Abstract
We explored the influence of pollination season and maturity of capsule on post-pollination capsule formation and in vitro asymbiotic seed germination, respectively. Three Phalaenopsis orchid hybrids, namely, ‘Athens’, ‘Moscow’ and ‘Lusaka’ flowers were artificially self-pollinated during winter, spring, summer and fall seasons and the impact of the pollination seasons was evident during capsule formation. It was observed that winter was the most suitable season for pollination of all the three Phalanaeopsis hybrids resulting in 80-88 % capsule formation. During summer, the pollination success rate was 24-28 %, but resulted in successful capsule formation. Season of pollination further delimited the germination efficiency of seeds harvested from capsules of variable maturity levels. Invariably, seeds collected from winter-pollinated capsules performed best in germination compared to other seasons, for instance, ‘Moscow’ seeds took less than 14 days to germinate from capsules developed following winter-pollination. Regarding the influence of capsule maturity on seed germination, we observed that seeds derived from 5-month mature capsules, invariably took least time to germinate than that of the 3-month or 7-month in all three hybrids, e.g., for ‘Moscow’ it was 13.9 days with a maximum of 90.3 % germination.
Keywords: Orchid, Phalaenopsis, Pollination, Seed germination
Introduction
Propagation by means of seeds signifies the ultimate competent system of orchid multiplication and breeding (Stewart and Kane 2006). Symbiotic seed germination poses cumbersome procedures, involving collection of root sections and isolation of fungi. In contrast, asymbiotic seed germination does not require isolation of microbes for orchid seed germination. Asymbiotic seed germination is a rather simple procedure, given that, isolation of microbes is not required for orchid seed gemination. Nevertheless, there still prevail conditions where seedlings with symbiotic germination are preferred. Orchid species or hybrids that are developed from asymbiotic seedlings relies on fungal symbionts that originates naturally, for the purpose involving seedling recruitment (Zettler 1997). Owing to probable environmental alterations at popular orchid growing zones, there remains a possibility that a target orchid species’ mycobionts may not/ may cease to exist at a location if the orchid itself is missing. In such conditions, symbiotically cultured seedlings can fulfill the purpose to serve as plant material and also as a basis for mycobiont inoculum; since bringing in contact a compatible mycobiont may well promote the development of self-sustaining orchid populace. Moreover, if there remain chances that symbiotic seedlings could develop more expeditiously than the asymbiotic ones, in that case seed germination via symbiotic means could possibly become the preferred way to produce orchids (Johnson 2007).
Phalaenopsis hybrids are the product of thorough and intensive breeding of plants with escalating commercial value as cut flowers and rather more as potted plants. In the horticulture industry, they have proved their worth to be the utmost marketable orchids hybrids owing to their attractive facets that includes producing large, colorful and durable flowers, along with their adaptability to normal room conditions. As stated by the revenue data of Flora Holland flower auction, Phalaenopsis remained nevertheless the top-selling potted plants in Europe. About 45000 cultivars of Phalaenopsis were recorded following an update of the Royal Horticultural Society that cataloged 643 incipient cultivars in 2010 (Reviewed by Lesar 2012). Orchid seeds are about microscopic in size. Since the initial observation of orchid seeds there prevails nearly 400 years gap up till its successful asymbiotic germination in 1921 by Knudson. From then on, propagation as well as breeding through hybridization of orchid is done globally. However, hybrids of initial growers could not even have presumed became achievable (Yam and Arditti 2009). The process that involves breeding fresh varieties of Phalaenopsis is rather overwhelmingly extensive and time taking. Contemporary hybrid seedlings are usually cultured from crosses between two superior parental cultivars with the intention to upgrade and refine the morphological and reproductive characters in combination with resistance against diseases (Tang and Chen 2007).
