Abstract
Background
In this study, we retrospectively analyzed the relationship between headache recurrence and serotonin 5-HT1B/1D receptor occupancy (Φ1B and Φ1D). Triptans marketed in Japan (sumatriptan, zolmitriptan, eletriptan, rizatriptan, naratriptan) were investigated.
Methods
Receptor occupancies were calculated from both the pharmacokinetic and pharmacodynamic data of triptans. We examined the relationships between recurrence rate and elimination half-lives, and Ф1B and Ф1D, as calculated from the time-course of plasma drug concentration obtained from other studies. The time until Ф1B and Ф1D became 50 % or less, 40 % or less, and 30 % or less was calculated as duration time to examine the relationship with recurrence rate.
Results
For Ф1B, eletriptan remained at a low level. For Ф1D, it was indicated that all triptans obtained an occupancy of 80 % or higher at maximum. For all items, though recurrence tended to be lower along with longer half-life, no significant statistical correlation was found. For both Ф1B and Ф1D, the recurrence rate tended to be lower as the duration became longer. In addition, a significant correlation was observed for Ф1D (p < 0.05). For clarifying the Ф value and time period most closely correlated with recurrence rate, recurrence and Ф1B and Ф1D at 6, 12, and 18 h after administration were calculated. The most significant correlation was observed between recurrence rate and Ф1D at 12 h after administration (p < 0.01).
Conclusions
As an index for evaluating headache recurrence following triptan administration, recurrence rate and Ф1D value at 12 h after administration were found to be most closely correlated and useful for analysis. Our results indicate that headache recurrence inhibition can be evaluated using these values.
Keywords: Triptan, Migraine, Headache recurrence, 5-HT1B receptor, 5-HT1D receptor, Receptor occupancy
Background
Triptans are serotonin 5-HT1B/1D agonists frequently used as migraine-abortive drugs. Presently, 5 different triptans are marketed in Japan, though lack of information is a problem for selection of the proper drug and that choice is largely dependent on only the experience of the attending physician. Even in the Japanese ‘Practical guidelines for chronic headaches’, it is noted that the triptans vary in regard to their pharmacological properties and therapeutic effects different with individual patients. For choice of the rational triptan, the only description states that the proper choice has not yet been elucidated, as there have been few closed studies with a satisfactory number of subjects [1]. Furthermore, it has been pointed out that the headache sometimes returned even after administration. Thus, it is considered indispensable to establish a theoretical dosage regimen for inhibiting recurrence. Differences in triptan’s therapeutic effects among individuals and drug characteristics, as well as lack of evidence for selecting a suitable drug for each patient are problems to be resolved. And it is important to establish an index for quantitative evaluation of the pharmacological and clinical effects of triptans.
Concerning headache recurrence following triptan administration, relationships between recurrence and elimination half-lives of plasma drug concentrations have been reported in other countries [2], whereas nearly nothing has been presented on this subject in Japan. Meanwhile, our department performed analysis of the clinical effects of triptans (headache relief rate) by focusing on serotonin 5-HT1B and 5-HT1D receptor occupancies (Ф), and we found that the parameter (AФ • AUCФ) of the area under the time curve of Ф (AUCФ) until the efficacy evaluation time point after correction by the velocity factor AФ (Фmax/Tmax) could serve as an index to correct the differences between drug and formulations, and uniformly evaluate them [3, 4].
In the present study, we performed a theoretical evaluation of headache recurrence following triptan administration in Japanese based on serotonin 5-HT1B and 5-HT1D receptor occupancies.
Methods
Collection of pharmacokinetic and pharmacodynamic parameters
Triptans marketed in Japan (sumatriptan, zolmitriptan, eletriptan, rizatriptan, naratriptan) were investigated. Data regarding pharmacokinetic and pharmacodynamics parameters, and headache recurrence rate (referred to as recurrence rate hereinafter) were obtained from published studies. In Japan, data obtained from various clinical trials performed domestically are included in the clinical data package for new drug registration, while drug properties and clinical trial results were extracted from those downloadable from the website of the regulatory authority the Pharmaceuticals and Medical Devices Agency (PMDA) (http://www.pmda.go.jp). When data were not available at that website, they were collected from published studies. For pharmacokinetic parameters, data for plasma drug concentration following administration of each drug, plasma elimination half-life, plasma unbound protein fraction, and the presence of active metabolite were collected from clinical trial results. For pharmacodynamics parameters, the dissociation constant Ki values for the serotonin 5-HT1B and 5-HT1D receptors were obtained.
Calculation of serotonin 5-HT1B and 5-HT1D receptor occupancies
On the basis of the receptor occupancy theory, the time-course changes of serotonin 5-HT1B and 5-HT1D receptor occupancies Ф (Ф1B and Ф1D) of the triptans were calculated.
