Abstract
Bixa orellana L. (Bixaceae) is a multipurpose tree grown for the production of commercially important dyes. In the present study, an efficient, reproducible protocol was developed for direct plant regeneration from in vitro derived petiole explants of Bixa orellana L. Murashige and Skoog medium (MS) supplemented with 2-isopentenyl adenine (9.8 μM) and naphthalene acetic acid (10.7 μM) was found to be optimum for production of high frequency of shoot organogenesis. Subculturing of the shoots onto the fresh MS medium containing similar concentrations of 2-iP (9.8 μM) and NAA (10.7 μM) produced elongated shoots. Elongated shoots when placed onto MS medium supplemented with 1.7 μM indole-3-acetic acid and 14.7 μM 2-iP produced optimal rooting. Rooted plantlets were acclimatized and transplanted to the field successfully. Histological investigation revealed the origin of shoot primordia, from sub-epidermal cells of petiole explants. The regeneration protocol developed in this study can be useful for mass in vitro propagation and effective genetic transformation of commercially important edible dye yielding tree species.
Keywords: Lipstick tree; Achiote; Adventitious roots; Histology, rooting, petiole
Introduction
Bixa orellana L. (Bixaceae) is a small evergreen perennial tree species native to tropical America and is widely cultivated in many other tropical countries including the southern parts of India (Srivastava et al. 1999). This tree species is commonly grown for the production of commercially important dyes bixin, norbixin contained in the mature seed coat. Annatto pigment is used as a natural dye for coloring various food products, cosmetic and pharmaceutical applications (Wealth of India 1990). Annatto ranks second in economic importance behind saffron with an estimated world consumption of 14,500 tons of seeds with an average cost of US$ 1100 per million ton of seeds (Satyanarayana et al. 2003). There is increasing demand for natural sources such as annatto pigment because of adverse effects of artificial colorants. The content of bixin generally varies from plant to plant as a result of its cross-pollinated nature (Aparnathi et al. 1990). Since annatto is one of the 13 basic pigments derived from natural sources that are currently permitted for food coloring by the US FDA, there is an ever increasing demand (Srinivasulu 1996).
The entire plant is highly medicinal hence it is widely used in Ayurveda (Caceres et al. 1995; Suhaila et al. 1996; Srivastava et al. 1999; Daniela et al. 2014). In spite of its economic and medicinal importance it has not been properly commercialized because of unavailability of plant material. Conventional propagation of the plant is through seeds and vegetative cuttings. The propagation rate of annatto through seed germination is unreliable due to low seed viability (20 %), poor germination rates (5–7 %), long generation cycle of seed production (~4 years), delayed seed germination (40 days) and seed dormancy (Eira and Mello 1997). The plant growth is influenced by favorable climatic conditions and requires soil rich in manganese for their seeds to germinate. Conventional multiplication via vegetative cuttings has limitations because of intense leaching of phenolic substances from the cut ends, which obscure rooting (Madhuri and D'Souza 2000).
An alternative approach to overcome the propagation limitations is the mass propagation through tissue culture using various in vitro derived annatto explants. Development of a simple and highly efficient regeneration procedure is a primary requisite for the improvement of productivity and significant reduction of plant heterogeneity. Although there are many published reports for in vitro micropropagation of B. orellana derived from various explants; they produced less number of shoot buds per explants and high frequency of shoot organogenesis has not been reported in this species (Madhuri and D'Souza 2000; Paiva Neto et al. 2002; Rivera-Madrid et al. 2006; Parimalan et al. 2008, 2009, 2011; Joseph et al. 2011; Siril and Joseph 2013; Ana Claudia et al. 2014). There are no successful reports on direct shoot organogenesis using petiole explants for this species. Therefore, the objective of the present work was to develop an in vitro regeneration method for B. orellana from petiole leaf explants. The usage of the petiole as an explant has potential for mass commercial micropropagation and genetic engineering in annatto.
Materials and methods
Plant material, disinfestation and seed germination
Mature seeds were collected from the fully ripe and dried fruits of 7-year old B. orellana growing in the Botanical garden, Sri Venkateswara University, Tirupati, India. Seeds were rinsed in running tap water for 2 min and then treated with 10 % Tween 20 (Sigma Aldrich, USA) for 5 min. The seeds were then treated with 5-60 % of sulphuric acid for 5–20 min for seed scarification. Removal of excess acid was through repeated rinsing with sterile double distilled water. Seeds were then surface sterilized in 70 % ethyl alcohol for 1 min, 0.1 % HgCl2 for 2 min, followed by rinsing twice with sterile double distilled water. Seeds were aseptically germinated in 150 ml conical flasks (Borosil, Mumbai, India) containing 20 ml Murashige and Skoog 1962 supplemented with 30 g/l sucrose and solidified with 8 g/l agar (Hi-Media, Mumbai,India). After 5–7 days of cultures in the dark, cultures were moved to light conditions (16 h photoperiod 40 μmol m−2 s−1 illuminated with cool white fluorescent tubes at 25 ± 2 °C).