Usual orchid hybrids may function as examples for promoting reintroduction programs, which can then be useful to endangered and threatened species. The paramount footstep in this procedure is determining proficient asymbiotic germination protocols to generate plants for successive investigation (Johnson 2007). Very few reports are available on seed germination of Phalanaeopsis species, and no report exists on the asymbiotic or symbiotic seed germination requirements of Phalaenopsis ‘Athens’, ‘Moscow’ and ‘Lusaka’, three popular hybrids of Middle East. According to Schwalliera et al. (2011) Phalaenopsis seeds are desiccation tolerant, showing their orthodox in their storage behavior, even with fairly high relative water content during harvest. It has been also advocated for further research for determining the endurance of seeds in storage and the maturation profile influencing germination, more precisely.
The aim of this research was (1) to assess the prospective of post-pollination capsule formation for the three hybrids depending on season of pollination, (2) to estimate the efficiency of asymbiotic seed germination for the three hybrids based on season of pollination and maturity stage of capsules. The data gathered from the present experiments will be utilized to propagate plants for orchid reintroduction approaches.
Materials and method
Plants
Thirty plants (of circa 20–25 cm height) from each of the Phalaenopsis hybrids, namely, ‘Athens’ (Fig. 1a), ‘Moscow’ (Fig. 1b) and ‘Lusaka’ (Fig. 1c) at their full bloom stage, growing in the greenhouse at the north of Iran, were employed in the present study.
Fig. 1.
Capsule formation and asymbiotic seed germination of three Phalaenopsis hybrids. Full grown flower of cultivar a ‘Athens’, b ‘Moscow’, c ‘Lusaka’ (Bar = 4 mm); Post-pollination capsule formation of cultivar d ‘Athens’, e ‘Moscow’, f ‘Lusaka’ pollinated during winter (Bar = 6 mm); Three-month old capsules showing low seed germination in g ‘Athens’, h ‘Moscow’, i ‘Lusaka’ pollinated during winter (Bar = 7 mm); Five-month old capsules showing high seed germination in j ‘Athens’, k ‘Moscow’, l ‘Lusaka’ pollinated during winter (Bar = 5 mm); Seven-month old capsules showing moderate seed germination in j ‘Athens’, k ‘Moscow’, l ‘Lusaka’ pollinated during winter (Bar = 7 mm)
Pollination
During the commencement of spring (max. 14 °C to min. 8 °C temperature, relative humidity max. 95 % to min. 71 %, and 12 h photoperiod), summer (max. 26 °C to min. 20 °C temperature, relative humidity max. 92 % to min. 67 %, and 14 h photoperiod), fall (max. 24 °C to min. 19 °C tempertaure., relative humidity max. 93 % to min. 69 %, and 12 h photoperiod) and winter (max. 14 °C to min. 6 °C temp., relative humidity max. 92 % to min. 65 %, and 10 h photoperiod), the flowers from two-year-old adult plants were labeled and artificially pollinated by detaching the anther cap and pollinia using forceps and subsequently placing the pollinia over the stigma of the same flower when they were completely opened. The number of post-pollination capsule formed was counted to assess the influence of pollination season on capsule production.
Harvesting
Undehisced capsules at their three (capsule started to swell and beginning to color), five (capsule swollen and light brown colored) and seven months (capsule fully swollen, dried and dark brown colored) after pollination stage were collected from plants of three Phalaenopsis cultivars (viz. ‘Athens’, ‘Moscow’ and ‘Lusaka’) whilst seed development and cell number amplified fast (Vujanovic et al. 2000). Ten capsules were harvested from a minimum of 10 plants for each hybrid. Stems were consistently cut with a scalpel roughly 1 cm underneath the pod juncture. Harvested pods were kept in self-sealing plastic sacs for carrying and pre-sterilization temporary storing to curtail dehydration.
Sterilization of capsules and collection of seeds
The capsules were washed under running tap water, and surface sterilized by dipping into 70 % (v/v) ethanol for for 30 s, followed by agitation for 15 min in a sodium hypochlorite (NaOCl) solution containing 2 % available chlorine and 0.05 % (v/v) Tween 20 in a completely aseptic condition under laminar air flow. Three times rinse with sterile double distilled water was done each time after employing surface sterilizing agent. The sterilized capsules were slit longitudinally with an alcohol-flamed surgical scalpel in a laminar-flow hood to isolate the seeds.