Triptan can be classified into two types. One is only the unchanged drug has pharmacological effect, and the other is both of unchanged drug and active metabolite has pharmacological effect.
When only the unchanged drug was involved in drug efficacy:
The plasma concentration of the unbound drug (Cf) after a single administration of the drug in Japanese subjects, and the dissociation constant Ki (Ki1B and Ki1D) of serotonin 5-HT1B and 5-HT1D were substituted in equation (1) to calculate the time course of Ф1B and Ф1D.
| 1 |
-
2)
When only one kind of active metabolite was present:
The changes in Ф1B and Ф1D were calculated by substituting the plasma unbound drug concentrations (Cf1 and Cf2) of the unchanged drug and active metabolite after a time of single-dose administration, and the dissociation constants of serotonin 5-HT1B and 5-HT1D (Ki1B1, Ki1D1 and Ki1B2, Ki1D2) in equation (2).
| 2 |
Relationship between headache recurrence and serotonin 5-HT1B and 5-HT1D receptor occupancies
The relationships between time-course changes of Ф1B and Ф1D were obtained, and headache recurrence rate (recurrence rate) were examined. For the definition of recurrence, migraine pain was rated as follows: grade 0, no pain; grade 1, mild pain; grade 2, moderate pain; grade 3, severe pain. The patients whose headache improved from grade 3 or 2 to grade 1 or 0 at 1-4 h after initial administration but returned to grade 3 or grade 2 within 24 h after administration were regarded as having recurrence. Data for patients who met the requirements were collected from clinical trial data published in the application summary and published reports. The headache recurrence rate (recurrence rate) was obtained by dividing the number of subjects with recurrence by the number of those with relief. Then the following items (1-3) were examined.
Relationships between headache recurrence rate (recurrence rate) and elimination half-lives of plasma drug concentrations, Ф1B, and Ф1D.
Relationships between headache recurrence rate (recurrence rate) and duration time of Ф1B and Ф1D.
Relationships between headache recurrence rate (recurrence rate) and Ф1B and Ф1D at 6, 12, and 18 h after administration.
Results
Extraction of pharmacokinetic and pharmacodynamics parameters for drugs and recurrence rate
Table 1 shows the generic name, formulation, elimination half-life (t1/2), molecular weight (MW), plasma unbound fraction (fu), and Ki values for the serotonin 5-HT1B and 5-HT1D receptors. For zolmitriptan, data for both the unchanged drug and active metabolite were collected, as an active metabolite that seemingly affected efficacy was considered to be present.
Table 1.
| Drug (formulation) | t1/2 (hr) | MW | fu | Ki (nM) | ||
|---|---|---|---|---|---|---|
| 5-HT1B | 5-HT1D | |||||
| Sumatriptan (subcutaneous injection) [19] | 1.46 | 295.40 | 0.66 | 12.59 | 12.59 | |
| Zolmitriptan (oral tablet) | Unchanged drug [12, 20] | 2.40 | 287.30 | 0.75 | a6.31 | a2.51 |
| Active metabolite [12, 20, 21] | 2.35 | 273.36 | 0.75 | a1.58 | a0.50 | |
| Eletriptan (oral tablet) [22] | 3.20 | 382.52 | 0.13 | 10.0 | 1.15 | |
| Rizatriptan (oral tablet) [23, 24] | 1.60 | 269.35 | 0.86 | 7.24 | 2.34 | |
| Naratriptan (oral tablet) [15] | 5.05 | 335.47 | 0.71 | 2.24 | 2.30 | |
aCalculated from radioligand test
Table 2 shows the dose and formulation for single-dose administration, and recurrence rate for each of the 5 triptans. The recurrence rate varied depending on the drug and formulation.
Table 2.
| Drug | Dose (mg/time) | Formulation | Recurrence rate (%) |
|---|---|---|---|
| Sumatriptan [25] | 3 | Subcutaneous injection | 54.8 [n = 31] |
| Zolmitriptan [12] | 2.5 | Oral tablet | 13.0 [n = 30] |
| Eletriptan [26] | 20 | Oral tablet | 10.0 [n = 51] |
| 40 | Oral tablet | 17.0 [n = 52] | |
| 80 | Oral tablet | 14.0 [n = 59] | |
| Rizatriptan [24] | 10 | Oral tablet | 31.7 [n = 41] |
| 10 | RPD oral tablet | 46.9 [n = 32] | |
| Naratritpan [27] | 1 | Oral tablet | 24.3 [n = 74] |
| 2.5 | Oral tablet | 11.9 [n = 84] |
RPD rapid dissolution
Time course of Ф after administration of each triptan
Changes in plasma drug concentrations after a single-dose administration for 5 triptans in Japanese subjects are shown in Fig. 1. These data included not only usual but also clinical dose data by which evaluation of recurrence rate was reported. Changes in serotonin 5-HT1B and 5-HT1D receptor occupancies (Ф1B and Ф1D) at the time of usual-dose administration calculated from the pharmacokinetic and pharmacodynamic parameters are shown in Fig. 2.