Shoot induction medium and culture conditions
From 2 weeks old seedlings, petiolated leaf explants were dissected and inoculated onto MS medium containing varying concentrations and combinations of plant growth regulators (PGRs) like cytokinins (BAP, 2-iP, Kn) and auxins (IAA, NAA) for shoot initiation (Table 1). The medium pH was adjusted to 5.8 prior to the addition of 0.8 g/l agar. Medium was distributed into culture tubes (23 × 150 mm, Borosil, India) and autoclaved at 105 K Pa and 121 °C for 20 min. After inoculation, the cultures were incubated in culture room conditions maintained at 25 ± 2 °C with a 16 h photoperiod at a light intensity of 40 μmol m−2 s−1 provided by cool white fluorescent tubes. Regenerating explants were sub-cultured to fresh medium of the same composition at an interval of 2 weeks. After 4 weeks of culture, the number of explants forming adventitious shoots were counted and recorded. Explants with more than four adventitious shoots were considered as multiple shoots and scored up in the present work for the percent frequency of explants producing multiple shoots.
Table 1.
Effect of various plant growth regulators (PGRs) on shoot organogenesis from petiolated leaf explants of Bixa orellana. The results are the mean ± S.E. of 10 replicates. Means with the same letters within columns are not statistically significant at P > 0.05
| Plant growth regulators (μM) | Responding explants (%) | Mean No shoots per explant | |||
|---|---|---|---|---|---|
| BAP | 2-iP | NAA | IAA | ||
| 4.4 | – | – | – | 22ab | 3.2 ± 0.6a |
| 6.6 | – | – | – | 26bcde | 3.4 ± 0.8a |
| 8.8 | – | – | – | 28cdef | 3.5 ± 0.4a |
| – | 4.9 | – | – | 36g | 3.1 ± 0.2a |
| – | 7.3 | – | – | 46ijkl | 3.0 ± 0.7a |
| – | 9.8 | – | – | 55m | 2.5 ± 0.3a |
| 4.4 | – | 5.3 | – | 24abcd | 3.4 ± 0.2a |
| 6.6 | – | 8.0 | – | 34fg | 3.6 ± 0.9a |
| 8.8 | – | 10.7 | – | 48kl | 3.8 ± 0.2a |
| 4.4 | – | – | 5.7 | 21a | 3.3 ± 0.2a |
| 6.6 | – | – | 8.5 | 30ef | 3.6 ± 0.9a |
| 8.8 | – | – | 11.4 | 42hi | 3.7 ± 0.2a |
| – | 9.8 | 5.3 | – | 62n | 25.0 ± 0.7b |
| – | 9.8 | 8.0 | – | 64n | 28.0 ± 0.6c |
| – | 9.8 | 10.7 | – | 80p | 36.0 ± 0.2d |
| – | 9.8 | – | 5.7 | 41hij | 4.4 ± 0.8a |
| – | 9.8 | – | 8.5 | 43ij | 4.2 ± 0.5a |
| – | 9.8 | – | 11.4 | 47l | 4.6 ± 0.8a |
Effect of basal media on shoot induction
After defining the optimal PGR treatments for shoot organogenesis, a further experiment was designed to assess the effect of basal salt formulation on adventitious shoot induction. Petiole explants were cultured on six different basal nutrient salt media: MS and ½ MS (half strength concentrations of the major and minor salts of the MS medium), B5 and ½ B5 (half strength concentrations of the B5 medium), WPM and ½ WPM (half strength concentrations of the WPM medium, Lloyd and McCown 1980) (Table 2). These media were prepared, autoclaved as above and were supplemented with 9.8 μM 2-iP and 10.7 μM NAA.
Table 2.