Seed culture and germination
Untreated seeds were harvested, gently teased from surface sterilized splitted-capsules using the scalpel and inoculated directly on the surface of Chen medium (Chen et al. 1999), solidified with 0.8 % agar, used as the seed germination medium (Balilashaki et al. 2014). The pH of the medium was adjusted to 5.7 with NaOH and HCl prior to autoclaving at 121 °C and 105 kg cm−2 for 15 min. Approximately 100 seeds were sown on the medium in each culture vessel. All the cultures were incubated at 25 ± 2 °C under cool white fluorescent light (50 μmol m−2 s−1) with a 16-h photoperiod. Seed germination percentages were recorded 60 d after in vitro sowing. The first stages in germination comprised of an engorgement of the embryo, succeeding split of the testa, and expansion of the embryo to a top-shaped protocorm. Germination was measured to have ensued when the embryo developed from the testa and the dimension of embryo amplified. The germination potential of mature and immature seed samples were scored under a light microscope. Seed germination percentage was calculated by the following formula: % seed germination = (Number of seeds forming spherule ÷ Total number of seeds) × 100 (Roy et al. 2011). The germination potential for independent seed samples was established concurrent to culture initiation.
Statistical analysis
The experiments were executed in a completely randomized design with 10 replicates per treatment. Each replicate comprised of five flower samples for the capsule formation experiments, and 100 seeds for the germination experiments. The data for capsule formation, days to germination initiation and germination frequency were analyzed through one-way Analysis of Variance (ANOVA) and the means were compared by the Tukey’s test (PC version Origin 7.0 Northampton, MA, USA) at 5 % probability levels using SPSS (Version 11, SPSS Inc. Chicago, USA) software package. Percentage of capsule formation and seed germination data were transformed using arcsine prior to ANOVA and converted back to the original scale to normalize variation (Compton 1994).
Results
Effect of pollination season on capsule formation
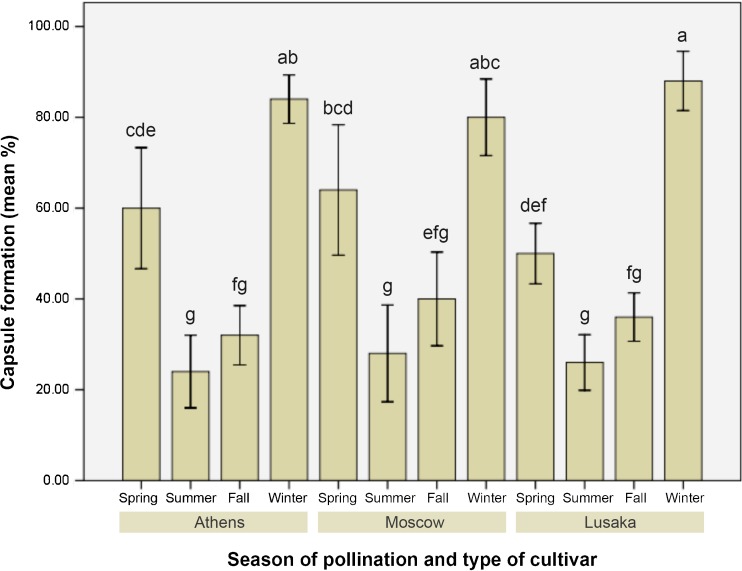
The present experiment showed some interesting results on how the capsule formation was directly influenced by season of pollination. It was evident that winter was the most suitable season of pollination for each of the three Phalanaeopsis hybrids showing a range of 80-88 % capsule formation (Fig. 2). Among the three hybrids under study, ‘Athens’ showed 84 % (Fig. 1d), ‘Moscow’ scored 80 % (Fig. 1e) whereas, ‘Lusaka’ scored the maximum of 88 % post-pollination capsule formation (Fig. 1f). Pollination during spring resulted in favor of ‘Athens’ and ‘Moscow’ showing 60 % or more capsule formation, nevertheless, revealed suppressive effect on the same for ‘Lusaka’ which performed best during winter (Fig. 2). It was significant to observe that fall and summer displayed a similar trend in regulating the success of pollination in terms of post-pollination capsule formation. Pollination during summer played an unfavorable role on its success where, merely 24-28 % pollination was successful and turned into capsules. Surprisingly, the least performer of winter season i.e., ‘Moscow’ proved its best during summer season with the maximum of 28 % capsule formation. However, there was no statistical significance on the differential performance of the three hybrids under study. Hence, it can be considered that irrespective of hybrid types the summer season is not suitable for pollination.