Fig. 1.
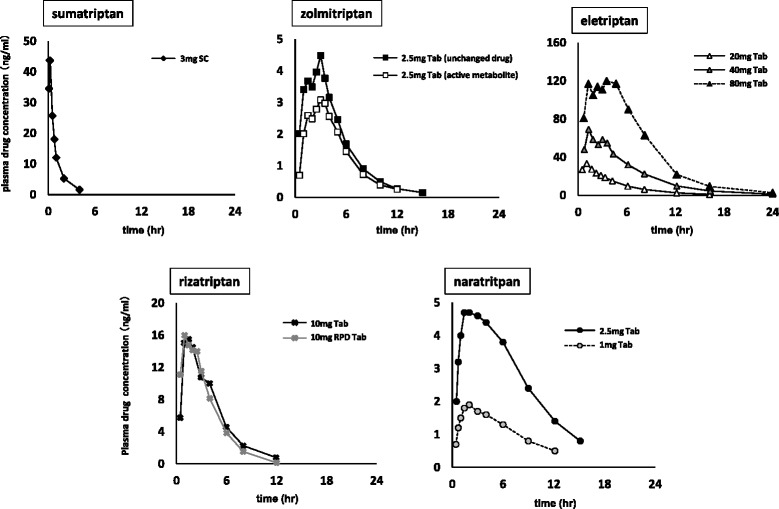
Changes in plasma drug concentrations after a single-dose administration in Japanese subjects. (SC: subcutaneous injection, Tab: oral tablet, RPD Tab: rapid dissolution oral tablet, solid line: usual-dose in Japan)
Fig. 2.
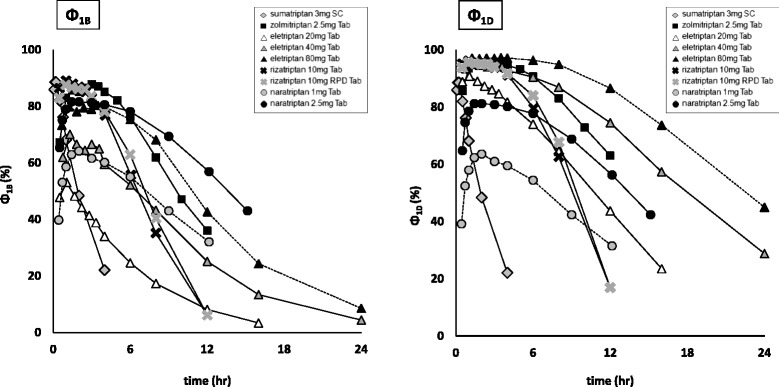
Changes in Ф1B and Ф1D at the time of usual-dose administration. (SC: subcutaneous injection, Tab: oral tablet, solid line: usual-dose in Japan)
For Ф1B, eletriptan remained at a low level. For Ф1D, it was indicated that all triptans obtained an occupancy of 80 % or higher at maximum. Moreover, the injectable form of sumatriptan showed a quicker occupancy decline than the other triptans for Ф1B and Ф1D.
Relationship with recurrence rate
Relationship between recurrence rate and elimination half-lives of plasma drug concentrations Ф1B and Ф1D
The relationships between recurrence rate and elimination half-lives, and Ф1B and Ф1D, as calculated from the time-course of plasma drug concentration obtained from other studies, are shown in Fig. 3. In our investigation of elimination half-life, zolmitriptan with active metabolites was excluded, as it could not be examined only on the basis of half-life in an unchanged drug.
Fig. 3.
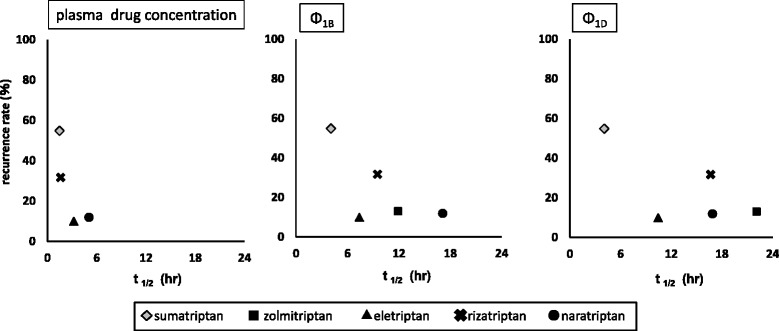
Relationships between headache recurrence rate and elimination half-lives of plasma drug concentration, Φ1B and Φ1D
For all items, though recurrence tended to be lower along with longer half-life, no significant statistical correlation was found.
