Abstract
Chickpea establishes symbiotic association with Mesorhizobium to fulfill its nitrogen (N) requirement. Integrating chickpea rhizosphere with potential native mesorhizobia and other plant growth promoting microorganisms can contribute multiple benefits to plants. The present investigation was undertaken to study interactions among Piriformospora indica (PI) with potential plant growth promoting rhizobacteria (PGPR) viz. Pseudomonas argentinensis (LPGPR1), Pseudomonas sp. (LPGPR2) along with national check Pseudomons sp. (LK884) and Mesorhizobium cicer (LGR33, MR) to examine the synergistic effect of consortium for improving growth, symbiotic efficiency, nutrient acquisition and yield in two chickpea (Cicer arietinum L.) varieties viz. desi PBG1 and kabuli BG1053. In-vitro, seed germination with consortium MR + PI + LPGPR1 was the best compatible treatment followed by MR + PI + LK884 and MR + PI + LPGPR2. Significant improvement in the growth, symbiotic parameters and grain yield was observed with MR + PI + LPGPR1 and MR + PI + LK884 treatments. Significantly high chlorophyll and leghaemoglobin content was recorded with MR + PI + LPGPR1 (1.57 and 1.64 mg g−1 fresh weight of leaves and 5.19 and 4.39 mg/g−1 fresh weight of nodules) in desi PBG1 and kabuli BG1053 chickpea varieties, respectively. At 90 DAS, MR + PI + LPGPR1 treatment significantly improved nodule dry weight (ranged between 84.0 and 141.7 mg plant−1) as compared to MR alone treatment (ranged between 62.3 and 123.3 mg plant−1). Data revealed significant increase in total nitrogen (N) and phosphorus (P) content of shoot with MR + PI + LPGPR1 by 1.2 and 1.5 fold, respectively over MR alone treatment. On the basis of overall mean, MR + PI + LPGPR1 significantly improved the yield by 8.2 % over Mesorhizobium alone application. It seems from foregoing study that tripartite combination of different micro-organisms can be explored as biofertilizer for improvement in chickpea productivity.
Keywords: Chickpea, Mesorhizobium cicer, Piriformospora indica, Pseudomonas spp. and Synergism
Introduction
Engineering the rhizosphere with native and non-native agriculturally beneficial microorganisms can overcome the use of chemical fertilizers in crop production in sustainable manner (Antoun and Prevost 2006). Chickpea is an important drought-tolerant food legume crop. India is the world leader in chickpea production followed by Pakistan and Turkey. It was grown in an area of 9.51 million hectares with production of 8.83 million tonnes and average yield of 929 kg/ha during rabi 2012–13 (Singh and Nadarajan 2013). Chickpea fixes about 141 Kg/ha/year molecular nitrogen (N) by establishing symbiotic association with bacterium Mesorhizobium of family Rhizobiaceae (Singh 2007). Legume yield and N accumulation are directly related to the magnitude and efficiency of symbiotic N2 fixation (SNF) in root nodules. Mesorhizobium strains vary naturally in their N2fixing capacity and adaptation to prevailing environmental stresses (Maatallah et al. 2002). Further, inoculation with selected Mesohizobium on chickpea having beneficial effect on yield is well documented in literature (L’taief et al. 2007; Rhomdhane et al. 2009; Jida and Aseefa 2012). Engineering the rhizosphere with other plant growth promoting micro-organisms can be applied as technological advancement in increasing survival and establishment of inoculated Mesorhizobium sp. in soil environment for improving SNF. Plant growth promoting rhizobacteria can positively influence plant growth by direct mechanisms viz. solubilization of nutrients, nitrogen fixation, production of growth regulators, etc. or by indirect mechanisms viz. stimulation of mycorrhizae, exclusion of pathogens or removal of toxic substances (Bashan and de-Bashan 2010). Piriformospora indica, a novel endophyte of Sebacinaceae family is an axenically cultivable fungus (Varma et al. 2001) which mimics the capabilities of typical Arbuscular-Mycorrhizal fungi (AMF). P.indica colonizes roots of wide variety of plant species and promotes their growth in a manner similar to mycorrhizal fungi (Shahollari et al. 2005). It mobilizes the insoluble phosphates and translocates phosphorus to the host in an energy dependent process with active involvement of acid phosphatases (Varma et al. 1999). P.indica colonization also has reminiscent effect on the buildup of microbial community structure in the rhizosphere. It enhances the seed production and stimulates active ingredients in plants allowing them to grow under adverse conditions (Bagde et al. 2010). Co-inoculation of P.indica with PGPR (Pseudomonas striata) had synergistic effect on its population build up and plant dry biomass with respect to single inoculation in chickpea (Meena et al. 2010). There had been reports in soybean where co-inoculation of AMF and Rhizobium increased N content, dry weight of plant and grain yield (Bhagyaraj and Verma 1995). Very limited information exists on interactions among P.indica, Pseudomonas sp. and Mesorhizobium cicer in chickpea rhizosphere. Multipartite interactions of diazotrophs, PGPR and AM or AM like fungi with host plants might synergistically improve the SNF and plant productivity in chickpea. The study was therefore planned with the objective to elucidate tripartite interactions of P.indica, PGPR and Mesorhizobium cicer on compatibility alongwith growth, nutrient acquisition, SNF and yield in chickpea.
Materials and methods
Chemicals and instruments
All the chemicals used in the present study were of analytical grade, obtained from M/S SRL, E–merk, CDH and Qualigens, Hi- media, India. Electronic balance (Axis), orbital incubator shaker (Mac), UV-Visible spectrophotometer (Techcomp), digital pH meter (Elico), cold centrifuge (Remi), magnetic strirrer (SESW), autoclave (NSW India), laminar flow (NSW India) and distillation (Mac) were used.
