Abstract
An efficient and reproducible in vitro propagation protocol has been established for Cadaba fruticosa (L.) Druce. Surface-sterilized nodal stem segments of mature plant were used as explants for culture establishment. Multiple shoots were optimally differentiated from the nodal stem explants through bud breaking on Murashige and Skoog (1962) medium containing 3.0 mg l−1 benzyladenine (BA). The effect of different plant growth regulators and minerals were studied on different stages of micropropagation procedure (i.e., explant establishment, shoot multiplication/growth and ex vitro rooting). Additionally, for enhancing shoot multiplication during subculture, MS medium was modified (MMS) with higher levels of magnesium, potassium and sulphate ions. Out of these, MMS3 medium containing 0.25 mg l−1 each of BA and Kin (N6-furfuryladenine), with 0.1 mg l−1 NAA (α-naphthalene acetic acid) was found the best for shoot multiplication (42.45 ± 3.82 per culture vessel). The in vitro regenerated shoots were rooted under ex vitro conditions on treating the shoot base with 500 mg l−1 of IBA (indole-3 butyric acid) for 3 min on sterile Soilrite®. The ex vitro rooted plants were hardened in the greenhouse and transferred to the field with ≈85 % survival rate. There were not any visual differences between wild and micropropagated plants in the field, although the later underwent significant changes during acclimatization. Micromorphological changes on leaf surface characters from in vitro to acclimatized plantlets were studied in terms of development of glandular trichomes, changes in vein spacing and vein structure in order to understand the nature of plant responses towards environmental conditions. The method developed and defined can be applied for commercial cultivation, which may be important for extraction of bioactive compounds and may facilitate conservation of this multipurpose endangered medicinal shrub.
Keywords: Cadaba fruticosa, Cadabicine, Endangered plant, Ex vitro rooting, Micropropagation, Modified MS medium
Introduction
Cadaba fruticosa (L.) Druce, a dense rambling, multipurpose endangered medicinal shrub, locally known as Dabi (Bhandari 1990) or Kodhab (Anonymous 2005) belongs to the family Capparaceae. This plant is endemic to Indian subcontinent (Bangladesh, India, Pakistan, Sri Lanka), Indo-China (Myanmar), and distributed throughout the tropical and sub-tropical regions of the world (Anonymous 2005; Amudha and Rani 2014). In India, it is designated as “Sthalavriksha” or temple tree (A sacred plant grown in temples for its numerous religious and medicinal values) which represents its esthetic values and helpful in its germplasm conservation (Gunasekaran and Balasubramanian 2012). C. fruticosa is known for its active constituents, i.e., cadabicine, cadabicine diacetate (Viqar Uddin et al. 1987), capparisine and α-β- dihydro ferulic acid (Aziz-Ur-Rehman 1990). This plant is known for its numerous medicinal values (Mythreyi et al. 2008; Amudha and Rani 2014; Shashikanth et al. 2014), therefore called as “Bahuguni” (In Hindi, Bahuguni means multiple uses), principally by ‘Jain Saints’ (a religious group of Indian subcontinent) who are always on move to spread their religious practices.
Despite the high medicinal and pharmacological importance, there are not any conventional methods known for propagation of C. fruticosa. Seed setting is poor due to ineffective pollination by a group of specific butterflies namely Colotis eucharis, C. danae, Anaphaeis aurota (Aluri 1990). Similarly, natural reproduction through seeds was also not observed in another species of Cadaba (C. heterotricha) due to non-viability of seeds (Abbas et al. 2010). Owing to its multipurpose importance, local people and pharmaceutical industries over-exploit the plant and sell in market as “Kodham Pulika” (ENVIS 2014). In addition, extensive habitat destruction for stone excavation is another cause by which status of this plant has been drastically affected (Abbas et al. 2010) and now, it is categorized as an endangered species by the Ministry of Environment, Sri Lanka (MOE 2012). Thus an effective strategy is required for rapid and large-scale multiplication of this potential medicinal plant. During last 2–3 decades, tissue culture methods involving micropropagation has been widely applied to propagate and conserve a number of endangered and medicinally important plant species (Rathore et al. 2005; Lodha et al. 2014a; Gashi et al. 2015). Abbas and Qaiser (2010) reported basic tissue culture protocol for conservation of one of the Cadaba species (C. heterotricha). Survey of literature reveals that, till date there is not any report of tissue culture protocol for Cadaba fruticosa. In the present communication, we report for the first time an efficient and reproducible micropropagation protocol from mature plant of C. fruticosa and the successful establishment of regenerated plants ex vitro. Moreover, micromorphological studies were also conducted to observe the changes taken place in leaf surface morphology which may be helpful in understanding the nature of plant responses towards environmental conditions.
Materials and methods
Plant material selection and decontamination
The morphologically/phenotypically healthy source plant used for the present study were selected from the Mehrangarh Fort (26°29′83″ N and 73°01′94″ E), Jodhpur, Rajasthan (India). Axillary shoots were taken periodically throughout the year to examine the effect of different seasons on culture initiation. Fresh hard nodal stem segments of 4–5 cm long were used as explant. These were decontaminated using [0.5 % (v/v)] solution of sodium hypochlorite for 10–12 min and treated with systemic fungicide 0.1 % (w/v) Bavistin (BASF India Limited, Mumbai, India) for 12–15 min followed by surface sterilization with 0.1 % (w/v) solution of HgCl2 (Hi-Media, India) for 3–4 min. Thereafter, treated explants washed thoroughly with sterilized water for 5–6 times to remove the traces of disinfectant in a laminar air hood.
Nutrient media and environmental conditions in growth chamber
Agar (Bacteriological grade, Qualigens Fine Chemicals, Mumbai, India) gelled (0.8 %) MS medium (MS; Murashige and Skoog’s 1962) with sucrose (3 %), with or without additives (50 mg l−1 ascorbic acid and 25 mg l−1 each of citric acid, adenine sulfate and arginine) were used for the present study. The pH of medium was attuned to 5.8 ± 0.02 (using 1 N KOH or HCl) before autoclaving at pressure 15 psi, temperature 121 °C for 15 min. Explants were inoculated vertically in culture tubes and placed in diffused light conditions (20–25 μ mol m−2 s−1 PFD) initially for 2–3 days, and thereafter transferred to culture room having high light intensity (40–50 μ mol m−2 s−1 PFD), provided by cool and white fluorescent tubes (Philips, India) for 16 h photoperiod per day, at 26 ± 2 °C and 55–60 % RH.
