentage | Estimatea | |
|---|---|---|
| RIDOC population | 100 % | 3227 |
| HCV Ab (+) | 26 % | 836 |
| HCV RNA (+) | 67 % | 559 |
| Sentenced population with 6 months or more remaining | 58 % | 327 |
| Treatment naive | 71 % | 232 |
| Treatment experienced | 29 % | 95 |
| Estimated distribution of genotype among HCV RNA (+) | ||
| Genotype 1 | 69 % | 225 |
| Genotype 2 | 8 % | 25 |
| Genotype 3 | 18 % | 57 |
| Genotype 4 | 6 % | 20 |
| Estimated distribution of staging by genotype | ||
| Stage 0 | 9 % | 30 |
| Genotype 1 | 20 | |
| Genotype 2 | 2 | |
| Genotype 3 | 5 | |
| Genotype 4 | 2 | |
| Stage 1–2 | 55 % | 178 |
| Genotype 1 | 123 | |
| Genotype 2 | 13 | |
| Genotype 3 | 31 | |
| Genotype 4 | 11 | |
| Stage 3–4 | 36 % | 119 |
| Genotype 1 | 82 | |
| Genotype 2 | 9 | |
| Genotype 3 | 21 | |
| Genotype 4 | 7 | |
aEstimates based on weighted percentages applied to cross-sectional census of RIDOC population as of February 2014. Percentages may not sum to 100 % due to rounding. Genotype distribution was applied to estimated number of chronic infections. Staging distribution was applied to estimated genotype frequencies. Treatment history was estimated among all HCV Ab(+) individuals
The results of our budget impact analysis are summarized in Table 4. If RIDOC were to initiate treatment for all sentenced inmates with 6 months or more remaining under current treatment guidelines, assuming entirely interferon-free regimens across all genotypes, it would cost an estimated $34 million—nearly twice the overall correctional healthcare budget and almost 13 times the pharmacy budget. Treating patients with any fibrosis would result in a budget impact of almost 12 times the pharmacy expenditures. Treating only advanced fibrosis patients would cost about $15 million, about three quarters the entire overall healthcare budget and over five times the allotted pharmacy budget. The estimated cost per patient cured ranges from $110 to 130,000 across all treatment strategies.
TABLE 4.
Estimated pharmacy and overall budget impact of HCV treatment guidelines by treatment strategy
| Treat all (n = 327) | Treat any fibrosis (n = 297) | Treat advanced fibrosis (n = 119) | |
|---|---|---|---|
| Estimated cures | 315 | 286 | 115 |
| Estimated total drug costsa | $34,170,063 | $31,454,139 | $15,188,440 |
| Budget impactb | |||
| RIDOC 2014 pharmacy budget: $2,723,669 | |||
| Proportion of pharmacy expenditures | 1254 % | 1155 % | 558 % |
| RIDOC 2014 overall healthcare budget: $19,889,269 | |||
| Proportion of overall healthcare expenditures | 172 % | 158 % | 76 % |
| Estimated cost per patient cured: $110,000–130,000 | |||
aTotal drug costs estimated from interferon-free regimens (SOF + RBV and SOF/LDV)
bBudget impact calculations based on public record of RIDOC 2014 expenditures
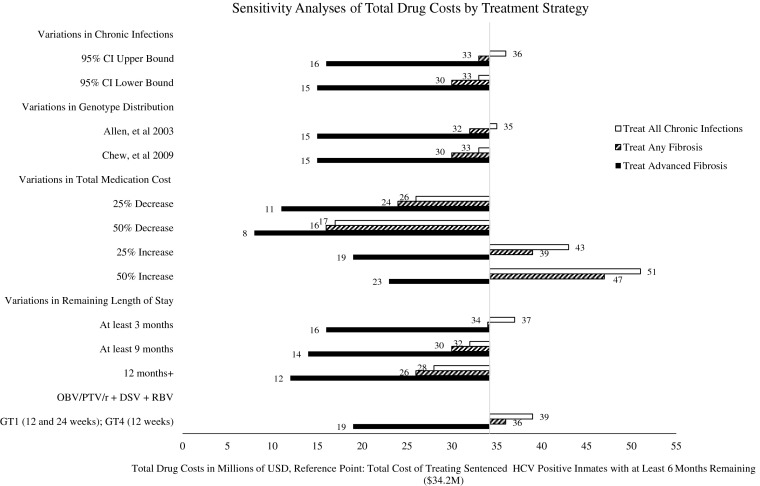
Our sensitivity analysis results are shown in Fig. 3. Our reference point is $34 million under current pricing for treating sentenced inmates with at least 6 months remaining. When hypothetical cost projections for newly available therapies were varied, we see significant deviations from our initial analysis. Under a 50 % cost reduction from current pricing, treating everyone would cost about $17 million and treating only advanced fibrosis patients would cost about $8 million. Relative to incarceration time, electing to treat only those with at least a year remaining would cost about $28 million for all chronic infections and $12 million for advanced fibrosis patients. Assuming that genotypes 1 and 4 are only treated with the recently approved OBV/PTV/r + DSV regimen with RBV rather than a SOF/LDV combination regimen, treating advanced fibrosis patients would cost about $19 million.