Procurement of micro-organisms
Recommended Mesorhizobium cicer (LGR-33), native strains of PGPR :-Pseudomonas argentinensis (LPGPR1) and Pseudomonas sp. (LPGPR2) were procured from Pulse Microbiology laboratory, Department of Plant Breeding and Genetics, Punjab Agricultural University (PAU), Ludhiana and reference strain of PGPR Pseudomons sp.(LK884) was procured from GB Pant University of Agricultural and Technology, Uttarakhand. Mesorhizobium and Pseudomonas spp. were sub-cultured on YEMA and King’s B media, respectively. Fungus Piriformospora indica was procured from Dr. S. S. Dudeja, Department of Microbiology, Chaudhary Charan Singh Haryana Agricultural University (CCSHAU), Hisar under AICRP-Chickpea Project and was sub-cultured on Potato dextrose agar (PDA) medium. All the cultures were stored at 4 °C for further use.
Procurement of seeds of chickpea
Seeds of chickpea desi PBG1and kabuli BG1053 varieties were procured from the Pulses Section, Department of Plant Breeding and Genetics, PAU, Ludhiana.
Assessment of compatibility of the tripartite combination for seed germination in chickpea
Prior to the field experiment compatibility of the tripartite mixture of Mesorhizobium cicer (LGR33, MR), Piriformospora indica (PI) and different Pseudomonas spp. was examined by seedling assay at Pulse Microbiology Laboratory, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana. A total of 6 microbial inoculant treatments were maintained viz. Mesorhizobium cicer (LGR33) (MR); P.indica (PI); MR + PI + LPGPR1; MR + PI + LPGPR2 and MR + PI + LK884 and un-inoculated Control. Healthy seeds of desi PBG1 and kabuli BG1053 chickpea varieties were selected, washed with running tap water and sterilized with 70 % alcohol for 1 min followed by 0.1 % HgCl2 for 3 min and rinsed thoroughly with six changes of sterilized water (Vincent 1970). Surface sterilized seeds were imbibed for 25 min in suspension of each of the above treatments. In co-inoculation treatments, Mesorhizobium cicer, P.indica and different PGPR strains were applied to chickpea seeds in ratio of 1:1:1. Treated seeds were allowed to germinate in Petridish containing 0.7 % water agar and three replications were maintained for each treatment. The plates were incubated at 28 °C for 9 days in the dark.
Evaluation of tripartite interactions among P.indica with PGPR and Mesorhizobium for improved growth, symbiotic efficiency, nutrient acquisition and yield in chickpea under field conditions
Field experiment was conducted in Rabi season (2011–12) with two varieties of chickpea viz. desi (PBG1) and kabuli (BG1053) at Pulses Research Farm, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana. Both varieties were treated with six microbial inoculant formulations in combination of single and multiple inoculants alongwith un-inoculated control. Each treatment had three replications. Experiment was designed in factorial randomized block design (FRBD) with 36 plots and 8 rows having row to row distance of 30 cm and plant to plant distance about 10 cm with net plot size 5.4 sq.m. Chickpea seeds of desi (PBG1) and kabuli (BG1053) varieties were sown at the rate of 18–20 kg/acre and 35–37 kg/acre, respectively. All the agronomic practices were followed for raising chickpea crop. The pooled mean of maximum and minimum temperatures during crop growth period was 23.9 and 9.3 °C, respectively. Experimental soil was loamy sand having pH (8.2) with low organic carbon (0.12 %) and available N (92.0 kg/acre), medium available P (18.4 kg/acre) and high available K (105 kg/acre).
Seed inoculation
Chickpea seeds of desi PBG1and kabuli BG1053 varieties were inoculated with recommended cultures of Mesohizobiumcicer (LGR33), different PGPR spp. (LK884, LPGPR1 and LPGPR2) alongwith P.indica as per treatment. Charcoal based cultures of different microorganisms (1 × 109 bacterial cells/g of carrier) were mixed with minimum amount of water to form thick slurry. The slurry was mixed with dry seeds (20 g and 40 g/Kg desi and kabuli chickpea seeds, respectively). In single and multiple inoculant treatments, Mesorhizobium cicer, different PGPR strains and P.indica were applied to chickpea seeds in equal proportions. Before sowing, inoculated seeds were air dried at room temperature under shade and sown within 2 h.
Observations
Emergence count was observed at 10 days after sowing (DAS). Observations for plant height, dry weight of shoot, number and dry weight of nodules were recorded at both vegetative and flowering stage (60 and 90 DAS, respectively). Leghaemoglobin content, total N and P uptake of shoot were recorded at 90 DAS. Chlorophyll content was recorded at 90 and 120 DAS and grain yield was recorded at the harvesting stage of crop.
Growth parameters
Data on emergence count was determined by recording number of emerged seedlings per meter row length from central row of each plot after leaving two border rows on each side. Three randomly selected plants from each plot were uprooted and height of shoots was measured from the base in cm after removing roots. Shoots of three randomly selected uprooted plants from each plot were sun dried and then oven dried at 60 °C for 2 days. Dry weight of shoot was recorded in g. Chlorophyll content of leaves were estimated as per method of Witham et al. (1971) at 645 and 663 nm wavelength.
Endophyte dependency
Endophyte dependency (ED) was recorded at 90 DAS. To determine the ED of chickpea, the formula given by Gerdemann (1975) was used:
Symbiotic parameters, nutrient acquisition and grain yield
Nodulation parameters (number and dry weight of nodules) were recorded by taking average of nodules carefully detached from three randomly uprooted plants. The detached nodules were oven dried at 60 °C for 2 days and the dry weight of nodules per plant was recorded in mg. Leghaemoglobin content was assayed according to Wilson and Reisenauer (1963) by reading absorbance of clear nodular tissue extract using Drabkin’s solution at 540 nm. Determination of total nitrogen content of shoot was carried out by Kjeldahl’s technique of McKenzie and Wallace (1954) with slight modification. Estimation of total phosphorous content of shoot in straw was carried out by digestion of straw using triacid mixture (HNO3: HClO4: H2SO4) (v/v) (Jackson 1973). Grain yield from each plot (g/plot) was recorded and the final grain yield was expressed in kg/ha.