Relationship between recurrence rate and duration time of Ф
The time until Ф1B and Ф1D became 50 % or less, 40 % or less, and 30 % or less was calculated as duration time to examine the relationship with recurrence rate. Table 3 shows the calculated duration time of Ф1B and Ф1D.
Table 3.
Calculated duration time of Ф
| [Φ1B] | ||||
| Drug | Dose (mg/time) (formulation) | Time until Ф1B became 50 % or less (hr) | Time until Ф1B became 40 % or less (hr) | Time until Ф1B became 30 % or less (hr) |
| Sumatriptan | 3 (subcutaneous injection) | 1.3 | 2.4 | 2.5 |
| Zolmitriptan | 2.5 (oral tablet) | 9.6 | 11.3 | 13.1 |
| Eletriptan | 20 (oral tablet) | 1.4 | 3.1 | 6.1 |
| 40 (oral tablet) | 6.7 | 8.9 | 11.1 | |
| 80 (oral tablet) | 11 | 12.8 | 14.9 | |
| Rizatriptan | 10 (oral tablet) | 8 | 9.6 | 11.3 |
| 10 (RPD oral tablet) | 7.2 | 8.1 | 9.2 | |
| Naratritpan | 1 (oral tablet) | 7.2 | 9.8 | 12.7 |
| 2.5 (oral tablet) | 13.7 | 15.8 | 18 | |
| [Φ1D] | ||||
| Drug | Dose (mg/time) (formulation) | Time until Ф1D became 50 % or less (hr) | Time until Ф1D became 40 % or less (hr) | Time until Ф1D became 30 % or less (hr) |
| Sumatriptan | 3 (subcutaneous injection) | 1.3 | 2.4 | 2.5 |
| Zolmitriptan | 2.5 (oral tablet) | 14.6 | 16.6 | 18.6 |
| Eletriptan | 20 (oral tablet) | 10.9 | 12.8 | 14.9 |
| 40 (oral tablet) | 18.3 | 21.2 | 24 | |
| 80 (oral tablet) | 22.9 | 26.7 | 31.1 | |
| Rizatriptan | 10 (oral tablet) | 12.2 | 13.8 | 15.5 |
| 10 (RPD oral tablet) | 9.3 | 10.1 | 10.8 | |
| Naratritpan | 1 (oral tablet) | 7.2 | 9.7 | 12.7 |
| 2.5 (oral tablet) | 12.4 | 15.6 | 17.9 | |
RPD rapid dissolution
Figure 4 shows the relationship with the duration time of Ф1B and Ф1D.
Fig. 4.
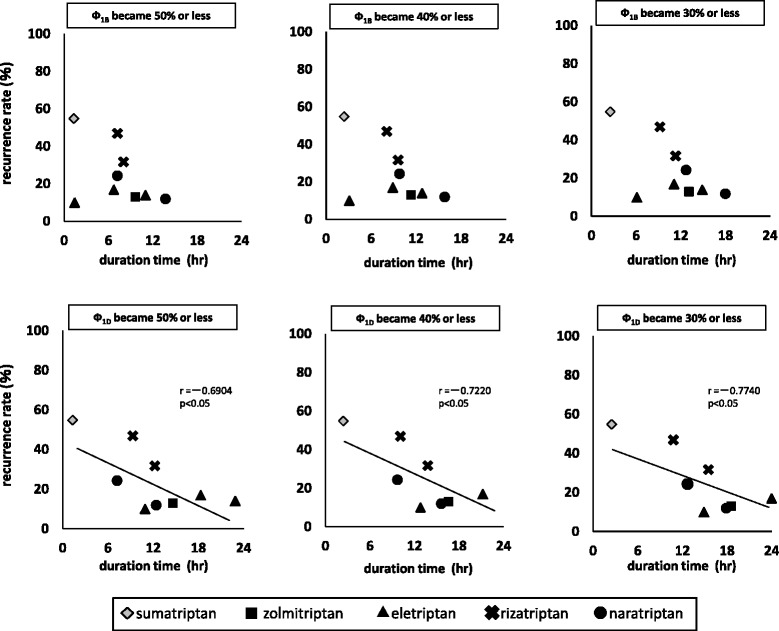
Relationships between headache recurrence and duration time of Φ1B and Φ1D
For both Ф1B and Ф1D, the recurrence rate tended to be lower as the duration became longer. In addition, a significant correlation was observed for Ф1D.
Relationship between recurrence rate and Ф1B and Ф1D at 6, 12, and 18 h after administration
For clarifying the Ф value and time period most closely correlated with recurrence rate, recurrence and Ф1B and Ф1D at 6, 12, and 18 h after administration were calculated. Table 4 shows calculated Ф1B and Ф1D values at 6, 12, and 18 h after administration.
Table 4.