FIG. 3.
Sensitivity analyses of total drug costs by treatment strategy.
Discussion
The improved efficacy of HCV therapy offers an important public health opportunity to curb the HCV epidemic by scaling up treatment. Treatment with new therapies, however, while it offers multiple potential benefits, is cost prohibitive, as observed with the significant budget impact of treating only those eligible for treatment based on length of stay, comprising 10 % of the total population. In the face of limited resources, prioritizing treatment for those with advanced fibrosis has been recommended given the potential for near term morbidity and mortality and the associated costs to the health system.5,10,14,46 This restrictive treatment scenario would incur costs that amount to 76 % of the current correctional health budget in Rhode Island. This scenario is similar to restrictions placed on treatment under Medicaid in the community and thus to some extent, reflects the community standard of care. Extending treatment to all persons with HCV would incur pharmacy costs nearly twice the current overall correctional health budget. It is clear that treating all those infected as a public health prevention strategy would require additional specific, substantial funding for hepatitis C treatment.
Cost effectiveness analyses for treatment of hepatitis C have focused on the potential of treatment to prevent development of cirrhosis as well as to prevent the complications of cirrhosis.39,47–49 The benefit of curing hepatitis C in this regard has been well established.1,5,10 It is important to recognize that these cost savings are to the system as a whole. Departments of corrections will realize those savings only for those likely to develop complications while incarcerated, which includes those with advanced fibrosis and those with very long-term sentences. Given the potential benefit to the community of effective management of hepatitis C in corrections, strategies for providing additional support from outside the existing correctional health budgets will be needed.
With the approval of the SOF/LDV combination and the co-formulated OBV/PTV/r + DSV, priced at $63,000 to 83,500 per patient, the total cost of treatment for non-cirrhotic genotype 1 patients has decreased 10–30 % compared to the previously recommended SOF + IFN + RBV regimen in late 2013 (Table 1). Though further reductions are expected, the pace of declining costs over subsequent years is unlikely to be as dramatic. Even with an additional 50 % reduction from the current lowest price for currently approved interferon-free treatment options, the total costs of treatment would remain over $8 million dollars, exceeding the current budget for pharmaceuticals for the correctional health system. Competition leading to price reductions is not expected to make wide-scale treatment in corrections feasible.
The reduction in treatment duration to 12 weeks or for some even 8 weeks (SOF/LDV) could afford the opportunity to treat unsentenced individuals and individuals with short-term stays in corrections. However, it is clear from this analysis that treating only those with longer stays (at least 12 months) is still cost prohibitive with current the budget at $28 million. Extending treatment to unsentenced individuals also creates the potential for release during treatment, lending to treatment interruption. Given the cost of these therapies, testing with linkage to care in the community is a better strategy for these individuals.