Shoot regeneration and multiplication
The initial experiment aimed to study the effect of plant growth regulators (PGRs) on shoot bud induction. MS medium containing different types (BA or Kin) and concentrations (1.0, 2.0, 3.0, 4.0 or 5.0 mg l−1) of cytokinins were tested for the shoot bud induction. MS basal medium (devoid of any PGRs) served as control.
The second experiment aimed to study the effect of the subsequent transfer of mother explant on different nutrient medium supplemented with various concentrations (0.5, 1.0, 1.5 or 2.0 mg l−1) of cytokinins (BA or Kin or 2-ip), for shoot multiplication. After 15 days of the culture establishment, mother explant along-with the regenerated shoots was transferred to these MS medium up to four passages having an interval of 4 weeks each.
The third experiment aimed to study the effect of PGRs and modified media on sub-culturing of in vitro raised shoots (after making a bunch of 4–6 shoots) on different combinations of PGRs and MS salts in medium. For this, BA or Kin alone (0.25, 0.50 or 1.0 mg l−1) or in combination with 2-iP (0.25 or 0.50 mg l−1) and NAA (0.1 mg l−1) were used. For further shoot multiplication and elongation, MS medium was modified (MMS) in terms of MgSO4.7H2O, (NH4)2SO4 and KCl having different concentrations and combinations as detailed in Table 1. The shoots cultures were regularly subcultured after an interval of 40–45 days and are being maintained for the last 3 years without any loss of vigor.
Table 1.
Various culture media used for shoot multiplication with different concentrations (mg l-1) of sulphates and potassium chloride
| MS salt | MS | MMS1 | MMS2 | MMS3 |
|---|---|---|---|---|
| MgSO4.7H2O | 370 | 540 | 540 | 540 |
| (NH4)2SO4 | – | – | 150 | 150 |
| KCl | – | – | – | 100 |
Ex vitro rooting of micropropagated shoots
In vitro raised healthy shoots from subsequent passages/subcultures were harvested individually and their bases (4–6 mm) were dipped in various types and concentrations (100, 300, 500 or 700 mg l−1) of auxins (IBA or NOA) alone and in combinations, for different time durations (1, 3, 5 or 7 min). Individual shoot devoid of any auxin treatment served as control. The auxin treated shoots and control were transplanted to bottles containing autoclaved Soilrite® (Kel Perlite, Bangalore, India) and irrigated with an aqueous sterile solution of quarter strength of MS basal salts (10–12 ml per bottle), at an interval of 4–5 days. These bottles were covered with polycarbonated caps and kept in the greenhouse near the pad section in order to maintain high RH (80–90 %) and low temperature (28 ± 2 °C) conditions.
Hardening and soil transfer
The caps of the bottles containing ex vitro rooted shoots were gradually loosened over a period of 2–3 weeks and eventually removed. Meanwhile, the bottles were also shifted from the pad section to the fan section (50–55 % RH, 36 ± 2 °C) of greenhouse for gradual hardening. These bottles (with open caps) were kept in the greenhouse for the next 4 weeks near the fan section. Successfully hardened plantlets were transplanted to the polybags (25 cm height × 10 cm diameter) containing field soil (collected from the site where the species grow naturally) and kept in the greenhouse for next 4 weeks. The hardened plants were finally transferred to the nursery.
Micromorphological studies of plantlets undergoing hardening
In another set of experiment, we compared the development of veins (vein density and pattern of venation) and glandular trichomes in leaves of in vitro growing shoots (after 4 weeks of subculture) and those shoots undergoing hardening in the greenhouse (after 6 weeks of rooting), as method developed by Rathore et al. (2013). A randomly selected plantlet was chosen from both the stages and the third to seventh leaves from the base were detached and fixed in 5 % (w/v) NaOH for 12–14 h. Thereafter, the leaves were kept in 70 % ethanol (v/v) for 24–48 h, until chlorophyll was removed and rinsed three times with distilled water. These were stained with 0.5 % (w/v) Safranine (Loba chemie, India), mounted in distilled water and examined and captured immediately under microscope (Leica DM3000, Germany). Images for this experiment were evaluated using the software Leica application suite V 3 1.0.
Experimental layout and statistical analysis
All the experiments were set up in a Randomized Block Design (RBD) for single factor experiment (Compton 1994) with a minimum of 20 replicates per treatment and the experiments were repeated three times. Observations like shoot number/length, root number/length were scored over a period of 2–5 weeks as per the experiment. The data were analyzed statistically using ANOVA (analysis of variance) and the differences among the mean values were compared with Duncan’s Multiple Range Test (DMRT) (P < 0.05) using SPSS ver. 17 (SPSS Inc., USA). All the results were expressed as mean ± SD of three experiments.