Statistical analysis
The data was analyzed using Analysis of Variance (ANOVA). Further mean separation of treatment effects was accomplished using Tukey's Honestly Significant Difference Test. All the data analysis was carried out using SAS software (Slaughter and Delwiche 2010).
Results and discussion
Legume roots are colonized by numerous rhizospheric microorganisms and these organisms have definite influence on the survival and nodulation ability of seed inoculated rhizobia. Rhizospheric microorganisms may not only influence the inoculated rhizobia adversely through saprophytic competition, but also help them in survival through synergism with increased nodulation and N2 fixation. Prior to field evaluation of tripartite interactions among P.indica with PGPR and Mesorhizobium cicer, in-vitro compatibility studies were conducted for seed germination for improved growth, symbiotic efficiency, nutrient acquisition and yield in chickpea under field conditions.
Assessment of compatibility of the tripartite combination for seed germination in chickpea
In the present study, compatibility of the tripartite combination was studied in–vitro for seed germination by agar plate assay method. It was found that germination was enhanced in all the mutiple inoculations as compared to the MR alone as well as un-inoculated control treatment (Plate 1). In-vitro derived shoots developed single thick and firm roots in multiple inoculated treatments. Data was supported with our previous study on the dry weight basis of fungal pellicle in single and multiple inoculant treatments (Mansotra P. 2012). Maximum dry weight of fungal pellicle was observed in the consortium of MR + PI + LPGPR1 (0.605 g) followed by MR + PI + LK884 (0.591 g) and MR + PI + LPGPR2 (0.578 g) as compared to the P. indica alone (0.515 g).
Plate 1.
Compatibility of P.indica with Mesorhizobium cicer along with Pseudomonas argentinensis (LPGPR1) a. MR + PI + LPGPR1 b. PI c Uninoculated Control
Growth parameters
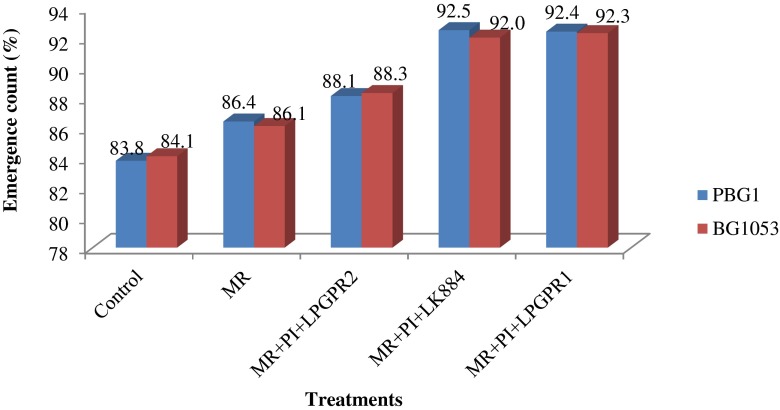
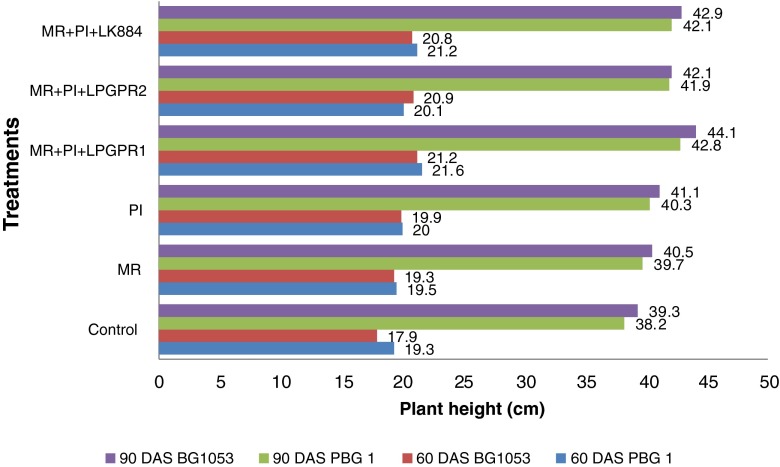
Maximum seed emergence was observed in multiple inoculant treatments (5.1–10.0 %) over the MR alone treatment. All the treatments revealed significant improvement in emergence count over the un-inoculated control (Fig. 1). All the treatments showed non-significant difference for plant height as compared to un-inoculated control at 60 and 90 DAS (Fig. 2). However, significant improvement in dry weight of the shoot was noticed with multiple inoculants viz. MR + PI + LPGPR1 (31.2 %) and MR + PI + LK884 (26.1 %) over the MR alone treatment at 90 DAS.
Fig 1.
Synergistic effect of different multiple inoculations on emergence count
Fig 2.
Synergistic effect of different multiple inoculations on plant height
It appears that synergistic effect of LPGPR1 might be due to release of IAA and further P.indica contributed in enhanced seed germination and plant height. Our results for improvement in seed germination are well in line with earlier findings in chickpea (Ponmurugan and Gopi 2006; Martinez-Viveroz et al. 2010 and Sharma et al. 2007) and cowpea (Lima et al. 2011). In earlier study, it was investigated that Mesorhizobium cicer and LPGPR1 used in the present study are known to produce IAA (Kaur and Sharma 2013). These results are further corroborated with the findings of Meena et al. (2010) on co-inoculation of the endophytic fungus Piriformospora indica with Pseudomonas striata positively affected population dynamics and plant growth in chickpea.