Calculated Ф values at 6, 12, and 18 h after administration
| [Φ1B] | ||||
| Drug | Dose (mg/time) (formulation) | 6 h after administration (%) | 12 h after administration (%) | 18 h after administration (%) |
| Sumatriptan | 3 (subcutaneous injection) | 0 | 0 | 0 |
| Zolmitriptan | 2.5 (oral tablet) | 76.3 | 36.1 | 3.3 |
| Eletriptan | 20 (oral tablet) | 25.3 | 8.7 | 1.5 |
| 40 (oral tablet) | 52.9 | 25.7 | 11.4 | |
| 80 (oral tablet) | 75.7 | 43.6 | 20.7 | |
| Rizatriptan | 10 (oral tablet) | 66.9 | 25.8 | 0 |
| 10 (RPD oral tablet) | 62.9 | 5.7 | 0 | |
| Naratritpan | 1 (oral tablet) | 54.3 | 32.1 | 10 |
| 2.5 (oral tablet) | 78.2 | 57.7 | 57.7 | |
| [Φ1D] | ||||
| Drug | Dose (mg/time) (formulation) | 6 h after administration (%) | 12 h after administration (%) | 18 h after administration (%) |
| Sumatriptan | 3 (subcutaneous injection) | 0 | 0 | 0 |
| Zolmitriptan | 2.5 (oral tablet) | 90.7 | 63.1 | 33 |
| Eletriptan | 20 (oral tablet) | 74.3 | 44.3 | 14.7 |
| 40 (oral tablet) | 90.7 | 75 | 50.7 | |
| 80 (oral tablet) | 96.4 | 87.1 | 67.1 | |
| Rizatriptan | 10 (oral tablet) | 86.2 | 51.8 | 15 |
| 10 (RPD oral tablet) | 83.6 | 16.4 | 0 | |
| Naratritpan | 1 (oral tablet) | 54.3 | 30.3 | 11.4 |
| 2.5 (oral tablet) | 77.8 | 57 | 29.3 | |
RPD rapid dissolution
The relationships between recurrence rate and Ф1B and Ф1D at each time point are shown in Fig. 5.
Fig. 5.
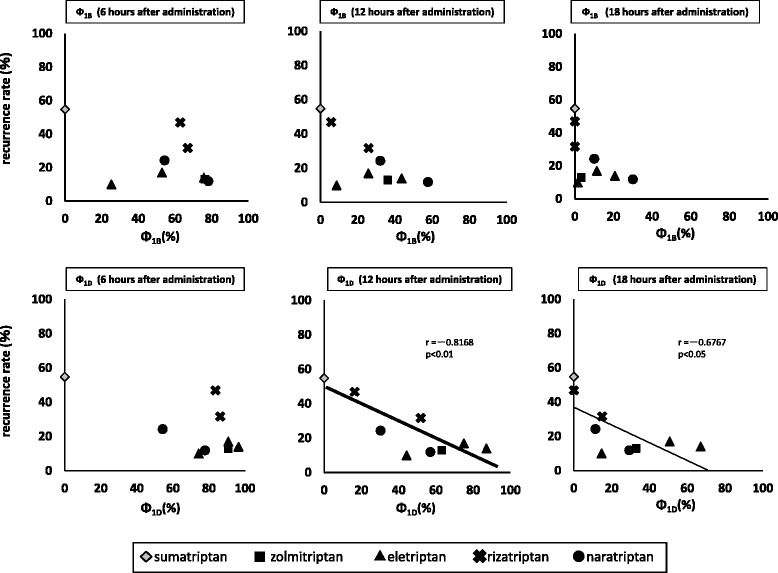
Relationships between headache recurrence and Φ1B and Φ1D at each time after administration
For Ф1B, the aspect differed depending on each time point, while for Ф1D, the recurrence rate tended to be lower when Ф1D was larger. Significant correlations with recurrence rate were observed at 12, and 18 h after administration. Notably, the most significant correlation was observed between recurrence rate and Ф1D at 12 h after administration.
Discussion
During migraine treatment, a headache sometimes returns after remission achieved by administration of triptans, which are serotonin 5-HT1B/1D receptor agonists. Accordingly it is indispensable to establish information related to proper use to prevent recurrence. In the present study, we performed theoretical evaluations for establishing proper use by calculating serotonin 5-HTIB and 5-HT1D receptor occupancies, and examined their relationship with headache recurrence.
Drug action is determined by drug concentration in the region of the site of action and binding to the target receptor. When drug action is elicited by a specific target receptor, it is important to kinetically clarify the relationship between them. In our previous study, we have reported that receptor occupancy is useful for theoretical evaluation of the standard therapeutic doses of various groups of drugs, and a more appropriate index for estimating drug action than dose or blood concentration [5–10].