Results and disscusion
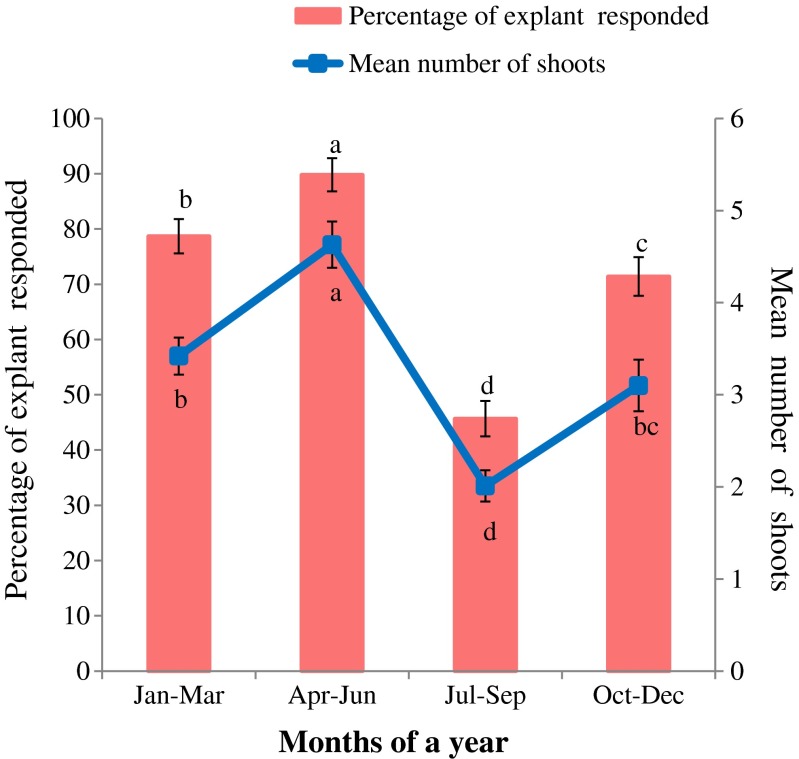
During culture initiation, extensive cleansing and decontamination of the nodal stem explants is required to establish the aseptic cultures because shoot segments bears glandular trichomes and may possess a few recalcitrant microbes between them (Rathore et al. 2013). In the present study, seasonal factors played a critical role in determining the percentage response of explants in culture (Rathore et al. 2005; Lodha et al. 2014a), as explants harvested before rainy season (i.e., April to June) in Rajasthan, India, were found most advantageous for culture initiation in terms of mean number of shoots (Fig. 1).
Fig. 1.
Effect of different seasons on establishment of cultures. Data were recorded after 2 weeks of inoculation. Data represent as mean ± SD. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Effect of growth regulators on bud breaking
On medium supplemented with BA (3.0 mg l−1), after 2 weeks each node produced 4.63 ± 0.42 of shoots with a mean length (3.45 ± 0.29 cm) (Fig. 2a; Table 2). It was observed that BA (2.0 mg l−1) was sub-optimal and BA (4.0 and 5.0 mg l−1) was inhibitory for shoot bud induction. However, on PGR-free MS basal medium nodal stem explants failed to induce axillary buds, therefore, it was mandatory to augment the culture medium with cytokinins to induce multiple shoot buds. It is well known that cytokinins stimulate plant cell divisions, participate in the DNA synthesis and control the cell cycle, which might be the reason for induction of adventitious buds (Gaspar et al. 2003). In the present study, BA is found superior over Kin in terms of percentage response and this may be attributed to its less resistance to the cytokinin oxidase; easy permeability, metabolism and induction of natural hormones (like zeatin) within the tissue (Lodha et al. 2014a). Similar results have also been reported in Capparaceous species like Capparis decidua (Deora and Shekhawat 1995), Maerua oblongifolia (Rathore et al. 2005) and Cleome gynandra (Rathore et al. 2013). In the present study, medium containing additives found better for shoot bud induction, shoot growth and further shoot multiplication than medium lacking additives (data not presented). In additives, ascorbic acid and citric acid act as a source of antioxidants; whereas L-arginine and adenine sulfate are known for its nitrogen reducing and additional cytokinin like properties (Patel et al. 2014; Zhang et al. 2015). In this study, it was focytokininund that initial incubation of explants under diffuse light for 2–3 days promoted activation of axillary meristem and thus bud breaking. For further growth, inoculated explants were transferred to culture room having high light intensity, as suggested by Lodha et al. (2014a).
Fig. 2.

In vitro propagation and ex vitro rooting for Cadaba fruticosa. a Bud breaking from nodal stem explants on MS medium having BA (3.0 mg l−1), after 2 weeks (Scale Bar 1.0 cm). b Re-culture of original explant on MS medium having BA (1.5 mg l−1), after 2 weeks (Scale Bar 2.0 cm). c & d Multiplication and maintenance of shoots on MMS3 medium supplemented with BA (0.25 mg l−1), Kin (0.25 mg l−1) and NAA (0.1 mg l−1), after 4th week of culture (Scale Bar 1.0 cm). e Ex vitro rooted shoots pulse-treated with IBA (500 mg l−1) for 3 min, after 3 weeks (Scale Bar 2.0 cm). f Acclimatization of rooted plantlets in the greenhouse conditions (Scale Bar 0.5 cm). g Successfully hardened plants of Cadaba fruticosa (Scale Bar 2.0 cm)
Table 2.
