Abstract
An efficient and rapid in vitro propagation system for Satureja avromanica, a rare and endangered folk medicinal plant of Iran was developed through the shoot tip and leaf disc explants. Nodal and leaf explants from wild plants were established on MS and WPM media supplemented with BA, BAP and TDZ (0, 0.1, 0.5, 1, 1.5, 2, 5 and 10 mgl−1) alone or by application of BA and TDZ (0, 2, 5 and 10 mgl−1) in combination with IBA and 2,4-D (0, 0.1, 0.5 and 1 mgl−1), respectively. Based on results, the highest mean shoot number (6.21) was obtained on MS medium supplemented with 2 mgl−1 BA. Regarding the shoot elongation, MS supplemented with 2 mgl−1 TDZ and MS containing 5 mgl−1 BA showed the longest shoots (4.82 and 4.39 cm, respectively) after 6 weeks of culture. As a matter of fact, increasing all three tested cytokinins levels led to enhancement of explant response frequency and regenerated shoot number. On the other side, WPM medium supplemented with 0.1 mgl−1 IBA was found suitable for rooting of regenerated shoots. RAPD molecular analysis revealed genetic stability of in vitro raised plants. In conclusion, individual application of BA, BAP and TDZ were in favor of S. avromanica direct shoot regeneration while treatment media with a combination of IBA and BA as well as 2,4-D and TDZ resulted in callogenesis in most explants. Finally, the in vitro raised plantlets were acclimatized and successfully established in the greenhouse conditions. Our developed protocol can be employed for the large-scale micropropagation and conservation of S. avromanica as a threatened medicinal plant.
Keywords: Lamiaceae, Micropropagation, Plant Growth regulators (PGRs), Plant conservation, Satureja avromanica
Introduction
Satureja is an important genus of the Lamiaceae family, which include 30 species, is primarily distributed in Mediterranean basin extended to Irano-Turanian geographical regions (Sefidkon and Jamzad 2005; Rechinger 1982). Among Satureja species, savory (Satureja hortensis L) is a famous medicinal and spice plant cultivated in many areas of the world which frequently are used as tea or additive in commercial and folk spice mixtures (Charles 2013).
Satureja avromanica Maroofi is a newly introduced perennial herb with violet flowers covered by papilla, yellowish sessile glands and long bracted with geographic distribution exclusively restricted to Kurdistan Mountains, West of Iran up to 800 ft (Maroofi 2010). S. avromanica is the only non-aromatic plant within about 3000 species of Lamiaceae family and just 150 individual plants have remained in the nature (Maroofi 2010). Therapeutic properties of S. avromanica are not well clarified however, it commonly is used in the folk remedies as a new source of medicinal bioactive compounds with human health benefits. S. avromanica stock is threatened in the wild as it being indiscriminately exploited. Low seed viability, poor germination and slow growth rate coupled with extensive harvesting are reasons for S. avromanica extinction and endangering of the natural population (Karimi et al. 2014).
In vitro conservation has proven to be an alternative tool for the rapid and efficient multiplication of rare and endangered medicinal and aromatic plant genotypes and in the present context, it can provide a continuous supply of plant materials from threatened germplasm species (Canter et al. 2006; Zhou and Wu 2006; Debnath, et al. 2006). To date, there has been an intensified effort to propagate and conserve medicinal plant species by in vitro culture techniques from various explant sources (Monemi et al. 2014; Gupta et al. 2014; Aremu et al. 2013; Siddique et al. 2010). Therefore, the main goal of the present study was to develop a holistic approach for in vitro multiplication and conservation of S. avromanica as a potential medicinal plant to obviate the dependence on the natural population for the supply of raw materials. In addition, individual and interactive effects of different auxin and cytokinins in the course of direct and indirect regeneration were studied.