The Federal Bureau of Prisons has released an interim set of guidelines for HCV treatment in prisons in which correctional institutions are encouraged to prioritize treatment for individuals with advanced fibrosis, liver transplant recipients, HIV co-infected individuals, individuals with HCV-related comorbidities, and individuals who were already on a treatment course prior to incarceration.46 These guidelines are being adopted as an initial framework in many departments of corrections, including RI. Efforts are currently underway to enhance testing and staging among those incarcerated with expansion of treatment within this framework, initially prioritizing treatment of sentenced individuals with advanced fibrosis patients and an adequate length of stay in order to complete treatment. With the recent expansion of treatment options as of early 2015, budget deficits are expected which will need to be resolved.50
The 15 % screening rate among the validation sample highlights the low uptake to screening overall in the correctional population. Though not an intentional policy, the lack of systematic screening for hepatitis C has passively limited uptake to treatment and by extension, treatment costs. Even if individuals are not treated while incarcerated, screening-incarcerated individuals can provide education and harm reduction opportunities and can trigger active linkage to care in the community at the time of release. Building active linkage programs following release offers a means of meeting public health goals and legal requirements with limited costs to the correctional health system, particularly for those awaiting trial or with indeterminate length of stay. New York’s continuity of care intervention in 2004 is an example of such a program, where the NY Department of Health and Department of Corrections collaborated to provide HCV therapy for those infected prisoners with short or indeterminate length of stay.51
While EMR data utilized in this budget impact analysis portray the most current information on the HCV burden in corrections, missing data for relevant HCV variables among all incarcerations present potential bias in estimating prevalence and other clinical distributions. Our analytic sample encompassed 15 % of the RIDOC population and included all potentially HCV-positive individuals based on recorded diagnoses. The rate of hepatitis C positivity among those without coded diagnoses was low. We relied on both physician-recorded diagnoses and lab results to assess HCV positivity, leading to potential bias if physicians noted HCV positivity based on inaccurate patient recall. In our validation sample, only 46 out of 305 patients (15 %) had identifiable records documenting HCV status. While oversampling ICD9-coded records may also have inflated our results, applying the appropriate weighting to each subsample yielded a 26 % prevalence estimate which is remarkably consistent with previously reported rates in Rhode Island.12,25,40 Only 42 % of patients had an available genotype. In the near term, this may impact costs but as more pangenotypic therapies become available in the next few years, genotype will become less of a cost driver. Only 19 % of our sample had a biopsy result but the fibrosis distribution of fibrosis scores was notably consistent with previously published data in an HCV cohort study on liver fibrosis prevalence.52 Persons with advanced fibrosis may be more likely to show symptoms and come to attention of providers, leading to a potential bias towards more advanced fibrosis among those biopsied. Even if the prevalence of advanced fibrosis was significantly less among those undiagnosed or not yet staged, the costs of treatment can still be expected to be prohibitive.
Our initial analysis assumes that all patients with active hepatitis C are candidates for treatment and does not account for variability in treatment options in a given setting. In practice, the ultimate decision to treat a patient varies as providers consider additional factors, comorbidities, and medical history that would either encourage or defer patients from a particular treatment option. Although missing data is a concern, uncertainty is expected with any budget impact analysis.53 Moreover, it is unlikely that missing data would substantially alter the conclusions given the large discordance between the treatment cost and the available correctional health budget, as observed in our sensitivity analysis with multiple varying parameters. The issue at hand is not the exact dollar amount that these projections provide, but the order of magnitude of the costs and the implications for corrections to undertake widespread HCV treatment in clinical settings.
Conclusion
Costs associated with hepatitis C are projected to escalate over the next several decades if substantial efforts are not made to increase testing and treatment uptake for high-risk individuals.7,8 Finding other means of financially supporting HCV treatment in corrections is critical if correctional institutions are to provide the community standard of care as opposed to the alternative of neglecting to treat. Nevertheless, we also contend that the rising cost of therapy goes beyond merely a “sticker shock” phenomenon and needs to be seriously reevaluated. Corrections have inherited an important public health opportunity to address the HCV epidemic. It is unrealistic, however, to expect correctional facilities to attempt widespread HCV treatment with the currently available budgets. As more all-oral regimens are approved, treatment strategies that are both realistically cost-effective and public health conscious will be needed. In addition, for the HCV epidemic to have any hope of eradication, treatment must be accessible and equitable, especially for more vulnerable populations.
Acknowledgments
We thank and acknowledge Pauline Marcussen, MS RHIA CCHP NCP, and the HCV Committee members at RIDOC for their support and guidance for this project.
Disclosures
Dr. Fred Vohr is currently the Director of Medical Programs at the Rhode Island Department of Corrections in Cranston, RI, where the research was conducted.