Effect of cytokinins (BA or Kin) on shoot bud sprouting from nodal stem explants of Cadaba fruticosa
| Cytokinins (mg l-1) | % explants responded | Shoot number (mean ± SD) | Shoot length (cm) (mean ± SD) | |
|---|---|---|---|---|
| BA | Kin | |||
| 0.0 | 0.0 | 0.0h | 0.0 ± 0.00g | 0.00 ± 0.00g |
| 1.0 | – | 39.5f | 1.71 ± 0.31ef | 0.84 ± 0.07f |
| 2.0 | – | 68.2c | 2.90 ± 0.48c | 1.87 ± 0.20d |
| 3.0 | – | 89.8a | 4.63 ± 0.42a | 3.45 ± 0.29a |
| 4.0 | – | 74.6b | 3.51 ± 0.51b | 2.70 ± 0.41b |
| 5.0 | – | 65.8c | 2.62 ± 0.45cd | 2.04 ± 0.22cd |
| – | 1.0 | 30.0g | 1.33 ± 0.33f | 0.75 ± 0.23f |
| – | 2.0 | 42.3ef | 1.90 ± 0.38e | 1.25 ± 0.23e |
| – | 3.0 | 58.2d | 2.91 ± 0.43c | 2.39 ± 0.14c |
| – | 4.0 | 48.1e | 2.35 ± 0.46d | 1.79 ± 0.24d |
| – | 5.0 | 39.3f | 2.00 ± 0.49e | 1.34 ± 0.18e |
Medium: MS + additives (50 mg l−1 ascorbic acid and 25 mg l−1 each of citric acid, adenine sulfate and arginine)
Data were recorded after 2 weeks of inoculation
Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Multiplication and maintenance of cultures
Effect of repeated transfer on shoot multiplication
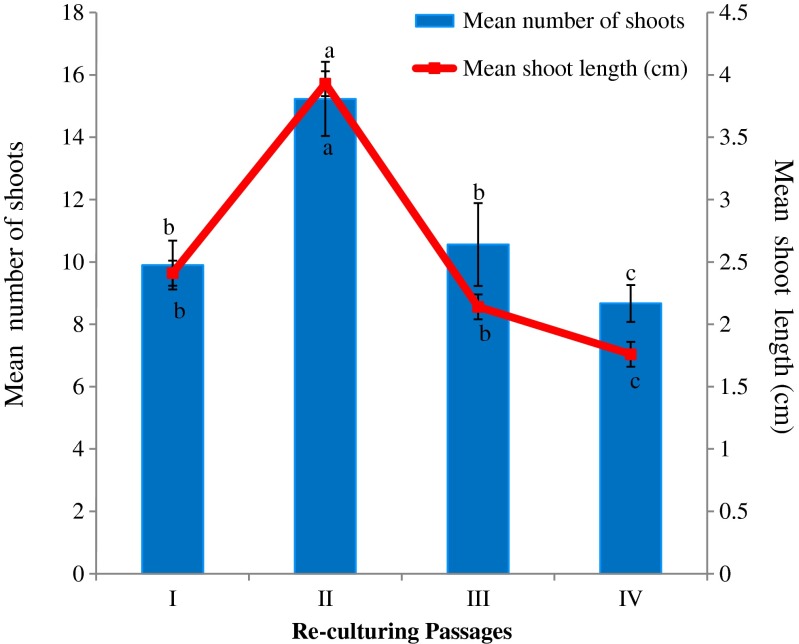
Shoot regeneration was influenced by re-culturing of initial/mother explants on fresh MS medium supplemented with various types and concentrations of cytokinins. Of these, MS medium containing BA (1.5 mg l−1) was most favorable and produced the optimum number of shoots (15.23 ± 1.19 per explant) after 2nd passage (Fig. 2b; Table 3). During 3rd and 4th passage, however, lesser number of shoots was formed with continuous decrement (Fig. 3). Re-culturing of original explant to fresh nutrient medium is an attractive way to produce new shoots in short time by rejuvenating and reinvigorating the basal dormant meristematic cells (Sánchez et al. 1997). This practice was successfully employed in a number of arid plant species (Rathore et al. 2005; Lodha et al. 2014a; Patel et al. 2014).
Table 3.
Effect of different cytokinins on shoot multiplication during re-culturing of in vitro raised shoots of Cadaba fruticosa, after 2nd passage
| Cytokinins (mg l-1) | Shoot number (mean ± SD) | Shoot length (cm) (mean ± SD) | ||
|---|---|---|---|---|
| BA | Kin | 2-iP | ||
| 0.0 | 0.0 | 0.0 | 0.00 ± 0.00i | 0.00 ± 0.00g |
| 0.5 | – | – | 5.76 ± 0.73e | 1.71 ± 0.34d |
| 1.0 | – | – | 9.90 ± 0.96b | 2.41 ± 0.42bc |
| 1.5 | – | – | 15.23 ± 1.19a | 3.93 ± 0.62a |
| 2.0 | – | – | 8.25 ± 0.82c | 2.75 ± 0.49b |
| – | 0.5 | – | 3.37 ± 0.98h | 1.16 ± 0.57f |
| – | 1.0 | – | 6.78 ± 0.87de | 1.37 ± 0.49e |
| – | 1.5 | – | 7.18 ± 0.77d | 2.10 ± 0.53c |
| – | 2.0 | – | 5.02 ± 0.99f | 1.64 ± 0.43d |
| – | – | 0.5 | 3.24 ± 0.97h | 1.17 ± 0.33f |
| – | – | 1.0 | 4.73 ± 0.93g | 1.38 ± 0.46e |
| – | – | 1.5 | 5.49 ± 0.84ef | 1.49 ± 0.41e |
| – | – | 2.0 | 5.84 ± 0.87e | 1.69 ± 0.34d |
Medium: MS + additives (50 mg l−1 ascorbic acid and 25 mg l−1 each of citric acid, adenine sulfate and arginine)
Data were recorded after 4 weeks of culture
Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Fig. 3.
Effect of re-culturing passages of original explant on shoot numbers and length. Medium: MS +1.5 mg l−1 BA. Data were recorded after 4 weeks of re-culture. Data represent as mean ± SD. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Effect of PGRs and modified media on shoot multiplication
Shoot regeneration was further increased by sub culturing of in vitro raised shoots (a clump of 4–6 shoots) on MS medium supplemented with different concentrations and combinations of PGRs. Out of various combination tested, MS medium having a combination of BA and Kin (0.25 mg l−1 each) and NAA (0.1 mg l−1) proved the optimum for shoot proliferation (≈28–30 shoots / culture vessel; Data not shown). Incorporation of auxin at a lower concentration (NAA 0.1 mg l−1) with cytokinins in subculturing medium showed synergetic effects on shoot multiplication, as a number of shoot buds were generated at the base of shoot clump. The probable attribute for this synergism is cross-talk mechanism of auxin and cytokinins which induce new bud primordia, subsequent bud formation and shoot regeneration in cultures (Lodha et al. 2014b; Zhang et al. 2015). This type of synergism has been well acknowledged in a number of plant species Gashi et al. 2015; Zhang et al. 2015 including C. heterotricha (Abbas and Qaiser 2010).