Regarding the in vitro propagation of related taxa, Arrebola et al. (1997) reported shoot multiplication of S. obavata from axillary buds. In a very recent study, indirect organogenesis from callus using hypocotyls explants developed for the S. hortensis and S. avoromanica (Karimi et al. 2014) however, this report is also insufficient to meet the need in time. The present article establishes a platform to achieve high-throughput shoot multiplication and direct organogenesis of S. Avoromanica by testing different concentrations of BA, BAP and TDZ individually and combination of IBA with BA and 2,4-D with thidiazuron.
Material and methods
Wild plants of S.avromanica were collected from Rocky Mountains of Kurdistan province (Avraman region, Belbar village) in Iran. Prior to any experimentation, taxonomic verification was performed by comparison to voucher specimens at the agriculture and natural resources research center of Sanandaj, Iran. Initially, shoot tip explants with 1 to 2 mm length and leaf disc (0.5 cm diameter) explants, were washed with tap water containing 0.1 % Tween 20 (w/v) for 20–30 min. Then surface sterilization was performed by soaking explants in 70 % ethanol solution for 30 s and immediate immersion in silver nanoparticle solution (100 mgl−1) for 30 s. After that, the explants were washed thrice with sterile double-distilled water and aseptically implanted on the culture media. The cultures were then incubated at 25 ± 1 °C under a 16-h photoperiod at an irradiance intensity of 40 μmol m−2 s−1. All variables were evaluated after a period of 30 days.
Murashige and Skoog (1962) and Woody plant medium (Loyd and McCown 1981) were used as basal media. To provide carbon source, 30 gl−1 commercial sucrose was used. Before the addition of agar and autoclaving, the pH of prepared media was adjusted to 5.8 with 0.1 N NaOH or 0.1 N HCl. Two series of experiments were conducted. In the first experiment, to find out optimum hormonal level for maximum shoot induction and multiplication, the effect of eight concentrations (0, 0.1, 0.5, 1, 1.5, 2, 5 and 10 mgl−1) of BA, BAP and TDZ cytokinins were evaluated. In the second experiment, MS and WPM media were supplemented with BA (0, 2, 5, 10 mgl−1) together with IBA (0, 0.1, 0.5 and 1 mgl−1) and TDZ (0, 2, 5, 10 mgl−1) together with 2,4-D (0, 0.1, 0.5 and 1 mgl−1) respectively, to evaluate the effect of cytokinin/auxin combination on the S. avromanica callogensis and shoot regeneration. To initiate rooting, individual shoots with an average length of 2 cm were excised and cultured on half strength MS and WPM medium supplemented with various concentrations of IBA. Shoot multiplication variables (frequency of shoot regeneration, number of shoots per explant, main shoot length) and rooting variables (number of roots and root length) as well as callus initiation frequency were recorded at the end of the 30-d culture period. To eliminate all traces of sucrose and culture medium, well-rooted plantlets were washed thoroughly with sterile water and then transplanted into plastic pots containing peat moss, coco peat and perlite in the ratio of 1:1:1 covered with perforated polythene bags to maintain humidity. Well irrigated and hardened plants were transferred to the greenhouse and kept under the nursery conditions for further growth. The experiment performed with four shoots per jar, and five replicate in each jar. Mean values of the various treatments were statistically analyzed using analysis of variance (ANOVA) and means were compared by Duncan’s multiple-range test (DMRT) at the 5 % probability level using SAS software (Version 9.1).
For the assessment of genetic fidelity, DNA was extracted from leaf samples of three randomly selected greenhouse acclimatized plants (SA) as well as mother plant of S. avromanica (SAM) using Doyle and Doyle (1978) protocol. Random amplified polymorphic DNA (RAPD) analysis was carried out based on the method described by Williams et al. (1990). PCR reactions were performed in a 20 μl reaction mixture containing 5 ng template DNA using Reddy® PCR master mix (Cinaclone, Karaj, Iran) according to the supplier’s manual. Three decamer oligonucleotide RAPD primers pair (OPS1, OPS2 and OPS3) were used for PCR amplification.