On pooled mean basis significantly high chlorophyll content in multiple inoculation with MR + PI + LPGPR1 (1.61 mg g−1 fresh weight of leaves) followed by MR + PI + LK884 (1.51 mg g−1 fresh weight of leaves) and MR + PI + PGPR2 (1.45 mg g−1 fresh weight of leaves) (Table 1) at 90 DAS was recorded with both varieties. Whereas, decrease in chlorophyll content was observed towards the crop maturity (120 DAS) in all the treatments. Our observations are well correlated with Ray and Valsalakumar (2010) who has reported that dual application of the AMF and the Rhizobium enhanced chlorophyll content in green gram.
Table 1.
Synergistic effect of different tripartite inoculations on dry weight of shoot and chlorophyll content in chickpea. Data are average value of three plants and following the analysis of variance (ANOVA) procedure, differences between desi PBG1 and kabuli BG1053 chickpea varieties, among different treatments and between varieties and treatments were determined using Critical difference (CD) at 5 % percent level of significance
| Treatments | Dry weight of shoot/plant (g) | Chlorophyll content (mg/g fresh weight of leaves) | ||||
|---|---|---|---|---|---|---|
| PBG 1 | BG1053 | Mean | PBG 1 | BG1053 | Mean | |
| Control | 2.60 (3.83) | 2.70 (3.42) | 2.65 (3.63) | 1.05 (0.653) | 1.09 (0.687) | 1.07 (0.670) |
| MR | 2.85 (3.98) | 3.05 (4.08) | 2.95 (4.03) | 1.23 (0.732) | 1.33 (0.738) | 1.28 (0.735) |
| PI | 2.74 (3.90) | 2.85 (3.97) | 2.79 (3.94) | 1.35 (0.764) | 1.38 (0.748) | 1.37 (0.756) |
| MR + PI + LPGPR1 | 3.56 (4.77) | 3.84 (5.82) | 3.70 (5.29) | 1.57 (0.834) | 1.64 (0.836) | 1.61 (0.835) |
| MR + PI + LPGPR2 | 3.11 (4.57) | 3.67 (5.09) | 3.39 (4.83) | 1.42 (0.775) | 1.47 (0.755) | 1.45 (0.780) |
| MR + PI + LK884 | 3.04 (4.58) | 3.29 (5.52) | 3.17 (5.05) | 1.48 (0.796) | 1.53 (0.799) | 1.51 (0.798) |
| Mean | 2.99 (4.27) | 3.26 (4.65) | 3.13 (4.46) | 1.35 (0.759 | 1.41 (0.766) | 1.38 (0.762) |
| CD 5 % | Variety (V): NS Treatment (T): NS (1.01) V × T: NS | Variety (V): 0.05 (0.006) Treatment (T): 0.09 (0.01) V × T: 0.13 (0.015) | ||||
*Data for dry weight of shoot/plant represents values at 60 DAS and that depicted in parenthesis for 90 DAS
Data for chlorophyll content represents values at 60 DAS and that depicted in parenthesis at 120 DAS
DAS denotes days after sowing
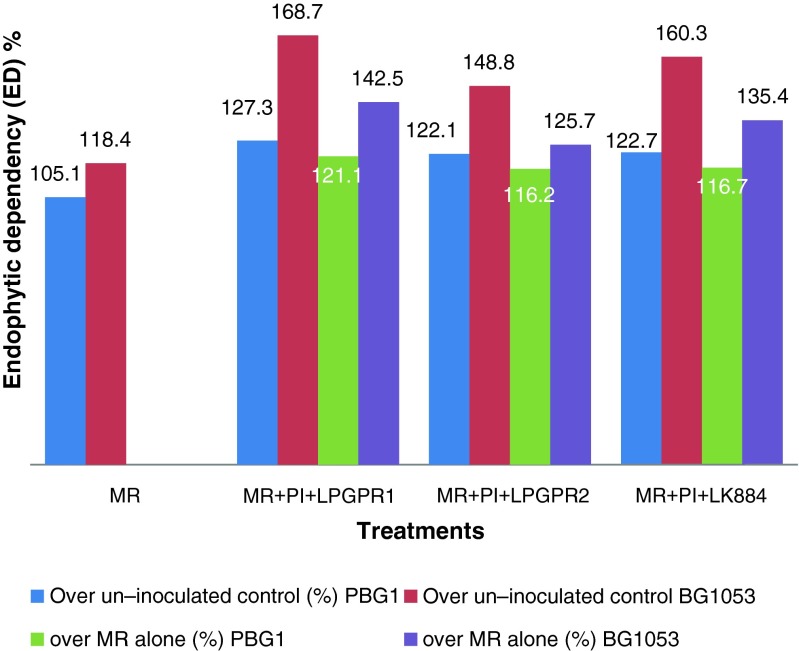
Endophytic Dependency (ED) is used as an index to compare receptivity of different plant species to individual endophytes or in combination. Multiple inoculation and MR alone treatments showed positive growth response over the uninoculated control (Fig. 3). Infective potential of both the native mycorrhiza as well as inoculated P.indica present in legume rhizosphere might have improved ED in MR alone treatment as compared to un-inoculated control. Further, MR + PI + LPGPR1 (121.1 and 142.5 %) recorded highest ED followed by MR + PI + LK884 (116.7 and 135.4 %) and MR + PI + LPGPR2 (116.2 and 125.7 %) in desi and kabuli chickpea, respectively. Similarly, high mycorrhizal dependence of Acacia spp. was also evidenced by Belay et al. (2013) for higher shoot and root dry weight compared to non-inoculated seedlings. Further, Vyas et al. (2008) reported enhanced ED with P.indica in swingle.