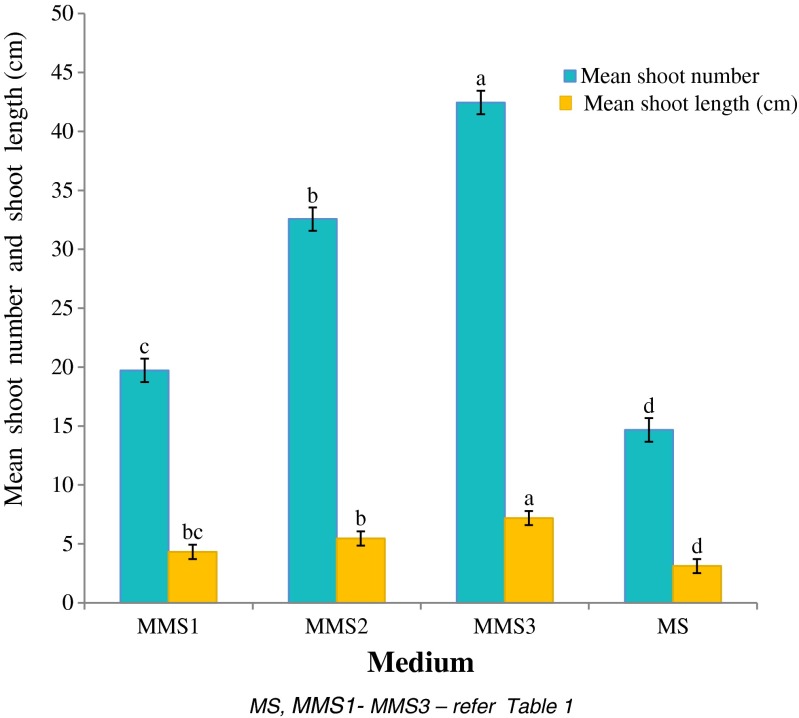
Once PGRs were optimized, cultures were transferred to MS modified media (MMS1 to MMS3) to study the effect of different salts concentration on further shoot multiplication. In the present study, MMS3 medium was found better than MS medium for shoot multiplication/growth (Fig. 4). On MMS3 medium (BA & Kin 0.25 mg l−1 each, NAA 0.1 mg l−1), a maximum number of shoots (42.45 ± 3.82 per culture vessel) of 7.19 ± 0.57 cm length were produced, after 4 weeks (Fig. 2c; Table 4). The promotive effect of MMS3 medium on shoot multiplication is probably due to high concentration of MgSO4.7H2O, (NH4)2SO4 and KCl. In salts, magnesium is an essential component of the chlorophyll molecule and also required for the activity of many enzymes. According to Mendonça et al. (2015), the additional Mg may reduce leaf chlorosis and abscission in plant tissue cultures. Similarly, potassium is required for protein synthesis and to maintain turgor pressure of the cell. Hence Mg and K play a predominant role in plant growth during shoot development (Ramage and Williams 2002; Hermans et al. 2004; Wada et al. 2013). Further, ammonium sulphate is suggested to promote the nitrate absorption and buffers the nutrient medium, thus improve the overall growth/health of cultures (Lodha et al. 2014a). Preece (1995) found interactions between PGRs and mineral nutrients in plant tissue culture medium, as well as effects of nutrient formulations on morphogenesis. Adjustment of mineral elements in the culture medium for micropropagation was found beneficial in “three ‘Vriesea bromeliads’ (Aranda-Peres and Martinelli 2009) and Picconia azorica (Mendonça et al. 2015). Similar results were also observed in hazelnuts (Nas and Read 2004), Dalbergia sissoo (Vibha et al. 2014) and Pyrus species (Wada et al. 2013). The cultures were maintained (Fig. 2d) by regular subculturing at an interval of 40–45 days.
Fig. 4.
Effect of modified medium compositions on shoot multiplication of Cadaba fruticosa. Medium: 0.8 % agar-gelled + 0.25 mg l−1 each of BA and Kin and 0.1 mg l−1 NAA. Data were recorded after 4 weeks of culture. Data represent as mean ± SD. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Table 4.
Effect of cytokinins-auxin synergisms on enhancement of shoot proliferation in Cadaba fruticosa
| Cytokinins (mg l-1) | Auxin (mg l-1) NAA | Shoot number (mean ± SD) | Shoot length (cm) (mean ± SD) | ||
|---|---|---|---|---|---|
| BA | Kin | 2-iP | |||
| 0.0 | 0.0 | 0.0 | 0.0 | 00.0 ± 0.00i | 0.00 ± 0.00g |
| 0.25 | – | – | 0.1 | 14.76 ± 1.71e | 4.12 ± 0.71d |
| 0.50 | – | – | 0.1 | 12.72 ± 1.78f | 3.91 ± 0.67de |
| 1.00 | – | – | 0.1 | 10.55 ± 0.93g | 2.97 ± 0.78f |
| – | 0.25 | – | 0.1 | 12.43 ± 0.92f | 3.52 ± 0.77e |
| – | 0.50 | – | 0.1 | 9.33 ± 0.86gh | 3.49 ± 0.84e |
| – | 1.00 | – | 0.1 | 8.27 ± 0.78h | 2.77 ± 0.89f |
| 0.25 | 0.25 | – | 0.1 | 42.45 ± 3.82a | 7.19 ± 0.57a |
| 0.50 | 0.50 | – | 0.1 | 34.24 ± 2.89b | 5.89 ± 0.54b |
| 0.25 | 0.25 | 0.25 | 0.1 | 29.43 ± 2.91c | 4.87 ± 0.64c |
| 0.50 | 0.50 | 0.50 | 0.1 | 21.75 ± 0.97d | 4.24 ± 0.68cd |
Medium: MMS3 + additives (50 mg l−1 ascorbic acid and 25 mg l−1 each of citric acid, adenine sulfate and arginine)
Data were recorded after 4 weeks of subculture
Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Ex vitro rooting of micropropagated shoots
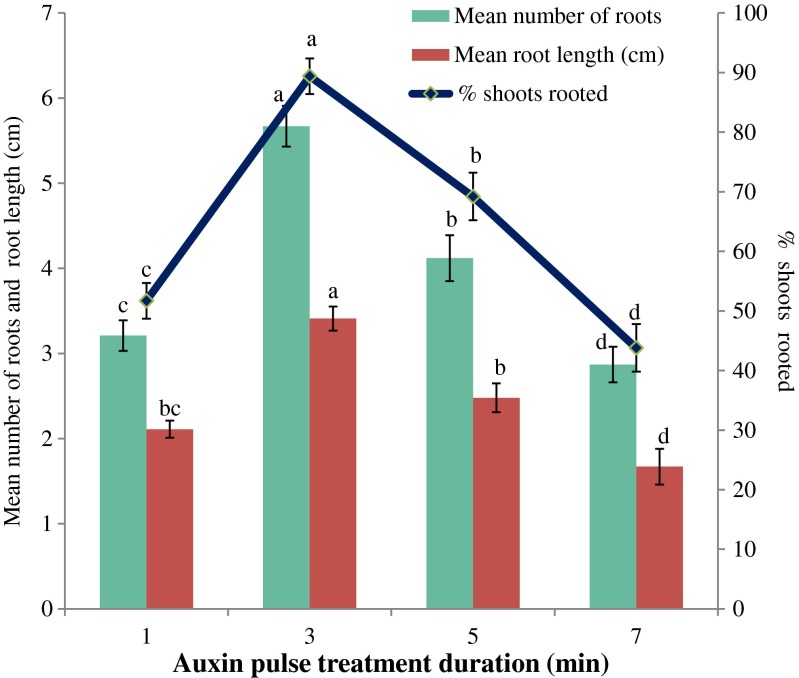
Of the type and concentration of auxins experienced, shoot bases treated with IBA (500 mg l−1) for 3 min was found the best (Fig. 5) and produced the optimum number of roots (5.67 ± 0.53 per shoot) with a mean length (3.41 ± 0.42 cm), after 3 weeks (Fig. 2e; Table 5). During ex vitro rooting, IBA alone was found better in terms of percentage rooting response in comparison to NOA and NOA + IBA combinations. The effectiveness of IBA in ex vitro rooting may be due to its steadiness greater than other auxins, easier uptake and transport, and successive gene activation (Ludwig-Muller 2000; Vengadesan and Pijut 2009). Similar findings of IBA in rooting has also been observed in Dalbergia sissoo (Vibha et al. 2014) and Cleome gyanandra (Rathore et al. 2013).