Fig. 2.
Seasonal effect on the pollination and capsule formation of Phalaenopsis ‘Athens’, ‘Moscow’, and ‘Lusaka’. Data represent (mean ± standard error bar) 5 flowers per treatment in 10 replicated experiments. Mean values with the same letter are not significant at P ≤ 0.05 based on one-way analysis of variance (ANOVA) followed by Tukey’s test. Data expressed as percentage were transformed using arcsine prior to ANOVA and converted back to the original scale for demonstration in the histobar (Compton 1994)
Effect of pollination season on germination
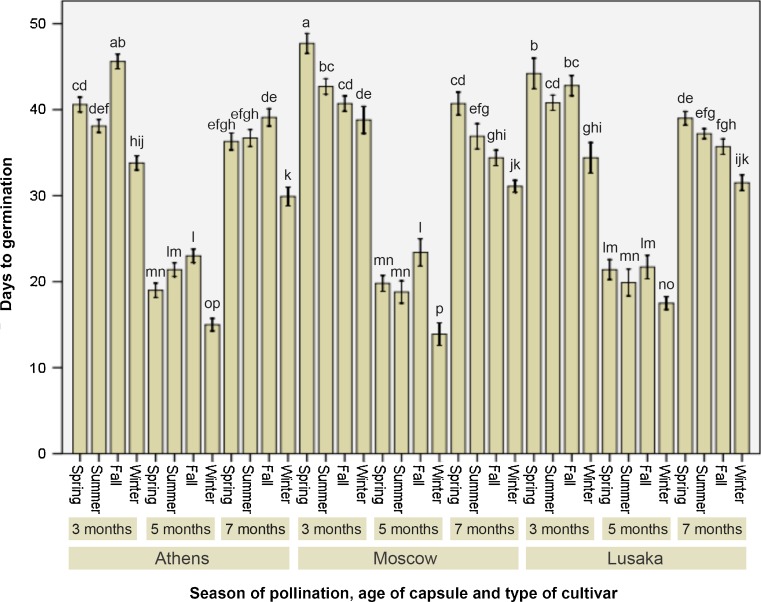
Season of pollination further regulated the germination ability of seeds derived from capsules with different maturity level. Invariably, seeds collected from capsules pollinated during winter performed best in terms of days to initiation of germination when compared to the other seasons (Fig. 3). Notably, the hybrid ‘Moscow’ that performed least in capsule formation after winter-pollination proved to be most efficient in earliest (less than 14 days) germination of seeds (Fig. 1h). Hence, it was evident that pollination season played its role differentially depending on the physiological events in plants. Though spring and summer are the pollination seasons, seeds have taken maximum time to germinate. For ‘Moscow’ the latest germination of spring-pollinated capsule-derived seeds was 47 days after inoculation and for ‘Lusaka’ it took 44.2 days to initiate the germination process. Fall as the pollination season resulted significantly faster germination when compared to spring or summer irrespective of types of hybrids. However, it was not the earliest as winter-pollinated capsule-derived seeds.
Fig. 3.