Effect of basal medium on Bixa orellana shoot organogenesis. a Regeneration frequency was determined after 4 weeks of culture (MS, 1/2MS, B5, 1/2B5, WPM, 1/2WPM) supplemented with 9.8 μM 2-iP and 10.7 μM NAA. The results were the mean ± S.E. of 10 replicates. Means with the same letters within columns are not statistically significant at P > 0.05.*Half strength concentrations of the major and minor salts of the MS, B5 and WPM
| Basal medium | Responding explants (%) | Mean No shoots per explant |
|---|---|---|
| MS | 80d | 36.0 ± 0.8d |
| ½MS* | 42c | 12.0 ± 0.9b |
| B5 | 75d | 22.0 ± 1.0c |
| ½B5+ | 34b | 9.0 ± 0.8ab |
| WPM | 30b | 8.0 ± 1.3a |
| ½WPM | 21d | 4.8 ± 1.3ab |
In vitro rooting and acclimatization of plantlets
Elongated shoots (2.5–4.5 cm long) were excised from the explants and transferred to full strength and half strength MS basal medium in the presence and absence of auxins (Table 3) for rooting and further elongation. After 3 weeks, the number of rooted shoots and the number of roots per shoot were recorded. Plantlets with 3–5 cm long roots were individually removed from the culture tubes. After washing the roots carefully with sterile double distilled water, plantlets were hardened by transplanting them into polythene bags containing autoclaved vermiculite and pelrite compost mixture (ratio 3:1). The plants were watered and maintained under green house conditions (25 ± 2 °C, 75–80 % relative humidity) for 4 weeks time.
Table 3.
Effect of auxins treatment on in vitro rooting of cultured shoots of Bixa orellana on MS medium. The results are the mean ± S.E. of 10 replicates. Means with the same letters within columns are not statistically significant at P > 0.05
| Medium | Plant growth regulators (μM) | Responding explants (%) | Mean No roots per explant | |||
|---|---|---|---|---|---|---|
| NAA | IAA | IBA | 2-iP | |||
| MS | 0.5 | – | – | 4.9 | 0.5a | 2.0 ± 0.6ab |
| 1.0 | – | – | 9.8 | 3.0a | 4.5 ± 0.8bc | |
| 1.6 | – | – | 14.7 | 3.5a | 4.8 ± 0.4bc | |
| – | 0.5 | – | 4.9 | 73e | 5.6 ± 0.5cde | |
| – | 1.1 | – | 9.8 | 78f | 6.8 ± 0.3de | |
| – | 1.7 | – | 14.7 | 75ef | 7.2 ± 0.2e | |
| – | – | 0.4 | 4.9 | 1.0a | 2.5 ± 0.7ab | |
| – | – | 0.8 | 9.8 | 0.9a | 2.6 ± 0.8ab | |
| – | – | 1.4 | 14.7 | 4.0a | 2.6 ± 0.5ab | |
| ½MS | 0.5 | – | – | 4.9 | 0.8a | 1.0 ± 0.6a |
| 1.0 | – | – | 9.8 | 0.9a | 1.0 ± 0.2a | |
| 1.6 | – | – | 14.7 | 2.5a | 2.8 ± 0.9ab | |
| – | 0.5 | – | 4.9 | 20b | 2.9 ± 0.7ab | |
| – | 1.1 | – | 7.3 | 32cd | 4.8 ± 0.5bc | |
| – | 1.7 | – | 9.8 | 38d | 5.2 ± 0.6cde | |
| – | – | 0.4 | 4.9 | 0.7a | 1.0 ± 0.9a | |
| – | – | 0.8 | 7.3 | 0.4a | 2.0 ± 0.8a | |
| – | – | 1.4 | 9.8 | 2.0a | 1.6 ± 0.6a | |
Histological studies
Leaf explants from 0 and 16 days following culture were fixed in an FAA solution (35 % formaldehyde/acetic acid/70 % ethanol (1:1:9, v/v/v). Following fixation, tissues were subjected for dehydration through a graded ethanol series (20, 30, 50, 70, and 90 %) sequentially for 20 min at each step and thrice in 100 % for 30 min. All tissues were embedded in paraffin wax following the protocol described previously (Johansen 1940). Transverse section of 10 μm in thickness were prepared using a rotatory microtome (LEICA 2045 Multicult) and then stained with safranin fast green and mounted on glass slides with Canada balsam and micrographed using a light microscope (Olympus AX-70, USA).
Experimental design and statistical analysis
Each culture tube with one shoot explant was considered as one replicate. Each treatment in each set of experiments consists of 20 replicates and each experiment was repeated three times. Standard error of means was calculated in each experiment. The data was statistically analyzed using one way analysis of variance (ANOVA) and means were compared using the Duncan’s multiple range test (DMRT). The p-values were considered statistically significant at p > 0.05 and p < 0.01.
Results
In vitro seed germination and multiple shoot production
Conventional propagation of Annatto through seeds is cumbersome due to their short life span, poor germination rate that restricts propagation. Hence it is of great interest to develop biotechnological methods to improve its cultivation. Although the micropropagation of this plant has been described, reports on the using petiolated explants for direct organogenesis have not been yet explored. Hence in the present study we used petiolar leaf explants by raising aseptic seedlings. In vitro seed germination was observed at 1 week after inoculation. The percent germination of the disinfected seeds was about 90–96 and 100 % contamination free seedlings were obtained using chemical scarification (10 % sulphuric acid for 5 min). Seeds are potentially good sources to produce disease free and fungal resistant plants as pathogens cannot penetrate into the seed although the plant is infected.