Fig 3.
Synergistic effect of different multiple inoculations on Endophytic dependency
Symbiotic parameters and nutrient acquisition
Nodulation is an important trait for effective SNF in leguminous plants. At 60 DAS, multiple inoculation registered significantly high number of nodules per plant with MR + PI + LPGPR1 and MR + PI + LK884 treatments as compared to MR alone in desi PBG1 and kabuli BG1053, respectively. At 90 DAS significantly high number of nodules were recorded with MR + PI + LPGPR1 by 15.7 % over MR alone treatment (Table 2). Further, all the multiple inoculations with different PGPR enhanced nodule dry weight significantly over Mesorhizobium cicer alone treatment at 60 DAS (Table 2). Whereas, at 90 DAS, MR + PI + LPGPR1 treatment significantly improved nodule biomass by 14.9 % over the Mesorhizobium cicer alone treatment as compared to other multiple inoculation treatments. This enhancement in effective nodulation could be due to higher metabolism in P.indica inoculated plants which have enabled them to provide greater amounts of carbohydrates to the rhizobia. Our findings are well corroborated with previous literature (Mishra et al. 2010; Rokhzadi and Toashih 2011; Verma et al. 2012) where PGPR are known to enhance ability of native rhizobia for higher nodulation in chickpea. Mobilization of phosphates from soil by native AMF and inoculated P. indica also might have benefited in development of efficient nodules in chickpea. Our results are in congruence with findings of Nautiyal et al. (2010) who also demonstrated consortia of P.indica and PGPR enhanced the nodulation ability by native rhizobia in chickpea. On the contrary, Tavasolee et al. (2011) reported that PGPR as bacterial strains have lowered nodule fresh mass due to competition for photosynthetic matter between bacteria and fungi in chickpea.
Table 2.
Synergistic effect of different tripartite inoculations on nodulation, total N and P content of shoot in chickpea. Data are average value of three plants and following the analysis of variance (ANOVA) procedure, differences between desi PBG1 and kabuli BG1053 chickpea varieties, among different treatments and between varieties and treatments were determined using Critical difference (CD) at 5 % percent level of significance
| Treatments | Number of nodules/plant | Dry weight of nodules/plant (mg) | Total N content (%) | Total P content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBG1 | BG1053 | Mean | PBG1 | BG1053 | Mean | PBG1 | BG1053 | Mean | PBG1 | BG1053 | Mean | |
| Control | 15. 7 (26.4) | 16.0 (39) | 15.8 (27.9) | 35.9 (88. 8) | 40.1 (90.6) | 38.0 (89.7) | 1.52 | 1.53 | 1.53 | 0.137 | 0.127 | 0.132 |
| MR | 20.0 (37.0) | 24. 3 (36.5) | 22.2 (36.8) | 60.2 (118.6) | 64.5 (127.9) | 62.3 (123.3) | 1.58 | 1.59 | 1.59 | 0.153 | 0.147 | 0.150 |
| PI | 16. 7 (29.0) | 18. 3 (31.3) | 17.5 (32.2) | 42.0 (103.9) | 45.0 (105.5) | 43.5 (104.7) | 1.55 | 1.56 | 1.56 | 0.172 | 0.173 | 0.172 |
| MR + PI + LPGPR1 | 31. 7 (43.2) | 33.0 (42.1) | 32.2 (42.6) | 80.0 (139.0) | 88.0 (144.3) | 84.0 (141.7) | 1.79 | 1.85 | 1.82 | 0.218 | 0.216 | 0.217 |
| MR + PI + LPGPR2 | 26. 7 (37.8) | 27. 7 (40.1) | 27.2 (38.9) | 69.6 (124.7) | 72.5 (136.8) | 71.1 (130.8) | 1.73 | 1.74 | 1.74 | 0.191 | 0.195 | 0.193 |
| MR + PI + LK884 | 28. 3 (40.7) | 30. 7 (42.3) | 29.5 (41.5) | 75.0 (134.8) | 80.0 (137.9) | 77.5 (136.4) | 1.77 | 1.80 | 1.78 | 0.203 | 0.205 | 0.204 |
| Mean | 23.2 (38.6) | 25.0 (35.7) | 24.1 (36.7) | 65.4 (122.8) | 70.0 (123.8) | 67.7 (120.5) | 1.66 | 1.68 | 1.67 | 0.179 | 0.177 | 0.178 |
| CD 5 % | Variety (V): 0.039 (NS) Treatment (T): 0.068 (0.007) V × T: 0.097 (NS) | Variety (V) 2.74 (NS) Treatment (T): 4.8 (14.3) V × T: NS | Variety (V): 0.039 Treatment (T): 0.068 V × T: 0.097 | Variety (V):NS Treatment (T) 0.007 V × T:NS | ||||||||
*Data for number and dry weight of nodules/plant represent values at 60 DAS and that depicted in parenthesis at 90 DAS
Data for total N and P content represent values at 90 DAS
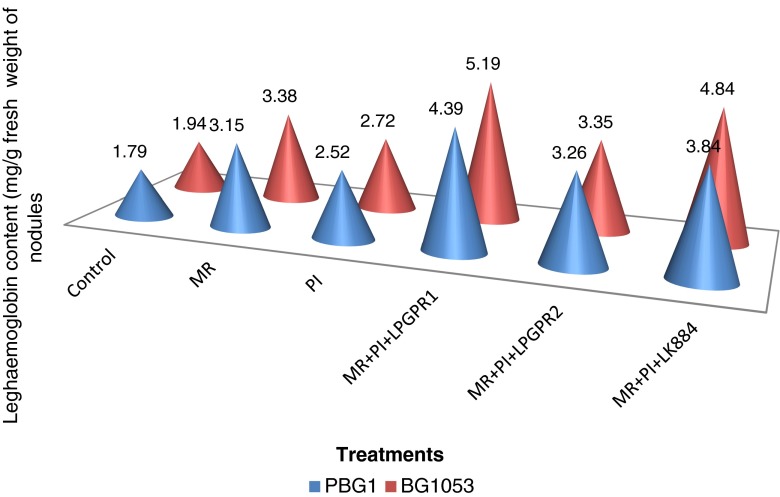