Seasonal effect on pollination and capsule age on days to germination of Phalaenopsis ‘Athens’, ‘Moscow’, and ‘Lusaka’ seeds. Data represent (mean ± standard error bar) 100 seeds per treatment in 10 replicated experiments. Mean values with the same letter are not significant at P ≤ 0.05 based on one-way analysis of variance (ANOVA) followed by Tukey’s test. Data expressed as percentage were transformed using arcsine prior to ANOVA and converted back to the original scale for demonstration in the histobar (Compton 1994)
Effect of capsule maturity on germination
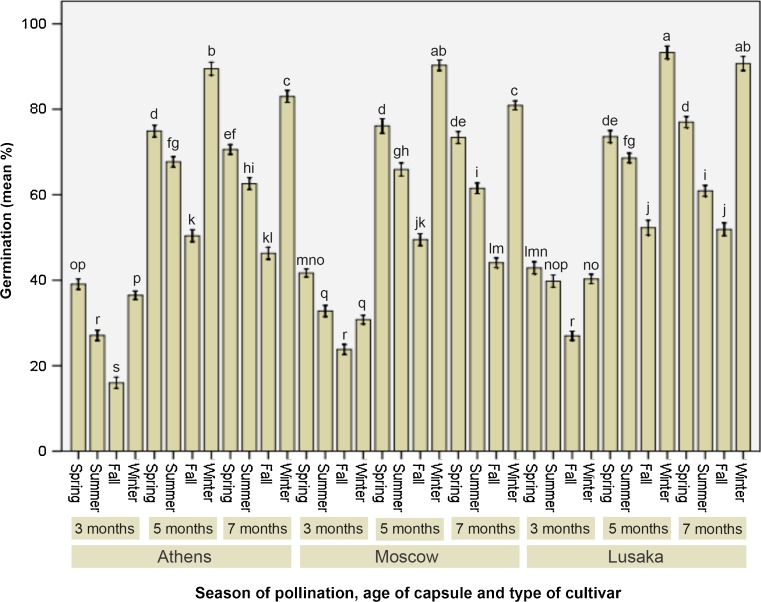
When it was about to observe the influence of capsule maturity, we observed a very interesting and unique pattern of influence on period taken to initiate seed germination. Seeds, derived from 5-month-old mature capsules, invariably took least time to germinate in all three hybrids (Fig. 3). For ‘Athens’ it was 15 days (Fig. 1g), for ‘Moscow’ it was 13.9 days (Fig. 1h) while 17 days was taken to commence the germination of seeds of ‘Lusaka’ (Fig. 1i). Nevertheless, there were variations within the hybrids depending on the season of pollination. Seeds collected from 3-months or 7-months old capsules showed significantly different results than that of the 5-month-old mature capsules. Both the 3- and 7-month capsules resulted in late germination of seeds. This effect was comparable for all the three hybrids under study. The differential response of the dissimilar maturity of capsules might be due to their physiological status during the concerned maturity stage. While 3-month-old capsules are immature, 7-month-old capsules are over-matured. Therefore, they could not quickly germinate like that of 5-month-old mature capsule-derived seeds. The physiological state at 5-month stage presumably was the optimum for attaining this earliest germination initiation and it was applicable irrespective of hybrid types. A comparable pattern was acquainted while studying the influence of pollination season and maturity stage of capsules on germination percentage as what we found in case of the other attribute of germination (days to germination initiation). Here the germination percentage was as highest as 89.5 for ‘Athens’ (Fig. 1j), 90.3 in case of ‘Moscow’ (Fig. 1k) and 90.7 in case of ‘Lusaka’ (Fig. 1l), (Fig. 4). Interestingly, all these three highest (corresponding to individual hybrids) germination percentages were from winter-pollinated and 5-month old capsules.
Fig. 4.
Seasonal effect on pollination and capsule age on germination percentage of Phalaenopsis ‘Athens’, ‘Moscow’, and ‘Lusaka’ seeds. Data represent (mean ± standard error bar) 100 seeds per treatment in 10 replicated experiments. Mean values with the same letter are not significant at P ≤ 0.05 based on one-way analysis of variance (ANOVA) followed by Tukey’s test. Data expressed as percentage were transformed using arcsine prior to ANOVA and converted back to the original scale for demonstration in the histobar (Compton 1994)
Discussion
A number of Phalaenopsis orchids have been successfully germinated using asymbiotic methods (Mweetwa et al. 2008; Paek et al. 2011; Lesar et al. 2012; Balilashaki et al. 2014; Shekarriz et al. 2014).