References
- 1.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31(8):1090–101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim W. The burden of hepatitis C in the United States. Hepatology. 2002;36(5):S30–4. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 3.Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: national health and nutrition examination survey 2001 through 2010. J Hepatol. 2014;60(4):691–8. doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD, Morgan RL, Beckett G, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 6. Ward JW. The hidden epidemic of hepatitis C virus infection in the United States: occult transmission and burden of disease. Top Antivir Med. 2013; 21(1): 15–9. [PMC free article] [PubMed]
- 7.Bornschlegel K, Holtzman D, Klevens M, Ward JW. Evaluation of hepatitis C virus infection testing and reporting—eight U.S. Sites, 2005–2011. MMWR. 2013;62(3):1–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BD, Jorgensen C, Zibbell JE, Beckett GA. Centers for disease control and prevention initiatives to prevent hepatitis C virus infection: a selective update. Clin Infect Dis. 2012;55(Suppl 1):S49–53. doi: 10.1093/cid/cis363. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan R, Liu SJ, Klevens RM, Holmberg SD. Indications for testing among reported cases of HCV infection from enhanced hepatitis surveillance sites in the United States, 2004–2010. Am J Public Health. 2013;103(8):1445–9. doi: 10.2105/AJPH.2013.301211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt DR, Saab S. Viral hepatitis in incarcerated adults: a medical and public health concern. Am J Gastroenterol. 2009;104(4):1024–31. doi: 10.1038/ajg.2008.143. [DOI] [PubMed] [Google Scholar]
- 11.Macalino G, Dhawan D, Rich J. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95(10):1739–40. doi: 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macalino GE, Vlahov D, Sanford-colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island Prisons. Am J Public Health. 2004;94(7):1218–23. doi: 10.2105/AJPH.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara B, Losikoff P, Huguenin L, Macalino GE, Rich JD, Gregory SH. Increasing hepatitis C prevalence and associated risk behaviors among incarcerated young adults. J Urban. 2013 doi: 10.1007/s11524-013-9807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaulding A, Kim A, Harzke A, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Baillargeon J, Snyder N, Soloway RD, et al. Hepatocellular carcinoma prevalence and mortality in a male state prison population. Public Health Rep. 2009;124:120–6. doi: 10.1177/003335490912400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58(4):1215–24. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough E, Kempf M, Graham L, et al. HIV and hepatitis B and C incidence rates in US correctional populations and high risk groups: a systematic review and meta-analysis. BMC Public. 2010;10(1):777. doi: 10.1186/1471-2458-10-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes AG, Taxman FS, Friedmann PD, Cropsey KL. HCV in incarcerated populations: an analysis of gender and criminality on risk. J Psychoactive Drugs. 2008;40(4):493–501. doi: 10.1080/02791072.2008.10400655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin CK, Hostetter JE, Hagan JJ. New opportunities for the management and therapy of hepatitis C in correctional settings. Am J Public Health. 2010;100(1):13–7. doi: 10.2105/AJPH.2008.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaulding A, Greene C, Davidson K, Schneidermann M, Rich J. Hepatitis C in state correctional facilities. Prev Med (Baltim) 1999;28(1):92–100. doi: 10.1006/pmed.1998.0418. [DOI] [PubMed] [Google Scholar]
- 21.Estelle v. Gamble, 429 US Supreme Court 97 (1976). Accessed July 15, 2014.
- 22.Rich JD, Allen SA, Williams BA. Responding to hepatitis C through the criminal justice system. N Engl J Med. 2014;370(20):1871–4. doi: 10.1056/NEJMp1311941. [DOI] [PubMed] [Google Scholar]
- 23.Ghany M, Strader D, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew KW, Allen SA, Taylor LE, Rich JD, Feller E. Treatment outcomes with pegylated interferon and ribavirin for male prisoners with chronic hepatitis C. J Clin Gastroenterol. 2009;43(7):686–91. doi: 10.1097/MCG.0b013e31818dd94c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen SA, Spaulding AC, Osei AM, Taylor LE, Cabral AM, Rich JD. Treatment of chronic hepatitis C in a state correctional facility. Ann Intern Med. 2003;138:187–91. doi: 10.7326/0003-4819-138-3-200302040-00010. [DOI] [PubMed] [Google Scholar]
- 26.Maru DS-R, Bruce RD, Basu S, Altice FL. Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis. 2008;47(7):952–61. doi: 10.1086/591707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterling RK, Hofmann CM, Luketic VA. Treatment of chronic hepatitis C virus in the virginia department of corrections: can compliance overcome racial differences to response? Am J Gastroenterol. 2004;99(5):866–72. doi: 10.1111/j.1572-0241.2004.30310.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson I, Gordon S, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. FDA approves Viekira Pak to treat hepatitis C. US Food and Drug Administration. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427530.htm. Published December 19, 2014. Accessed February 1, 2015.