Our preliminary screening of various cytokinins (BAP, Kinetin and 2-iP) showed effective initiation of shoot buds from the petiole region of the petiolated leaf explants within 18–21 days of inoculation in the MS medium fortified with 2-iP (data not shown). The initiation of shoot buds from the petiole explants was followed by the development of twisting and wilting of leaf blade. Hence, the yellowish leaf lamina were dissected and discarded to avoid displacement of petiole explants from the medium and subsequent elongation of shoot buds. These explants when placed onto the medium supplemented with 2-iP (9.8 μM) and NAA (10.7 μM) produced a maximum of 36.0 ± 0.3 shoots/ explants in 4 weeks (Table 1, Figs. 1 top panel; 2 top panel). The better shoot organogenic response was observed with petiolated leaf explants grown in B5 medium containing 2-iP (9.8 μM) and NAA (10.7 μM) which produced a large number of 22 shoots/ explants as compared to WPM (Table 2). Subculturing of the shoots onto the fresh MS medium containing similar concentrations of PGRs (10.7 μM NAA and 9.8 μM 2-iP) produced elongated shoots (Fig. 1 bottom panel). The combination of 2-iP with NAA responded better when compared to the combination of 2-iP and IAA for shoot buds production and subsequent elongation.
Fig. 1.
In vitro plant regeneration from petiole leaf explants of Bixa orellana L. (Top panel) Adventitious shoot initiation of petioles on MS medium supplemented with 9.8 μM 2-iP and 10.7 μM NAA after 18–20 days of post-inoculation (Bottom panel) Multiple shoot proliferation in MS medium supplied with 9.8 μM 2-iP and 10.7 μM NAA after 4 weeks interval
Fig. 2.
Different stages of multiple shoots (Top panel) and their elongation (Bottom panel) from petioles on MS medium supplemented with 9.8 μM 2-iP and 10.7 μM NAA
In vitro rooting and acclimatization of plantlets
Elongated shoots of 3.0–4.6 cm long were inoculated onto the MS half and full strength media supplemented with different auxins (IAA, NAA and IBA) and cytokinin (2-iP) for rooting (Fig. 2 bottom panel, Table 3). Among the different concentrations and combinations of auxins tested, 1.7 μM IAA with 14.7 μM 2-iP was more effective in rapid root induction within 3 weeks. This treatment not only induced more number of roots (7.2 ± 0.2) but also promoted root length as well (Fig. 3a,b). Elongated shoots upon inoculation onto the MS half and full strength media supplemented with only auxins (IAA, NAA and IBA) produced basal callus that obscured the root development.
Fig. 3.
In vitro rooting and Hardening of Bixa orellana L. A, B Regenerated shoots rooted on MS solid medium fortified with 14.7 μM 2-iP and 1.7 μM IAA. C Hardened plantlets regenerated from petiole explants established in polythene bags
Healthy plantlets (20 nos) with 3–5 cm long roots were individually removed from the culture tubes. After washing the roots carefully with sterile double distilled water, in vitro rooted plantlets were hardened (Fig. 3c) by transplanting them into plastic pots containing autoclaved vermiculite and pelrite compost mixture (ratio 3:1). The plants were watered and maintained under greenhouse conditions (25 ± 2 °C; 75–80 % relative humidity) for 4 weeks wherein the survival rate was 95 %. Following hardening, the plants were gradually transferred to the field for subsequent development into mature plants. Thus, rooted plants were successfully acclimatized in vermiculite with gradual decrease in air humidity and 90 % of transferred plantlets survived without any morphological variations.