In our experiment, we observed a decline in germination frequency of 7-month old capsules in comparison to that of the 5-month old, which is also similar to the result of Zhang et al. (2013). This trait is similar to the earlier reports on orchids where mature developed seeds were more complex to germinate in vitro than immature seeds (St-Arnaud et al. 1992; de Pauw and Remphrey 1993). This profile is also comparable to the report of Lee et al. (2005) on C. formosanum, in which they suggested the collection of capsules at 3–4 month-old-stage to enhance the germination. The variable results regarding maturity of the capsule in our study might be due to the diverse time intervals taken by each stage to reach the optimum development of the embryo suitable for germination. Such an opinion has also been elicited by Zhang et al. (2013) earlier in Cypripedium macranthos. As per the conclusion of Zhang et al. (2013) the variable results maturity of the capsule in our study might be due to the diverse time spans taken by each maturity stages to reach the optimum embryo development stage, suitable for germination.
Another important reason might be the physical characteristics of seed coat. This rigid inner seed coat is recognized as ‘carapace’ and could be noticed in mature seeds of quite a number of orchids (Rasmussen 1995; Lee et al. 2005; Yamazaki and Miyoshi 2006). During our experiment, the accumulation of hydrophobic cuticular substance and phenolic compounds in their walls might occur. According to Yamazaki and Miyoshi (2006) with the advancement of seeds towards maturity, the walls of carapace become cutinized and lignified. Eventually, the seed coat-inflicted dormancy occurs owing to the impermeability of seeds following hydrophobic cutinization and lignification.
The present report offers a consistent protocol for ensuring high pollination success in terms of capsule formation and seed culture from pre-mature capsules based on a distinct time frame of Phalaenopsis cultivars viz. ‘Athens’, ‘Moscow’ and ‘Lusaka’. Collecting the immature seeds at 5-month maturity stage that were pollinated during winter might circumvent the seed coat-imposed dormancy and boost the germination efficiency.
Acknowledgements
Authors concede the research assistance from the Faculty of Agricultural Sciences and Engineering, College of Agriculture and Natural Resources, University of Tehran and express appreciation to the anonymous reviewers and the editor of this article for their critical comments and suggestions.
Authors’ contribution
K. Balilashaki, R. Naderi - Conceived the idea, designed and executed the experiments; S. Gantait - Carried out the statistical analysis; S. Gantait, M. Vahedi - Wrote the manuscript. All the authors have read and approved the manuscript prior submission.
Conflict of interest
The authors declare that there is no conflict of interest and no financial gain from this article.
Abbreviations
- ANOVA
Analysis of variance
References
- Balilashaki K, Naderi R, Kalantari S, Vahedi M. Efficient in vitro culture protocols for propagating Phalaenopsis ‘Cool Breeze’. Plant Tissue Cult Biotech. 2014;24:191–203. doi: 10.3329/ptcb.v24i2.23552. [DOI] [Google Scholar]
- Chen JT, Chang C, Chang WC. Direct somatic embryogenesis on leaf explants of Oncidium Gower Ramsey and subsequent plant regeneration. Plant Cell Rep. 1999;19:143–149. doi: 10.1007/s002990050724. [DOI] [PubMed] [Google Scholar]
- Compton ME. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tiss Organ Cult. 1994;37:217–242. [Google Scholar]
- de Pauw MA, Remphrey WR (1993) In vitro germination of three Cypripedium species in relation to time of seed collection, media, and cold treatment. Can J Bot 71:879–885