- 31.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 32.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 33.Pol S, Vallet-Pichard A, Corouge M. Treatment of hepatitis C virus genotype 3-infection. Liver Int. 2014;34(Suppl 1):18–23. doi: 10.1111/liv.12405. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 35.Shiffman ML, Benhamou Y. HCV F1/F2 patients: treat now or continue to wait. Liver Int. 2014;34(Suppl 1):79–84. doi: 10.1111/liv.12408. [DOI] [PubMed] [Google Scholar]
- 36.Reau NS, Jensen DM. Sticker shock and the price of new therapies for hepatitis C: Is it worth it? Hepatology. 2014;59(4):1246–9. doi:10.1002/hep.27039. [DOI] [PubMed]
- 37.Japsen B. As Pricey Hepatitis Pill Harvoni Joins Sovaldi, States Erect Medicaid Hurdles. Forbes. 2014. http://www.forbes.com/sites/brucejapsen/2014/10/10/as-hepatitis-pill-harvoni-joins-sovaldi-states-erect-medicaid-hurdles/. Published October 10 2014. Accessed Novemer 23, 2014.
- 38.Associated Press. Oregon Medicaid targets expensive hepatitis drug. The Washington Post. 2014. http://www.washingtonpost.com/national/health-science/oregon-medicaid-targets-expensive-hepatitis-drug/2014/07/31/4af1855a-1912-11e4-88f7-96ed767bb747_story.html. Published July 31, 2014. Accessed November 23, 2014.
- 39.Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD. Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in u.s. Incarcerated populations: a cost-effectiveness analysis. Ann Intern Med. 2014;161(8):546–53. doi: 10.7326/M14-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammett T. Adopting more systematic approaches to hepatitis C treatment in correctional facilities. Ann Intern Med. 2003;138(3):235–6. doi: 10.7326/0003-4819-138-3-200302040-00021. [DOI] [PubMed] [Google Scholar]
- 41.Chew K, Allen SA, Taylor LE, Rich JD, Feller E. Treatment outcomes with pegylated interferon and ribavriin for male prisoners with chronic hepatitis C. J Clin Gastroenterol. 2009;43(7):686–91. doi: 10.1097/MCG.0b013e31818dd94c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregory B, Van Horn C, Kaprielian VS. 8 steps to a chart audit for quality. Fam Pract Manag. 2008;15(7):A3–8. [PubMed] [Google Scholar]
- 43.State of Rhode Island and Providence Plantations. Budget Fiscal Year 2014 Volume IV - Public Safety, Natural Resources, and Transportation. http://www.budget.ri.gov/Documents/PriorYearBudgets/OperatingBudget2014/BudgetVolumeIV/2_DepartmentOfCorrections.pdf. Accessed April 15, 2014.
- 44.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2015;384(9956):1756–65. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 45.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N Engl J Med. 2014:1889–1898. doi:10.1056/NEJMoa1402454. [DOI] [PubMed]
- 46.Federal Bureau of Prisons. Interim Guidance for the Management of Chronic Hepatitis C Infection. http://www.bop.gov/resources/pdfs/hepatitis_c_current.pdf. Accessed July 15, 2014.
- 47.Sutton AJ, Edmunds WJ, Sweeting MJ, Gill ON. The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. J Viral Hepat. 2008;15(11):797–808. doi: 10.1111/j.1365-2893.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 48.Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis. 2013;56(10):1382–93. doi: 10.1093/cid/cit069. [DOI] [PubMed] [Google Scholar]
- 49.Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;2015:1–20. doi: 10.1111/apt.13081. [DOI] [PubMed] [Google Scholar]
- 50.Sanger-Katz M. Why the Hepatitis Cure Sovaldi is a Budgetary Disaster for Prisons. New York Times. http://www.nytimes.com/2014/08/07/upshot/why-the-hepatitis-cure-sovaldi-is-a-budgetary-disaster-for-prisons.html?_r=0&abt=0002&abg=1. Published August 7 2014. Accessed November 23, 2014.
- 51.Klein S, Wright L, Birkhead G, et al. Promoting HCV treatment completion for prison inmates: New York State’s hepatitis C continuity program. Public Health Rep. 2007;122(2):83–8. doi: 10.1177/00333549071220S216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349(9055):825–32. doi: 10.1016/S0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 53.Mauskopf JA, Sullivan SD, Annemans L. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices—budget impact analysis. Value Health. 2007;10(5):336–47. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]