For collecting pharmacokinetic parameters and recurrence data, we selected only clinical trials that used Japanese subjects. Regarding pharmacodynamic parameters, we collected Ki values that showed an affinity to the serotonin 5-HT1B and 5-HT1D receptors based on pharmaceutical interview forms and literature from new drug application data summaries. Only for zolmitriptan, the Ki value could not be obtained from literature. However, it was reported that the Ki value was equivalent to the IC50 value of the radioactive ligand displacement trial [11], thus we used it as substitute for the Ki value in our analysis. Furthermore, since one kind of active metabolite considered to affect efficacy was present for zolmitriptan, we collected data regarding the unchanged drug and active metabolite for the present analysis.
In this study, therefore using the pharmacokinetic data of a Japanese subject, it was necessary to examine the data for clinical trials in Japan. However, the information that has been published in clinical trials of Japanese was less. In the future, in order to analyze in more detail, it is required to include more data for clinical trials.
The definition of recurrence was found to vary among the clinical trials examined. Notably, the method for determining the time period until headache relief following the initial administration differed. In the present analysis, for comparing recurrence rates among the 5 triptans according to the same definition, we used the following: the ratio of patients who had headache relief 1-4 h after the initial administration but had headache return within 24 h after administration.
In clinical trials of zolmitriptan, there was no difference found in regard to recurrence rate between patients who had headache relief 2 h after administration and those who had relief 4 h after administration [12]. Accordingly, we employed that in our analysis based on the assumption that the method for determining the time period until headache relief may not cause a dispersion in recurrence rate. In the future, it is expected that analysis precision might be further improved if comparisons can be made by using data collected according to a unified definition of recurrence. Using our definition to analyze clinical trial data of Japanese patients we obtained recurrence rates for sumatriptan (3 mg subcutaneous injectable) (headache relief at 1 h after administration), zolmitriptan (2.5-mg tablets), eletriptan (20-, 40-, 80-mg tablets), rizatriptan (10-mg tablets), RPD (10-mg tablets) (headache relief at 2 h after administration), and naratriptan 1- and 2.5-mg tablets (headache relief at 4 h after administration). The rate was relatively low for eletriptan and naratriptan, whereas it was high for rizatriptan. Also, the injectable form had a higher rate of recurrence than tablets. Thus, our findings clarified that recurrence rate varied among the kind and administered form of the drugs.
In the present analysis, the drug concentration near the subject receptor was speculated to be at the same level as that of the plasma unbound drug concentration. Triptans act on the serotonin 5-HT1B and 5-HT1D receptors, and they are thought to work in three main ways: 1) peripheral inhibition of release of vasodilator neuropeptides; 2) modulation of second-order neurons centrally in the trigeminocervical pathway; and 3) vasoconstriction [13]. There are no studies that performed direct measurements of drug concentration near those receptors, thus we speculate that the drug transits to plasma after administration and the unbound form from plasma protein penetrates through the vascular wall to reach the action site [14]. Smatriptan and naratriptan especially are scarcely passed through the blood–brain barrier (BBB) and were weakly distributed in the central nervous system [15, 16]. However, they elicited the same therapeutic effect for migraine as other triptans. Furthermore, it is reported that triptans also acted on 5-HT1B/1D receptors in the trigeminal and dorsal root ganglion cells where BBB was lacked rather than in the sites with BBB in the trigeminovascular system [17]. Accordingly, we did not take concentration in other tissues such as the brain into consideration. As certain findings could be obtained on the basis of these speculations, we considered that they had some validity. More detailed analysis would be possible if the actual concentration near the receptors could be measured at the time of administration in human subjects.
On the basis of data collected regarding changes in plasma drug concentration in Japanese patients, as well as pharmacokinetic and pharmacodynamics parameters, we calculated the time-course changes of receptor occupancies (Ф1B and Ф1D). As the dose varied depending on the kind of triptan, it was considered difficult to make a quantitative evaluation on the basis of plasma drug concentration. However, that of recurrence rate using Ф was considered possible and applicable as a unified index irrespective of drug kind. For Ф1B, eletriptan maintained a low level in whole, whereas for Ф1D, it was observed that all of the tested triptans attained an occupancy of 80 % or higher at maximum. As compared to the other triptans, sumatriptan injectable had a short half-life of plasma drug concentration, and showed a quicker decline of occupancy for both Ф1B and Ф1D.
As for headache recurrence following triptan administration, a study conducted with western subjects noted that a certain relationship could be observed between elimination half-life and recurrence rate [2]. Thus, we first investigated the relationship between recurrence rate and elimination half-life of plasma drug concentration. For both, longer half-life was related to lower recurrence rate, though the differences were not statistically significant. In addition, the drug which has an active metabolite like zolmitriptan, could not be compared with other drugs based on elimination half-life of plasma drug concentration.