Histological studies
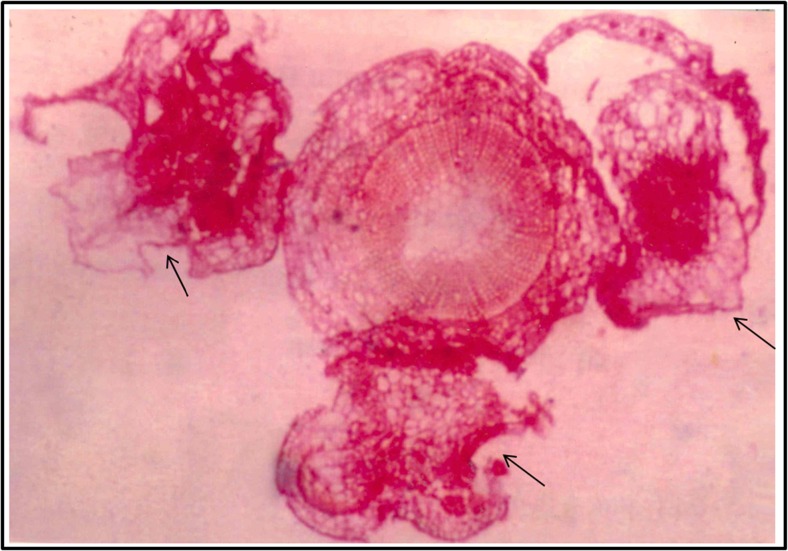
Histological examination of cross sections of petiole segments revealed no pre-existing meristems at the time of seedling. The mitotic cell divisions were most apparent in the sub-epidermal and cortical tissues of petiole explants after 16 days of placement onto the medium. Light microscopic observations revealed the development of many shoot buds directly from cut ends of the petiole explants (Fig. 4).
Fig. 4.
Histology of petiole explants of Bixa orellana L. Transverse section of petiole showing superficial cell proliferation in the epidermal and subepidermal layers after 16 days of culture. The arrowheads showing the initiation of shoot primordia
Discussion
In this study, we report for the first time successful shoot organogenesis from petiole explants of in vitro germinated seedlings of annatto. The use of in vitro derived plant seedlings provides juvenile explants that often have a better regenerability than explants derived from mature tissue (Harding et al. 1996). Moreover the plant material derived from in vitro cultures is more desirable to use for transformation purposes because they are aseptic. Previous reports on B. orellana organogenesis are few and they produced less than 10 shoots per explants and the shoot organogenic response from leaf explants have been attempted earlier but were not successful in shoot induction (Paiva Neto et al. 2002; Parimalan et al. 2008; 2009, 2011; Joseph et al. 2011; Siril and Joseph 2013; Ana Claudia et al. 2014). There are several factors known to be important for shoot organogenesis in vitro (Brown and Thorpe 1986), in particular, the type of explants, age of plant (donor of explant), nutrient medium components and the plant genotype (Kamal et al. 2007). The importance of cultivar/genotype in organogenesis has been reported in several species (Hosokawa et al. 2001).
Adventitious shoots were obtained in highest numbers from petiole sections of Oxalis tuberosa using 3 mg/l BAP and 3 mg/l NAA, but the best regeneration from internode sections occurred when they were grown on a medium with 3 mg/l Zeatin and 3 mg/l NAA. The presence of 2-iP in B5 medium produced four to six shoots per nodal explants as reported by D’Souza and Sharon (2001). Paiva Neto et al. (2002) reported the shoot organogenesis from rooted hypocotyls and hypocotyl segments as an explant source and they produced three and two shoot buds per explants, respectively on the medium containing thidiazuron (TDZ) and zeatin. Nevertheless, problems with conversion of TDZ-induced shoots into complete plantlets such as poor elongation of shoots and inadequate rooting have been reported (Murthy and Saxena 1998). Interestingly, we could achieve the best organogenesis response (adventitious shoot regeneration and elongation) and produced 36 shoots per petiole explants grown in MS medium fortified with 2-iP (9.8 μM) and NAA (10.7 μM). 2-iP was stated as a potent hormone in inducing maximum number of shoots in not only Bixa (D’Souza and Sharon 2001) but also in a wide range of plant species (Sergio et al. 2000; Bhaskaran and Jayabalan 2005; Hiregoudar et al. 2006; Sujatha and Ranjitha Kumari 2007; Ping Luo et al. 2009; Sivanesan et al. 2008; Hussain et al. 2008; Aruna et al. 2012; Krishnareddy and Pulliah 2012; Sonali et al. 2013).
Adventitious shoot regeneration using leaf explants is established for several woody plant species, such as Paulownia tomentosa (Corredoira et al. 2008), Prunus serotina (Liu and Pijut 2008) Citrus sinensis (Khan et al. 2009), Pistacia vera (Tilkat et al. 2009), blackberry (Sandhya and Mahalaxmi 2009), Morus alba (Kodandaram et al. 2013). Similar to earlier observations, kinetin did not promote shoot multiplication in any of the combinations tested in the present investigation (Madhuri and D'Souza, 2000). MS medium was reported to be effective for Bixa orellana plant tissue culture (Ramamurthy et al. 1999; Paiva Neto et al. 2002; Parimalan et al. 2008, 2009, 2011; Joseph et al. 2011; Siril and Joseph 2013; Ana Claudia et al. 2014), except a few reports like, Madhuri and D'Souza (2000) where B5 medium has been used. In this study half-strength MS, half-strength B5 and half-strength WPM media were less effective in promoting shoot organogenesis (Table 2) which is consistent with previous observation (Ramamurthy et al. 1999).