Fig. 5.
Effect of IBA (500 mg l−1) pulse treatment durations on ex vitro rooting of Cadaba fruticosa. Data were recorded after 3 weeks of rooting. Data represent as mean ± SD. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Table 5.
Effect of IBA and NOA pulse treatment on ex vitro root induction from shoots of Cadaba fruticosa
| Auxins (mg l-1) | % shoots rooted | Number of roots (mean ± SD) | Root length (cm) (mean ± SD) | |
|---|---|---|---|---|
| IBA | NOA | |||
| 0.0 | 0.0 | 0.0i | 0.00 ± 0.00i | 0.00 ± 0.00i |
| 100 | – | 61.4e | 2.41 ± 0.69d | 1.29 ± 0.50e |
| 250 | – | 73.7d | 3.45 ± 0.73c | 2.52 ± 0.63b |
| 500 | – | 89.4a | 5.67 ± 0.53a | 3.41 ± 0.42a |
| 700 | – | 76.2c | 3.93 ± 0.75bc | 2.00 ± 0.57c |
| – | 100 | 24.6h | 0.27 ± 0.79h | 0.24 ± 0.26h |
| – | 250 | 39.5g | 0.97 ± 0.68fg | 0.57 ± 0.15g |
| – | 500 | 56.4f | 2.12 ± 0.67de | 1.23 ± 0.76e |
| – | 700 | 42.7g | 1.37 ± 0.84f | 0.93 ± 0.39f |
| 100 | 100 | 62.2e | 2.39 ± 0.74d | 1.53 ± 0.82cd |
| 250 | 250 | 81.5b | 4.39 ± 0.93b | 2.33 ± 0.79bc |
| 500 | 250 | 71.9d | 3.39 ± 0.79c | 1.83 ± 0.84c |
Auxin pulse treatment duration: 3 min
Data were recorded after 3 weeks of auxin treatment
Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Rooting under ex vitro conditions is an attractive technique because it does not need any additional acclimatization prior to the field transfer (Pospóšilová et al. 1999; Osório et al. 2013; Lodha et al. 2014b). It also requires lesser time and resources; and it is estimated that ex vitro rooting can lower the cost of a micropropagation protocol up to 70–75 % (Lodha et al. 2014a; Patel et al. 2014). Moreover, ex vitro rooted plantlets have more vigor to tolerate environmental pressures during the hardening procedure (Vengadesan and Pijut 2009; Vibha et al. 2014).
Hardening and soil transfer
The temperature and RH gradient in greenhouse coupled with gradual opening of polycarbonated caps (of bottles) assisted in hardening of the plantlets (Fig. 2f). Initially the hardened plants were transplanted to black polybags (Fig. 2g) and kept in greenhouse for next 4 week. Thereafter, the hardened plants were transferred to the nursery. The eventual success of a micropropagation protocol depends on the ability to transfer the plants from culture medium to pots, and then to the field with a significant survival rate. In the present study, micropropagated C. fruticosa survived in the field with ≈85 % survival rate and did not show any visual detectable variation in morphological and growth characteristics when compared with their respective donor plants, although micropropagated plants underwent significant changes during acclimatization.
Micromorphological studies of micropropagated plants
In another set of experiment, we studied the micromorphological changes from in vitro to acclimatized conditions. Under in vitro conditions, the trichomes were less frequent and underdeveloped (Fig. 6a) which became fully developed in hardened plants (Fig. 6b). Besides, the leaves under in vitro condition have lesser developed and fewer veins / vein islet (Fig. 6c and e) in comparison to plants under the hardened phase (Fig. 6d and f). Earlier, Rathore et al. (2013) also reported the changes in venation patterns in Cleome gynandra. Our study supports the fact that, under in vitro condition plants encountered special conditions of humidity and light which resulted in altered morphology, physiology and anatomy (Preece 2010). After ex vitro transfer, these plantlets might easily be impaired by sudden changes in environmental conditions, and so need a period of acclimatization to correct the abnormalities (Pospóšilová et al. 1999; Osório et al. 2013). The discussed vein clearing experiment shows that how the vein density increases during the hardening period and this reflects the gradual acclimatization process, which triggered changes necessary for survival and the maintenance of normal growth under field conditions.