In Japan, there was a report which considered the relationship between efficacy time of triptans and recurrence rate [18]. Thus, we examined the relationship between recurrence rate and duration time of Ф after administration. As the Ф1B value of eletriptan was maintained at the relatively low level of 53.1 % at a maximum, our analysis was performed by setting the index of duration time as the time until Ф1B and Ф1D became 50 % or less, 40 % or less, and 30 % or less. For Ф1B, no statistically significant correlation could be obtained. For Ф1D, recurrence rate tended to be lower as duration time was longer, which showed a statistically significant correlation. Thus, it considered likely that the time required to maintain Ф1D higher than a certain level might have an effect on recurrence rate.
Furthermore, for elucidating the Ф value and time period most closely correlated with recurrence rate, we examined the relationship between recurrence rate and Ф at predetermined time points after administration in subjects who had headache recurrence within 24 h after administration. However, for plasma drug concentration, time-course data up to 24 h after administration could not be calculated, as they were not available. Accordingly, we focused on Ф until 6-18 h after administration, and analyzed the relationship between recurrence rate and Ф every 6 h. No correlation was found between Ф1B and recurrence rate, whereas a significant correlation was observed between Ф1D and recurrence rate, with the most notable at 12 h after administration. On the basis of these findings, it is suggested that higher Ф1D at 12 h after administration is related to lower recurrence rate.
Conclusions
Based on our findings in this study of receptor occupancy, it is suggested that Ф1D exerts a greater effect on headache recurrence rate after triptan administration in Japanese subjects as compared to Ф1B and that recurrence may be inhibited to a greater degree when Ф1D is maintained at a higher level. Notably, as an index for evaluating headache recurrence following triptan administration, recurrence rate and Ф1D value at 12 h after administration were found to be most closely correlated and useful for analysis. Our results indicate that headache recurrence inhibition can be evaluated using these values.
Funding
No grants or fellowships are supporting the writing of the paper.
Abbreviations
- 5-HT
5-hydroxytriptamine
- AUC
Area under the curve
- Φ
Receptor occupancy
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KT conceptualized and designed the study, acquired and analysis the data, and drafted the manuscript. RT conceptualized and designed the study, acquired and analysis the data, and drafted the manuscript. MT conceptualized and designed the study, acquired and analysis the data, and drafted the manuscript. MW conceptualized and designed the study. YK conceptualized and designed the study, and critically revised the manuscript. YY conceptualized and designed the study, and critically revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kentaro Tokuoka, Email: tokuoka.kentaro@hachioji-hosp.tokai.ac.jp.
Risa Takayanagi, Email: risat@ps.toyaku.ac.jp.
Mioko Toyabe, Email: pv305vg@gmail.co.jp.
Masayuki Watanabe, Email: watanabe.masayuki@hachioji-hosp.tokai.ac.jp.
Yasuhisa Kitagawa, Email: kitagawa.yasuhisa@hachioji-hosp.tokai.ac.jp.
Yasuhiko Yamada, Email: yamada@ps.toyaku.ac.jp.
References
- 1.Societas Neurologica Japonica and Japanese headache society . Japanese guidelines for the management of primary headache. Tokyo: IGAKU-SHOIN Ltd; 2013. [Google Scholar]
- 2.Géraud G, Keywood C, Senard JM. Migraine Headache Recurrence: Relationship to Clinical, Pharmacological, and Pharmacokinetic Properties of Triptans. Headache. 2003;43(4):376–388. doi: 10.1046/j.1526-4610.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- 3.Takayanagi R, Tokuoka K, Suzuki Y, Watanabe M, Kitagawa Y, Yamada Y. Analysis of Drug Efficacy of Sumatriptan for Acute Migraine Based on Receptor Occupancy. Japanese Journal of Headache. 2012;39(1):91–97. [Google Scholar]
- 4.Tokuoka K, Takayanagi R, Suzuki Y, Watanabe M, Kitagawa Y, Yamada Y. Theory-based analysis of clinical efficacy of triptans using receptor occupancy. J Headache Pain. 2014;15:85. doi: 10.1186/1129-2377-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura M, Sawada Y, Yamada Y, Nakamura K, Iga T. Prediction of therapeutic doses of sulfonylureas based on receptor occupancy theory. Xenobio Metabo Disp. 1992;7:233–241. [Google Scholar]