Previous studies with IAA and IBA were found ineffective in root induction and only NAA at 0.5 mg/l promoted maximum number as well as longer roots (Madhuri and D'Souza 2000). Contrary to the previous observations, the results obtained with only auxins supplied MS medium in this study showed significant development of basal calli that hinders the growth of healthy root system. However, we could obtain successful healthy root development without induction of basal calli in MS half and full strength media supplied with combination of cytokinin (2-iP) and auxin (IAA) in 3 weeks. The supplementation of cytokinin in the growth medium was sufficient to abrogate the development of calli at the basal end of the elongated shoots. Thus, the formation of efficient root system from the elongated shoots were successfully achieved, plantlets were hardened, and maintained in the greenhouse. It is well known that phytohormones play a vital role in regulating plant development and the flexible shaping of the plant architecture. However, our knowledge of the mechanisms involved in the hormonal crosstalk is still poorly understood. Generally in the regulation of root development, many hormonal pathways are involved, with auxin and cytokinin being the principal players. Ruzicka et al. (2009) revealed a unique mechanism of auxin–cytokinin interaction and proposed a model for regulation of the auxin–cytokinin balance that is critical for root organogenesis. The study of histological section from 16 days old petiolated leaf explants clearly suggests the direct regeneration of shoot bud from the cut ends of petiole explants.
Conclusion
These are the first successful attempts to establish direct shoot organogenesis from the petiole explants using 2-iP and NAA. This protocol provides an efficient method for adventitious shoot regeneration that may enable efficient genetic transformation towards the improvement of traditional breeding and commercial micropropagation of economically important edible dye yielding tree species. Our study provides a successful and rapid technique that can be used for ex situ conservation.
Acknowledgements
We thank Dept. of Botany, SV University,Tirupati for providing us required chemicals and facilities. We highly appreciate our lab members for critical reading of the manuscript. The authors are highly grateful to the UMK for their logistical support under Grant No. R/SGJP/A07.00/00710A/001/2012/000081).
Author contributions
Conceived and designed the experiments: AM,KKC,GRG. Performed the experiments: AM, KKC. Analyzed the data: KKC,AM,GRG. Wrote the paper: KKC, AM,GRG.
Conflict of interest
Authors declare that there is no competing interest.
Abbreviations
- NAA
α-naphthalene acetic acid
- 2-iP
2-isopentenyl adenine
- IAA
Indole-3-acetic acid
- BAP
6-benzylaminopurine
- PGR
Plant growth regulator
- WPM
woody plant medium
Contributor Information
Arifullah Mohammed, Email: arifullahmd@gmail.com.
Kishore K. Chiruvella, Email: drkishorekumaar@gmail.com
Nima D. Namsa, Email: ndnamsa@yahoo.co.in
Rama Gopal Ghanta, Email: ghantargopal@yahoo.co.in.
References
- Ana Claudia FC, Diego IR, Lourdes L, Marília CV, Marcio GCC, Vespasiano BPN, Wagner CO. In vitro organogenesis from root culture segments of Bixa orellana L. (Bixaceae) In Vitro Cell Dev Biol. 2014;50(1):76–83. doi: 10.1007/s11627-013-9580-2. [DOI] [Google Scholar]
- Aparnathi K, Lata R, Sharma R. Annatto (Bixa orellana L.,): its cultivation preparation and usage. Int J Trop Agric. 1990;8:80–88. [Google Scholar]
- Aruna V, Kiranmai C, Karuppusamy S, Pulliah T. Influence of aseptic seedling explants on in vitro shoot multiplication of Caralluma adscendens var. attenuate Wight. Afr J Plant Sci. 2012;6:290–294. [Google Scholar]
- Bhaskaran P, Jayabalan N. An efficient micropropagation system for Eclipta alba - a valuable medicinal herb. In Vitro Cell Dev Biol Plant. 2005;41:532–539. doi: 10.1079/IVP2005667. [DOI] [Google Scholar]
- Brown DCW, Thorpe TA. In: Plant regeneration by organogenesis. Vasil IK, editor. London: Academic; 1986. pp. 49–65. [Google Scholar]