Fig. 6.
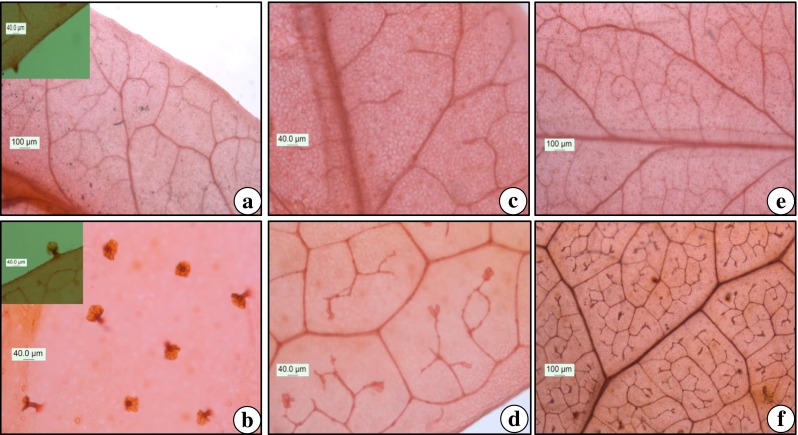
Micromorphological studies of Cadaba fruticosa. a Glandular trichomes in leaves of in vitro raised shoot and b hardened plant. c & e Venation pattern in leaves of in vitro raised shoot and d & f hardened plant. (Scale Bar 40.0 μm, 100.0 μm)
Conclusion
In conclusion, we report here for the first time an efficient in vitro propagation system for C. fruticosa-a plant of high medicinal and industrial importance. The high rate of shoot multiplication achieved in this protocol is due to selection of an appropriate medium (MMS3) and an optimum combination of PGRs. Micropropagated shoots were rooted by ex vitro technique which made this protocol cost effective, less-labor intensive/easy to use, and significantly contributes for its transplant survival rate. In addition, results of micromorphological investigation could help in understanding the nature of plant responses towards environmental conditions. Therefore, the discussed protocol could be of great value for commercial propagation, conservation and may facilitate extraction of bioactive compounds from this multipurpose and endangered medicinal plant.
Acknowledgments
DL gratefully acknowledges the financial support from Council of Scientific and Industrial Research (CSIR), New Delhi in the form of Junior and Senior Research Fellowship (JRF-SRF). AKP is thankful to the University Grant Commission (UGC), New Delhi, for providing Special Assistance Program (SAP) in the form of Centre for Advanced Study (CAS) to the Department of Botany, Jai Narain Vyas University, Jodhpur (INDIA).
Author’s contribution
D. Lodha designed and performed the experiments and wrote the first draft of the manuscript. A. K. Patel helped in data analysis and organized it in figures and tables. N. S. Shekhawat guided the research and edited the final version of the manuscript.
Abbreviations
- BA
6-benzyladenine
- IBA
Indole-3-butyric acid
- 2-iP
N6–(2-isopentenyl) adenine
- Kin
Kinetin (N6-furfuryladenine)
- MS
Murashige and Skoog (1962) medium
- MMS
Modified MS medium
- NAA
α-naphthalene acetic acid
- NaOH
Sodium hydroxide
- NOA
Naphthoxyacetic acid
- PFD
Photon Flux Density
- PGRs
Plant Growth Regulators
- RH
Relative Humidity
Contributor Information
Deepika Lodha, Phone: +91-7597319493, Email: deeplodha113@gmail.com.
N. S. Shekhawat, Email: biotechunit@gmail.com
References
- Abbas H, Qaiser M. In vitro conservation of Cadaba heterotricha stocks, an endangered species in Pakistan. Pak J Bot. 2010;42:1553–1559. [Google Scholar]
- Abbas H, Qaiser M, Alam J. Conservation status of Cadaba heterotricha Stocks (Capparaceae): an endangered species in Pakistan. Pak J Bot. 2010;42:35–46. [Google Scholar]
- Aluri RJS. Studies on pollination in India: a review. Proc Natl Acad Sci India. 1990;56:375–388. [Google Scholar]
- Amudha M, Rani S. Assessing the bioactive constituents of Cadaba fruticosa (L.) Druce through GC-MS. Int J Pharm Pharm Sci. 2014;6:383–385. [Google Scholar]
- Anonymous (2005) The wealth of India- publication and information directorate, Council of Scientific and Industrial Research, Volume-I A, New Delhi
- Aranda-Peres AN, Martinelli AP. Adjustment of mineral elements in the culture medium for the micropropagation of three Vriesea bromeliads from the Brazilian Atlantic Forest: the importance of calcium. Hortic Sci. 2009;44:106–112. [Google Scholar]
- Aziz-Ur-Rehman (1990) Studies in chemical constituents of Cadaba fruticosa. Pakistan Research Repository, PhD thesis, University of Karachi, Karachi
- Bhandari MM (1990) Flora of the Indian Desert. In: MPS Reports, Jodhpur, India
- Compton ME. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult. 1994;37:217–242. [Google Scholar]
- Deora NS, Shekhawat NS. Micropropagation of Capparis decidua (Forsk.) Edgew.-a tree of arid horticulture. Plant Cell Rep. 1995;15:278–281. doi: 10.1007/BF00193736. [DOI] [PubMed] [Google Scholar]
- ENVIS (2014) The Environmental Information System, Ministry of Environment & Forests, India (http://envis.frlht.org/raw-drug.php?show=235; accessed on 13th April 2015)
- Gashi B, Abdullai K, Sota V, Kongjika E. Micropropagation and in vitro conservation of the rare and threatened plants Ramonda serbica and Ramonda nathaliae. Physiol Mol Biol Plant. 2015;21:123–136. doi: 10.1007/s12298-014-0261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar TH, Kevers C, Faivre-Rampant O, Crevecoeur M, Penel C, Greppin H, Dommes J. Changing concepts in plant hormone action. In Vitro Cell Dev Biol Plant. 2003;39:85–106. doi: 10.1079/IVP2002393. [DOI] [Google Scholar]
- Gunasekaran M, Balasubramanian P. Ethnomedicinal uses of Sthalavrikshas (temple trees) in Tamil Nadu, Southern India. Ethnobot Res Appl. 2012;10:253–268. [Google Scholar]