- 6.Yamada Y, Sawada Y, Takayanagi R, Ito K, Nakamura K, Iga T. Prediction of therapeutic doses of antipsychotic drugs as Dopamine D2 receptor antagonists: Approach based on receptor occupancy theory. Xenobio Metabo Disp. 1993;8:247–261. [Google Scholar]
- 7.Yamada Y, Matsuyama K, Takayanagi R, Kotaki H, Sawada Y, Iga T. Kinetic Analysis of Therapeutic Doses of β-Blockers for Angina Pectoris Based on Receptor Occupancy Theory-Relationship between β-Receptor Occupancy or Vasodilative Action and Dose. YAKUGAKU ZASSHI. 1999;119:495–501. doi: 10.1248/yakushi1947.119.7_495. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Sugiura M, Higo K, Ozeki T, Takayanagi R, Okuyama K, Yamamoto K, Satoh H, Sawada Y, Iga T. Receptor occupancy theory-based analysis of antiemetic effects and standard doses of 5-HT3 receptor antagonists in cancer patients. Cancer Chemother Pharmacol. 2004;54:185–190. doi: 10.1007/s00280-004-0798-x. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi R, Mizushima H, Ozeki T, Yokoyama H, Iga T, Yamada Y. Analysis of pharmacological effects of drugs used for treatment of urinary disturbance based on anticholinergic and smooth muscle-relaxing effects. Biol Pharm Bull. 2007;30:1297–1300. doi: 10.1248/bpb.30.1297. [DOI] [PubMed] [Google Scholar]
- 10.Ayuhara H, Takayanagi R, Okuyama K, Yoshimoto K, Ozeki T, Yokoyama H, Yamada Y. Receptor occupancy theory-based analysis of interindividual differences in antiemetic effects of 5-HT3 receptor antagonists. Int J Clin Oncol. 2009;14:518–524. doi: 10.1007/s10147-009-0912-5. [DOI] [PubMed] [Google Scholar]
- 11.Akarawut W, Lin CJ, Smith DE. Noncompetitive Inhibition of Glycylsarcosine Transport by Quinapril in Rabbit Renal Brush Border Membrane Vesicles: Effect on High-Affinity Peptide Transporter. J Pharmacol Exp Ther. 1998;287(2):684–690. [PubMed] [Google Scholar]
- 12.Summary basis of approval of ZOMIG (2005) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.pmda.go.jp/shinyaku/P200100027/index.html. Accessed 15 April 2015
- 13.Johnston MM, Rapoport AM. Triptans for the management of migraine. Drugs. 2010;70(12):1505–1518. doi: 10.2165/11537990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Yamada Y, Sawada Y, Iga T. Prediction of adverse effect of β-blocking agents. Jpn Pharmacol Ther. 1995;23(1):27–33. [Google Scholar]
- 15.AMERGE Tablets Product Information (2011) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.info.pmda.go.jp/go/pack/2160007F1020_1_05/. Accessed 15 April 2015
- 16.Perry CM, Markham A. Sumatriptan. An updated review of its use in migraine. Drugs. 1998;55(6):889–922. doi: 10.2165/00003495-199855060-00020. [DOI] [PubMed] [Google Scholar]
- 17.Tomita M, Suzuki N, Sakai F. The Distribution of 5-HT1B Receptors and the Effect of Anti-migraine Drugs Triptan in Trigeminovascular System of the Rat. Kitasato Med. 2002;32:173–178. [Google Scholar]
- 18.Hamada J. Significance of naratriptan based on the pharmacokinetic and pharmacological profile. Progress in Medicine. 2010;30:2437–2442. [Google Scholar]
- 19.IMIGRAN Injection/ Kit Subcutaneous Injection Product Information (2008) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.info.pmda.go.jp/go/pack/2160402G1026_1_05/. Accessed 5 March 2015
- 20.ZOMIG Tablets Product Information (2015) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.info.pmda.go.jp/go/pack/2160004F1027_1_13/. Accessed 15 April 2015
- 21.Dixon R, Warrander A. The clinical pharmacokinetics of zolmitriptan. Cephalalgia Suppl. 1997;18:15–20. doi: 10.1177/0333102497017S1803. [DOI] [PubMed] [Google Scholar]
- 22.RELPAX Tablets Product Information (2012) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.info.pmda.go.jp/go/pack/2160005F1021_2_03/. Accessed 15 April 2015
- 23.MAXALT Tablets/RPD Tablets Product Information (2015) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.info.pmda.go.jp/go/pack/2160006F1026_2_11/. Accessed 15 April 2015
- 24.Summary basis of approval of MAXALT Tablets/RPD Tablets (2003) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.pmda.go.jp/shinyaku/P200300017/index.html. Accessed 15 April 2015
- 25.Fukuuchi Y, Teramoto J, Tatsuoka Y, Yamaguchi M, Shimizu T, Urashima T, Nishioka H, Iwasaki M. Clinical Evaluation of Imigran® Kit Subcutaneous Injection 3mg (Sumatriptan Succinate) in Patients with Migraine and Ciuster Headache. J Clin therapeutics Med. 2008;24(9):809–824. [Google Scholar]
- 26.Summary basis of approval of RELPAX Tablets (2002) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.pmda.go.jp/shinyaku/P200200017/index.html. Accessed 15 April 2015
- 27.Summary basis of approval of AMERGE Tablets (2008) [in Japanese] Pharmaceuticals and Medical Devices Agency, Tokyo. http://www.pmda.go.jp/shinyaku/P200800004/index.html. Accessed 15 April 2015