- Caceres A, Menindez H, Mendez E, Cohobon E, Samayoa Blanca E, Jauregul E, Peralto E, Carrillo G. J Ethanopharmacol. 1995;48:85–88. doi: 10.1016/0378-8741(95)01288-O. [DOI] [PubMed] [Google Scholar]
- Corredoira E, Ballester A, Vieitez AM. Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tissue Org Cult. 2008;95:197–208. doi: 10.1007/s11240-008-9433-6. [DOI] [Google Scholar]
- D’Souza MC, Sharon M. In vitro clonal propagation of annatto (Bixa orellana L.) In Vitro Cell Dev Biol Plant. 2001;37:168–172. doi: 10.1007/s11627-001-0029-7. [DOI] [Google Scholar]
- Daniela AV, Marina SAV, Túlio FAM, Fernanda NR, Márcia RO, Camilo FOF, Petrônio FF, Margareth FFM, José MBF. Traditional uses, chemical constituents, and biological activities of Bixa orellana L. A review. Sci World J. 2014 doi: 10.1155/2014/857292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eira MTS, Mello CMC. Bixa orellana L. seed germination and conservation. Seed Sci Tech. 1997;35:373–380. [Google Scholar]
- Harding K, Benson EE, Roubelakis-Angelakis KA. Methylated DNA changes associated with initiation and maintenance of Vitis vinifera in vitro shoot and callus cultures: a possible mechanism for age-related changes. Vitis. 1996;35:79–85. [Google Scholar]
- Hiregoudar LV, Murthy HN, Bhat JG, Nayeem A, Hema BP, Hahn EJ, Paek KY. Rapid clonal propagation of Vitex trifolia. Biol Plant. 2006;50:291–294. doi: 10.1007/s10535-006-0023-3. [DOI] [Google Scholar]
- Hosokawa K, Koiwa H, Yamamura S. In vitro adventitious shoot formation on petioles of commercial cultivars of Delphinium. Sci Hortic. 2001;90:143–150. doi: 10.1016/S0304-4238(00)00249-1. [DOI] [Google Scholar]
- Hussain TM, Chandrasekhar T, Gopal GR. Micropropagation of Sterculia urens Roxb., an endangered tree species from intact seedlings. Afr J Biotechnol. 2008;7:95–101. [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. [Google Scholar]
- Joseph N, Siril EA, Nair GM. An efficient in vitro propagation methodology for Annatto (Bixa orellana L.) Physiol Mol Biol Plants. 2011;17:263–270. doi: 10.1007/s12298-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal GB, Illich KG, Asadollah A. Effects of genotype, explant type and nutrient medium components on canola (Brassica napus L.) shoot in vitro organogenesis. Afr J Biotechnol. 2007;6(7):861–867. [Google Scholar]
- Khan EU, Zheng Fu X, Wang J, Fan OJ, Huang XS, Zhang GN, Shi J, Liu JH. Regeneration and characterization of plants derived from leaf in vitro culture of two sweet orange (Citrus sinensis (L.) Osbeck) cultivars. Sci Hortic. 2009;120:70–76. doi: 10.1016/j.scienta.2008.10.004. [DOI] [Google Scholar]
- Kodandaram R, Karaba NN, Jyothi SM, Radha SS, Mala VR, Syed MHQ. In vitro plant regeneration of Morus indica L. cv. V1 using leaf explant. Am J Plant Sci. 2013;4:2001–2005. doi: 10.4236/ajps.2013.410249. [DOI] [Google Scholar]
- Krishnareddy PV, Pulliah T. In vitro conservation of Ceropegia elegans, an endemic plant of south India. Afr J Biotechnol. 2012;16:12443–12449. [Google Scholar]
- Liu X, Pijut PM. Plant regeneration from in vitro leaves of mature black cherry (Prunus serotina) Plant Cell Tissue Org Cult. 2008;94:113–123. doi: 10.1007/s11240-008-9393-x. [DOI] [Google Scholar]
- Lloyd GB, McCown BH. Commercially feasible micropropagation of mountain laurel (Kalmia latifolia) by use of shoot tip culture. Proc Int Plant Prop Soc. 1980;30:421–427. [Google Scholar]
- Madhuri S, D'Souza MC (2000) In vitro clonal propapagation of annatto (Bixa orellana L.). Curr Sci 78:1532–1535
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murthy BNS, Saxena PK. Somatic embryogenesis and plant regeneration of Neem (Azadirachta indica A. Juss) Plant Cell Rep. 1998;17:469–475. doi: 10.1007/s002990050427. [DOI] [PubMed] [Google Scholar]