There was significant improvement in leghaemoglobin content with multiple inoculations of MR, P.indica with LPGPR1 and LK884 as compared to MR alone treatment while it differed non-significantly with LPGPR2 (Fig. 4). Higher nodulation and N2 fixation increased the occupancy of effective nodules which might have increased the leghaemoglobin content. Similarly a positive correlation between leghaemoglobin content and nitrogen fixation was reported by Kunal and Sharma (2012) in chickpea due to MR inoculation in chickpea. Further, increased P nutrition in AMF associated legumes (Artursson et al. 2006) is one of the reasons for increased nodulation in such plants. AMF are known to increase the amount of N availability of legumes by stimulating nodule development (Varennes and Goss 2007) with good amount of leghaemoglobin content in the present investigation. P. indica is considered as potent phosphate solublizer (Varma et al. 2001) which might have contributed in enhancement of effective nodulation and leghaemoglobin content through improved N uptake in chickpea. LPGPR1 is known to produce siderophores (Kaur and Sharma 2013) which might have enhanced leghaemoglobin content through facilitated uptake of iron which is a key constituent of leghaemoglobin protein. P.indica mimics the functional activities of AMF and also reported to stimulate N uptake metabolism through increased colonization in Arabidopsis roots (Sherameti et al. 2008).
Fig 4.
Synergistic effect of different multiple inoculations on leghaemoglobin content
Multiple inoculations recorded significantly high total N and P content of shoot as compared to MR alone in both varieties of chickpea (Table 2). Significantly high N content was recorded in MR + PI + LPGPR1 (1.79 % and 1.85 %) followed byMR + PI + LK884 (1.77 % and 1.80 %) and MR + PI + LPGPR2 (1.73 % and 1.74 %) in desi PBG1 and kabuli BG1053, respectively over MR alone treatment. Improvement in total N content is well in line with previous findings in chickpea (Nautiyal et al. 2010) and soybean (Lima et al. 2011) where different bio--inoculant combinations as P.indica + PGPR, AMF + Azospirillum and Bradyrhizobium sp. + Glomus etunicatum, respectively. Higher P content was observed in P. indica alone treatment as compared to Mesorhizobium cicer alone. It can be due to the potency of P. indica for efficient phosphate solublization and mobilization. Improvement of P content in P. indica treatments in present study was well documented with earlier findings of Malla et al. (2004) who reported that P. indica contained substantial amounts of an acid phosphatase which had the potential to solubilize phosphate in the soil and delivered it to the host plant. Moreover, PGPR might contribute to soil phosphate pool available for extraradical hyphae of AM like fungi to pass on to the plant, especially in soils with low P bioavailability. In congruence with this, significantly high P content was recorded in MR + PI + LPGPR1 treatment (0.218 and 0.216 %) followed by MR + PI + LK884 (0.203 and 0.205 %) and MR + PI + LPGPR2 (0.191 and 0.195 %) in desi PBG1and in kabuli BG1053 chickpea varieties, respectively. The investigation has been found coherent between the results of Nautiyal et al. (2010) who reported that consortia of P. indica and PGPR along with native chickpea rhizobia increased the P content.
Grain yield
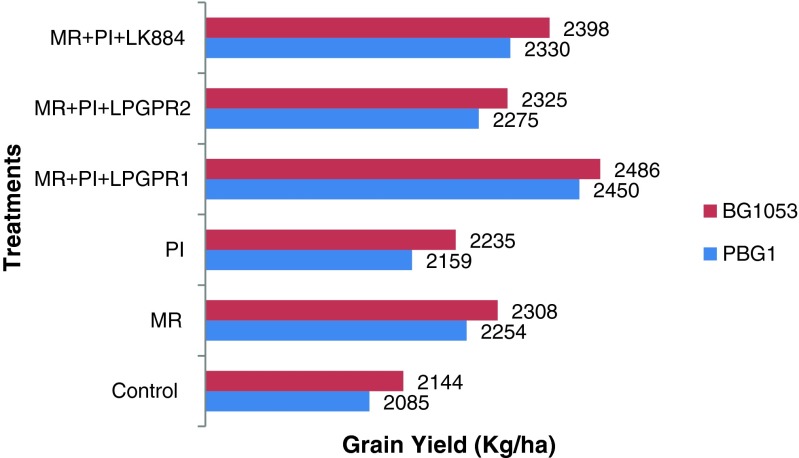
On the basis of overall mean, increase in yield due to multiple inoculations was in the range of 1–8.2 % over MR alone application. Significant increase in grain yield was recorded with MR + PI + LPGPR1 (2450 and 2486 kg/ha) over the MR alone treatment (2254 and 2308 kg/ha) in desi PBG1 and kabuli BG1053, respectively (Fig. 5). This might have improved colonization niches through root proliferation to introduced Mesorhizobium in the rhizosphere of chickpea by reflecting better nodulation and yield (Kunal and Sharma 2012). Further increase in dual and multiple inoculations can be due to IAA production and phosphate solubilization activity by the PGPR. Increased root growth results in improved acquisition of water and nutrients as a result of symbiotic interaction with P. indica. The efficient root colonization by P.indica leading to greater absorption of water and nutrients enhanced its effectiveness on chickpea alongwith enhanced P and K nutrition (Vyas et al. 2008). Higher phosphate contents in plants inoculated with P.indica (Meena et al. 2010) and enhanced photosynthetic rates of the colonized plants might have contributed in yield improvement in chickpea (Achatz et al. 2010). Similar study revealed that the co-inoculation of Rhizobium and AM fungus improved plant vigour and nutrient uptake and dramatically increased the yield of green gram (Ray and Valsalakumar 2010).