- Hermans C, Johnson GN, Strasser RJ, Verbruggen N. Physiological characterisation of magnesium deficiency in sugar beet: acclimation to low magnesium differentially affects photosystems I and II. Planta. 2004;220:344–355. doi: 10.1007/s00425-004-1340-4. [DOI] [PubMed] [Google Scholar]
- Lodha D, Patel AK, Rai MK, Shekhawat NS. In vitro plantlet regeneration and assessment of alkaloid contents from callus cultures of Ephedra foliata (Unth phog), a source of anti-asthmatic drugs. Acta Physiol Plant. 2014;36:3071–3079. doi: 10.1007/s11738-014-1677-7. [DOI] [Google Scholar]
- Lodha D, Rathore N, Kataria V, Shekhawat NS. In vitro propagation of female Ephedra foliata Boiss. & Kotschy ex Boiss.: an endemic and threatened Gymnosperm of the Thar Desert. Physiol Mol Biol Plant. 2014;20:375–383. doi: 10.1007/s12298-014-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Muller J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000;32:219–230. doi: 10.1023/A:1010746806891. [DOI] [Google Scholar]
- Mendonça D, Luna S, Bettencourt S, Lopes MS, Monteiro L, Neves JD, Monjardino P, Machado AC. In vitro propagation of Picconia azorica (Tutin) Knobl. (Oleaceae) an Azorean endangered endemic plant species. Acta Physiol Plant. 2015;37:47. doi: 10.1007/s11738-015-1797-8. [DOI] [Google Scholar]
- MOE . The national red list 2012 of Sri Lanka; conservation status of the fauna and flora. Colombo: Ministry of Environment; 2012. [Google Scholar]
- Murashige T, Skoog FA. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Mythreyi R, Sasikala E, Geetha A, Madhavan V. Antipyretic activity of leaves of Cadaba fruticosa (L.) Druce. Pharmacol Online. 2008;3:136–142. [Google Scholar]
- Nas MN, Read PE. A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic. 2004;101:189–200. doi: 10.1016/j.scienta.2003.10.004. [DOI] [Google Scholar]
- Osório ML, Gonçalves S, Coelho N, Osório J, Romano A. Morphological, physiological and oxidative stress markers during acclimatization and field transfer of micropropagated Tuberaria major plants. Plant Cell Tissue Organ Cult. 2013;115:85–97. doi: 10.1007/s11240-013-0343-x. [DOI] [Google Scholar]
- Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS. In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: an endemic and endangered edible plant species of the Thar Desert. Sci Hortic. 2014;165:175–180. doi: 10.1016/j.scienta.2013.10.039. [DOI] [Google Scholar]
- Pospóšilová J, Tichá I, Kadleček P, Haisel D, Plzáková Š. Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant. 1999;42:481–497. doi: 10.1023/A:1002688208758. [DOI] [Google Scholar]
- Preece J. Can nutrient salts partially substitute for plant growth regulators? Plant Tissue Cult Biotechnol. 1995;1:26–37. [Google Scholar]
- Preece JE (2010) Acclimatization of plantlets from in vitro to the ambient environment. Encycl Ind Biotechnol 1–9
- Ramage CM, Williams RR. Mineral nutrition and plant morphogenesis. In Vitro Cell Dev Biol Plant. 2002;38:116–124. doi: 10.1079/IVP2001269. [DOI] [Google Scholar]
- Rathore JS, Rathore MS, Shekhawat NS. Micropropagation of Maerua oblongifolia- a liana of arid areas. Phytomorphology. 2005;55:241–247. [Google Scholar]
- Rathore NS, Rathore N, Shekhawat NS. In vitro propagation and micromorphological studies of Cleome gynandra: a C4 model plant closely related to Arabidopsis thaliana. Acta Physiol Plant. 2013;35:2691–2698. doi: 10.1007/s11738-013-1301-2. [DOI] [Google Scholar]
- Sánchez MC, San-José MC, Ferro E, Ballester A, Vieitez AM. Improving micropropagation conditions for adult-phase shoots of chestnut. J Hortic Sci. 1997;72:433–443. [Google Scholar]
- Shashikanth J, Mohan CH, Reddy PR. A potent folklore medicinal plant: Cadaba fruticosa (L.) Druce. Res Rev J Bot Sci. 2014;3:30–33. [Google Scholar]
- Vengadesan G, Pijut PM. In vitro propagation of northern red oak (Quercus rubra L.) In Vitro Cell Dev Biol Plant. 2009;45:474–482. doi: 10.1007/s11627-008-9182-6. [DOI] [Google Scholar]
- Vibha JB, Shekhawat NS, Mehandru P, Dinesh R. Rapid multiplication of Dalbergia sissoo Roxb.: a timber yielding tree legume through axillary shoot proliferation and ex vitro rooting. Physiol Mol Biol Plant. 2014;20:81–87. doi: 10.1007/s12298-013-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viqar Uddin A, Kaniz F, Azia-ur-Rahman A, Shoib A. Cadabacine and cadabacine diacetate from Crataeva nurvala and Cadaba farinosa. J Nat Prod. 1987;50:1186. [Google Scholar]
- Wada S, Niedz RP, DeNoma J, Reed BM. Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cell Dev Biol Plant. 2013;49:356–365. doi: 10.1007/s11627-013-9508-x. [DOI] [Google Scholar]
- Zhang A, Wang H, Shao Q, Xu M, Zhang W, Li M (2015) Large scale in vitro propagation of Anoectochilus roxburghii for commercial application: pharmaceutically important and ornamental plant. Ind Crop Prod 70:158–162