- Paiva Neto VB, Mota TR, Otoni WC. Direct organogenesis from hypocotyl-derived explants of annatto (Bixa orellana) Plant Cell Tissue Org Cult. 2002;75:159–167. doi: 10.1023/A:1025063906822. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Ravishankar GA. Mass multiplication of Bixa orellana L. through tissue culture for commercial propagation. Ind Crop Prod. 2008;28:122–127. doi: 10.1016/j.indcrop.2008.01.012. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA. Micropropagation of Bixa Orellana using phytohormones and triacontanol. Biol Plant. 2009;53:347–350. doi: 10.1007/s10535-009-0064-5. [DOI] [Google Scholar]
- Parimalan R, Giridhar P, Ravishankar GA. Enhanced shoot organogenesis in Bixa orellana L. in the presence of putrescine and silver nitrate. Plant Cell Tissue Org Cult. 2011;105:285–290. doi: 10.1007/s11240-010-9865-7. [DOI] [Google Scholar]
- Ping Luo J, Wawrosch C, Kopp B. Enhanced micropropagation of Dendrobium huoshanense C.Z. Tang et S.J. Cheng through protocorm-like bodies: the effects of cytokinins, carbohydrate sources and cold pretreatment. Sci Hort. 2009;123:258–262. doi: 10.1016/j.scienta.2009.08.008. [DOI] [Google Scholar]
- Ramamurthy N, Savithramma N, Usha R, Swamy PM. Multiple shoot induction and regeneration of Japhra (Bixa orellana L.) through axillary bud derived callus cultures. J Plant Biol. 1999;26:231–235. [Google Scholar]
- Rivera-Madrid R, Escobedo-GM RM, Balam-Galera E, Vera-Ku M, Harries H. Preliminary studies toward genetic improvement of annatto (Bixa orellana L.) Sci Hortic. 2006;106:165–172. doi: 10.1016/j.scienta.2006.03.011. [DOI] [Google Scholar]
- Ruzicka K, Simaskova M, Duclercq J, Petrasek J, Zazímalova E, Simon S, Friml J, van Montagu MCE, Benkova E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci U S A 106(11):4284–4289 [DOI] [PMC free article] [PubMed]
- Sandhya G, Mahalaxmi V. In vitro high frequency direct plant regeneration from whole leaves of blackberry. Sci Hortic. 2009;120:22–26. doi: 10.1016/j.scienta.2008.09.010. [DOI] [Google Scholar]
- Satyanarayana A, Prabhakara RPG, Rao DG. Chemistry, processing and toxicity of annatto (Bixa orellana L.) J Food Sci Technol. 2003;40:131–141. [Google Scholar]
- Sergio E, Simone B, Fernando F, Serafini LA. Clonal micropropagation of Roman chamomile. J Herbs Spices Med Plants. 2000;7:35–42. doi: 10.1300/J044v07n02_04. [DOI] [Google Scholar]
- Siril EA, Joseph N. Micropropagation of annatto (Bixa orellana L.) from mature tree and assessment of genetic fidelity of micropropagated plants with RAPD markers. Physiol Mol Biol Plants. 2013;19(1):147–155. doi: 10.1007/s12298-012-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan I, Hwang SJ, Jeong BR. Influence of plant growth regulators on axillary shoot multiplication and iron source on growth of Scrophularia takesimensis Nakai-a rare endemic medicinal plant. Afr J Biotechnol. 2008;7:4484–4490. [Google Scholar]
- Sonali J, Iyyakkannu S, Byoung RJ. Effect of cytokinins on in vitro multiplication of Sophora tonkinensis. Asian Pac J Trop Biomed. 2013;3(7):549–553. doi: 10.1016/S2221-1691(13)60111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu C. In: Annatto- the natural colour in the supplement to cultivation and utilization of medicinal plants. Hananda SS, Kaul MK, editors. New Delhi: National Institute of Science Communication; 1996. pp. 537–543. [Google Scholar]
- Srivastava A, Shukla YN, Jain SP, Kumar S. Chemistry, pharmacology and uses of Bixa orellana: A review. J Med Arom Plant Sci. 1999;21:1145–1154. [Google Scholar]
- Suhaila M, Saka S, Sharkawy SH, Ali AM, Muid S. Antimycotic screening of 58 Malaysian plants against plant pathogens. Pestic Sci. 1996;47:259–264. doi: 10.1002/(SICI)1096-9063(199607)47:3<259::AID-PS413>3.0.CO;2-N. [DOI] [Google Scholar]
- Sujatha G, Ranjitha Kumari BD. Effect of phytohromone on microprogation of Artemisia vulgaris L. Acta Physiol Plant. 2007;29:189–195. doi: 10.1007/s11738-006-0023-0. [DOI] [Google Scholar]
- Tilkat E, Onay A, Yıldırım H, Ayaz E. Direct plant regeneration from mature leaf explants of pistachio. Pistacia L Sci Hortic. 2009;121:361–365. doi: 10.1016/j.scienta.2009.02.007. [DOI] [Google Scholar]
- Wealth of India . Bixa orellana. India: CSIR; 1990. pp. 141–143. [Google Scholar]