Fig 5.
Synergistic effect of different multiple inoculations on grain yield
Compatibility studies on Mesorhizobiumsp. cicer and P. indica with different PGPR (Pseudomonas sp.) in-vitro as well as in-vivo resulted in improved growth, symbiotic parameters, nutrient acquisition and grain yield in chickpea. Tripartite combination of inoculants MR + PI + LPGPR1 significantly improved all the above mentioned parameters followed by MR + PI + LK884 and MR + PI + LPGPR2. It seems from foregoing study that tripartite combination can be explored as biofertilizer for improved productivity in chickpea. However, extensive and more detailed mechanisms should be searched before bioaugmentation of multiple microbial inoculants as biofertilizer for enhanced plant growth promotion and sustainable productivity in chickpea.
References
- Achatz B, Kogel KH, Franken P, Waller F. Piriformospora indica mycorrhization increases grain yield by accelerating early development of barley plants. Plant Signal Behav. 2010;5:1685–1687. doi: 10.4161/psb.5.12.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun H, Prevost D. Ecology of plant growth promoting rhizobacteria. In: Siddiqui ZA, editor. PGPR: Biocontrol and fertilization. Berlin: Springer; 2006. pp. 1–38. [Google Scholar]
- Artursson V, Finlay RD, Jansson JK. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Env Microbiol. 2006;8:1–10. doi: 10.1111/j.1462-2920.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- Bagde US, Prasad R, Varma A. Interaction of Mycobiont: Piriformospora Indica with medicinal plants and plants of economic importance. Afr J Biotechnol. 2010;9:9214–9226. [Google Scholar]
- Bashan Y, de-Bashan LE. How the plant growth-promoting bacterium Azospirillum promotes plant growth-a critical assessment. Adv Agron. 2010;108:77–136. doi: 10.1016/S0065-2113(10)08002-8. [DOI] [Google Scholar]
- Belay Z, Vestberg M, Assefa F. Diversity and abundance of arbuscular-mycorrhizal fungi associated with acacia trees from different land use systems in Ethiopia. Afr J Microbiol Res. 2013;7:5503–5515. [Google Scholar]
- Bhagyaraj DJ, Varma Interaction between arbuscular mycorrhizal fungi and plants: their importance in sustainable agriculture in arid and semi-arid tropics. Adv Microb Ecol. 1995;14:119–142. doi: 10.1007/978-1-4684-7724-5_3. [DOI] [Google Scholar]
- Gerdemann JW. Vescicular–arbuscular mycorrhizae. In: Torrey JG, Clarkson DT, editors. The development and function of roots. London: Academic; 1975. pp. 575–591. [Google Scholar]
- Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall of India; 1973. [Google Scholar]
- Jida M, Assefa F. Phenotypic diversity and plant growth promoting characteristic of Mesorhizobium species isolated from chickpea (Cicer arietinum L.) growing areas Ethiopia. Afr J Biotechnol. 2012;11:7483–7493. [Google Scholar]
- Kaur N, Sharma P. Screening and characterization of native Pseudomonas sp. as plant growth promoting rhizobacteria in chickpea (Cicer arietinum L.) rhizosphere. Afr J Microbiol Res. 2013;7:1465–1474. [Google Scholar]
- Kunal, Sharma P. Influence of pesticide treated seeds on survival of Mesorhizobium cicer, symbiotic efficiency and yield in chickpea. Plant Protect Sci. 2012;48:37–43. [Google Scholar]
- L’taief B, Sifi B, Gtari M, Zaman-Allah M, Lachaal M. Phenotypic and molecular characterization of chickpea rhizobia isolated from growing different areas of Tunisia. Can J Microbiol. 2007;53:427–434. doi: 10.1139/w06-127. [DOI] [PubMed] [Google Scholar]
- Lima AST, Xavier TF, Lima CEP, Oliveira JP, Mergulhão ACS, Figueiredo MVB. Triple inoculation with Bradyrhizobium, Glomus and Paenibacillus on cowpea (Vigna unguiculata [L.] Walp.) development. Braz J Microbiol. 2011;42:919–926. doi: 10.1590/S1517-83822011000300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatallah J, Berraho EB, Sanjuan J, Lluch C. Phenotypic characterization of rhizobia isolated from chickpea (Cicer arietinum) growing in Moroccan soils. Agronomie. 2002;22:321–329. doi: 10.1051/agro:2002013. [DOI] [Google Scholar]
- Malla R, Prasad R, Kumari R, Giang PH, Pokharel U, Oelueller R, Varma A. Phosphorus solubilizing symbiotic fungus: Piriformospra indica. Endocytobio Cell Res. 2004;15:579–600. [Google Scholar]
- Mansotra P (2012) Studies on interactions among Piriformospora indica with potential plant growth promoting rhizobacteria and Mesorhizobium in chickpea (Cicer arietinum L.). Dissertation, Punjab Agricultural University
- Martinez-Viveros O, Jorquera M, Crowley D, Gajardo G, Mora M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr. 2010;10:293–219. doi: 10.4067/S0718-95162010000100006. [DOI] [Google Scholar]
- McKenzie HA, Wallace HA. The Kjeldahl determination of nitrogen. Aust J Chem. 1954;16:55–79. doi: 10.1071/CH9540055. [DOI] [Google Scholar]