- Johnson TR, Stewart SL, Dutra D, Kane ME, Richardson L. Asymbiotic and symbiotic seed germination of Eulophia alta (Orchidaceae)—preliminary evidence for the symbiotic culture advantage. Plant Cell Tiss Organ Cult. 2007;90:313–323. doi: 10.1007/s11240-007-9270-z. [DOI] [Google Scholar]
- Lee YI, Lee N, Yeung EC, Chung MC. Embryo development of Cypripedium formosanum in relation to seed germination in vitro. J Am Soc Hortic Sci. 2005;130:747–753. [Google Scholar]
- Lesar H, Čeranič N, Kastelec D, Luthar Z. Asymbiotic seed germination of Phalenopsis Blume orchids after hand pollination. Acta Agricult Slovenica. 2012;99:5–11. doi: 10.2478/v10014-012-0001-8. [DOI] [Google Scholar]
- Mweetwa AM, Welbaum GE, Tay D. Effects of development, temperature, and calcium hypochlorite treatment on in vitro germinability of Phalaenopsis seeds. Sci Hortic. 2008;117:257–262. doi: 10.1016/j.scienta.2008.03.035. [DOI] [Google Scholar]
- Paek KY, Hahn EJ, Park SY (2011) Micropropagation of Phalaenopsis Orchids via Protocorms and Protocorm-Like Bodies. In: Trevor TA, Edward CY (Eds.), Plant Embryo Culture: Methods and Protocols, Methods in Molecular Biology, vol. 710, Springer, pp. 293–306 [DOI] [PubMed]
- Rasmussen HN. Terrestrial Orchids: From Seed to Mycotrophic Plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Roy AR, Patel RS, Patel VV, Sajeev S, Deka BC. Asymbiotic seed germination, mass propagation and seedling development of Vanda coerulea Griff ex. Lindl. (Blue Vanda): An in vitro protocol for an endangered orchid. Sci Hortic. 2011;128:325–331. doi: 10.1016/j.scienta.2011.01.023. [DOI] [Google Scholar]
- Schwalliera R, Bhoopalanb V, Blackman S. The influence of seed maturation on desiccation tolerance in Phalaenopsis amabilis hybrids. Sci Hortic. 2011;128:136–140. doi: 10.1016/j.scienta.2010.12.019. [DOI] [Google Scholar]
- Shekarriz P, Kafi M, Deilamy SD, Mirmasoumi M. Coconut water and peptone improve seed germination and protocorm like body formation of hybrid Phalaenopsis. Agric Sci Dev. 2014;3:317–322. [Google Scholar]
- St-Arnaud M, Lauzer D, Barabe D. In vitro germination and early growth of seedling of Cypripedium acaule (Orchidaceae) Lindleyana. 1992;7:22–27. [Google Scholar]
- Stewart SL, Kane ME. Asymbiotic germination and in vitro seedling development of Habenaria macroceratitis (Orchidaceae), a rare Florida terrestrial orchid. Plant Cell Tiss Organ Cult. 2006;86:147–158. doi: 10.1007/s11240-006-9098-y. [DOI] [Google Scholar]
- Tang CY, Chen WH. Breeding and development of new varieties in Phalaenopsis. In: Chen WH, Chen HH, editors. Orchids Biotechnology. Singapore: World Scientific Publishing Co. Pvt. Ltd; 2007. pp. 1–22. [Google Scholar]
- Vujanovic V, St-Arnaud M, Barabé D, Thibeault G. Viability testing of orchid seed and the promotion of colouration and germination. Ann Bot. 2000;86:79–86. doi: 10.1006/anbo.2000.1162. [DOI] [Google Scholar]
- Yam TW, Arditti J. History of orchid propagation: a mirror of the history of biotechnology. Plant Biotechnol Rep. 2009;3:1–56. doi: 10.1007/s11816-008-0066-3. [DOI] [Google Scholar]
- Yamazaki J, Miyoshi K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae) Ann Bot. 2006;98:1197–1206. doi: 10.1093/aob/mcl223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettler LW. Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana. 1997;18:188–194. [Google Scholar]
- Zhang Y, Lee YI, Deng L, Zhao S. Asymbiotic germination of immature seeds and the seedling development of Cypripedium macranthos Sw., an endangered lady’s slipper orchid. Sci Hortic. 2013;164:130–136. doi: 10.1016/j.scienta.2013.08.006. [DOI] [Google Scholar]