- Meena K, Mesapogu S, Kumar M, Yandigeri MS, Singh G, Saxena AK. Co-inoculation of the endophytic fungus Piriformospora indica with the phosphate-solubilising bacterium Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol Fertil Soils. 2010;46:169–174. doi: 10.1007/s00374-009-0421-8. [DOI] [Google Scholar]
- Mishra M, Kumar U, Mishra PK, Prakash V. Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Adv Biol Res. 2010;4:92–96. [Google Scholar]
- Nautiyal CS, Chauhan P, Dasgupta SM, Seem K, Varma A, Staddon WJ. Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488, Piriformospora indica DSM 11827, and Cicer arietinum L. World J Microbiol Biotechnol. 2010;26:1393–1399. doi: 10.1007/s11274-010-0312-z. [DOI] [Google Scholar]
- Ponmurugan P, Gopi C. Distribution pattern and screening of phosphate solubilising bacteria Isolated from different food and forage crops. J Agron. 2006;5:600–604. doi: 10.3923/ja.2006.600.604. [DOI] [Google Scholar]
- Ray JG, Valsalakumar N. Arbuscular Mycorrhizal Fungi and Piriformospora indica individually and in combination with Rhizobium on green gram. J Plant Nutr. 2010;33:285–298. doi: 10.1080/01904160903435409. [DOI] [Google Scholar]
- Rhomdhane SB, Aouani ME, Trabelsi M, De Lajudie P, Mhamdi R. Selection of high nitrogen-fixing rhizobia nodulating chickpea (Cicer arietinum) for semi-arid Tunisia. J Agron Crop Sci. 2009;194:413–420. [Google Scholar]
- Rokhzadi A, Toashih V. Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plan growth promoting rhizobacteria. Aust J Crop Sci. 2011;5:44–48. [Google Scholar]
- Shahollari B, Varma A, Oelmuller R. Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J Plant Physiol. 2005;162:945–958. doi: 10.1016/j.jplph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Sharma P, Jeenie, Singh P. Synergism among phosphate solubilising bacteria, Rhizobium and PGPR in green gram (Vigna radiata (L.) Wilczek) and black gram (Vigna mungo (L.) Hepper) Microbiol World. 2007;9:41–44. [Google Scholar]
- Sheramati I, Triapthi S, Varma A, Oelmuller R. The root colonizing endophyte Piriformospora indica confers drought tolerance in Arabdidopsis by stimulating the expression of drought stress-related genes in leaves. Mol Plant Microbe Interact. 2008;21:799–807. doi: 10.1094/MPMI-21-6-0799. [DOI] [PubMed] [Google Scholar]
- Singh N P (2007) Project Coordinater’s reports (2006–07): In: All India Coordinated Research Project in Chickpea, Indian Institute of Pulses Research, Kanpur, pp 19–20
- Singh NP, Nadarajan N. Growth, stability and future outlook of chickpea subsector in India- A march towards nutritional security. Lukhnow: Army printing press; 2013. pp. 90–99. [Google Scholar]
- Slaughter SJ and Delwiche LD (2010). The Little SAS Book for Enterprise Guide 4.2. Cary, USA: SAS Institute. (www.sas.com)
- Tavasolee A, Aliasgharzad N, Jouzani GS, Mardi M, Asgharzadeh A. Interactive effects of arbuscular mycorrhizal fungi and rhizobial strains on chickpea growth and nutrient content in plant. Afr J Biotechnol. 2011;10:7585–7591. [Google Scholar]
- Varennes A, Goss MJ. The tripartite symbiosis between legumes, rhizobia and indigenous mycorrhizal fungi is more efficient in undisturbed soil. Soil Biol Biochem. 2007;39:2603–2607. doi: 10.1016/j.soilbio.2007.05.007. [DOI] [Google Scholar]
- Varma A, Verma S, Sudha Sahay N, Britta B, Franken P. Piriformospora indica - a cultivable plant growth promoting root endophyte with similarities to arbuscular mycorrhizal fungi. Appl Environ Microbiol. 1999;65:2741–2744. doi: 10.1128/aem.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Singh A, Sudha, Sahay NS, Sharma J, Roy A, Kumari M, Rana D, Thakran S, Deka D, Bharti K, Hurek T, Blechert O, Rexer KH, Kost G, Hahn A, Maier W, Walter M, Strack D, Kranner I. Piriformospora indica: an axenically culturable mycorrhiza-like endosymbiotic fungus. In: Hock B, editor. The mycota. Berlin: Springer; 2001. pp. 125–150. [Google Scholar]
- Verma JP, Yadav J, Tiwari KN. Enhancement of nodulation and yield of chickpea by co-inoculation of indigenous Mesorhizobium spp. and plant growth—promoting rhizobacteria in eastern Uttar Pradesh. Commun Soil Sci Plan. 2012;43:605–621. doi: 10.1080/00103624.2012.639110. [DOI] [Google Scholar]
- Vincent JM. A manual for the pratical study of root-nodule bacteria. Oxford: Blackwells Scientific Publications; 1970. [Google Scholar]
- Vyas S, Nagori R, Purohit SD. Root colonization and growth enhancement of micropropagated (Feronia lemonia L.) Swingle by Piriformospora indica- A cultivable root endophyte. Inter J Plant Develop Biol. 2008;2:128–132. [Google Scholar]
- Wilson DO, Reisenauer Determination of leghaemoglobin in legume nodules. Anal Biochem. 1963;6:27–30. doi: 10.1016/0003-2697(63)90004-6. [DOI] [Google Scholar]
- Witham PH, Dyes DF, Delvin RM. Chlorophyll absorption of spectrum and quantitative determination. In: Hock B, editor. Experimental plant physiology. New York: Von Nostr and Reinhoed Company; 1971. pp. 51–56. [Google Scholar]