Results and discussion
Shoot multiplication
To find out the effects of cytokinin type and concentration on shoot regeneration, shoot tip explants of S. avromanica were inoculated on MS and WPM media fortified with various concentrations (0.0–10 mgl−1) of BA, BAP and TDZ as cytokinin regulators (Table 1). Although the explants cultured on MS and WPM media lacking phytohormone had some background regeneration but S. avromanica shoot regeneration was more effectively occurred with the shoot tip explants using the most tested cytokinin concentrations. Initiation of these adventitious shoot buds after 3 weeks and 100 % regeneration rate of the explants in some treatments, proving successful establishment of shoot cultures. Among three different cytokinins types and eight applied concentrations, the highest observed direct shoot regeneration rate (6.21) were obtained with MS medium fortified with 2 mgl−1 BA, while the highest values of main shoot length (4.82 and 4.39 cm) were obtained with explants treated on MS medium supplemented with 5 mgl−1 (Table 1). The frequency of explant response and axillary shoot number increased progressively when BA, BAP and TDZ concentration was enhanced (Table 1). In general, cytokine could significantly influence the multiplication of S. avromanica, and in course of regenerated shoot number, TDZ was better than BAP but less effective than BA. Our results substantiate with previous studies, where BA was the best PGR for the stimulation of multiple shoot formation, e.g. Saturea obovata (Arrebola et al. 1997), Curculigo orchioides (Francis et al. 2007), Satureja horetnsis (Karimi et al. 2014). The mechanism for multiple shoot formation can be due to suppression of apical dominance which is a general role of cytokinins (Hwang et al. 2012).
Table 1.
Effect of different concentrations of BA, BAP and TDZ on shoot regeneration parameters from shoot tip explants of Satureja avoromanica after 30 days of culture on MS and WPM media containing 0.7 % agar and 3 % commercial sugar
| Cytokinin (mgl−1) | Frequency of response (%) | Axillary Shoots Number | Main Shoot Length (cm) | |||
|---|---|---|---|---|---|---|
| MS | WPM | MS | WPM | MS | WPM | |
| 0 | 15 | 10 | 1.33 ± 0.0N | 1 ± 0.0N | 0.95 ± 0.31U | 0.98 ± 0.35U |
| BA | ||||||
| 0.1 | 70 | 65 | 2.07 ± 0.1I-N | 1.84 ± 0.55J-N | 1.88 ± 0.4P-R | 0.91 ± 0.28U |
| 0.5 | 75 | 70 | 3.40 ± 0.5C-F | 2.61 ± 1.2F-K | 2.70 ± 0.45I-K | 1.46 ± 0.4T |
| 1 | 80 | 80 | 4.25 ± 1.0BC | 2.35 ± 0.9J-L | 2.65 ± 0.92J-L | 1.55 ± 0.28ST |
| 1.5 | 90 | 85 | 4.55 ± 0.8B | 2.76 ± 1.33E-J | 2.89 ± 0.96HI | 1.72 ± 0.59Q-S |
| 2 | 90 | 90 | 6.16 ± 2.2A | 3.33 ± 1.0DEF | 3.37 ± 1.1EF | 2.98 ± 0.27GH |
| 5 | 100 | 95 | 4.05 ± 0.8BCD | 2.21 ± 0.68H-M | 4.39 ± 1.35B | 2.45 ± 0.37LM |
| 10 | 100 | 100 | 3.55 ± 1.2CDE | 2.10 ± 0.55I-N | 3.87 ± 0.73C | 2.00 ± 1.11PO |
| BAP | ||||||
| 0.1 | 67 | 44 | 1.35 ± 0.35MN | 1.84 ± 0.92J-N | 1.91 ± 0.15P-Q | 0.87 ± 0.2U |
| 0.5 | 70 | 65 | 2.06 ± 0.25I-N | 2.00 ± 0.64I-N | 2.44 ± 0.17LM | 1.59 ± 0.36ST |
| 1 | 75 | 67 | 2.62 ± 0.7F-K | 1.92 ± 0.65J-N | 2.53 ± 0.34KL | 1.58 ± 0.6ST |
| 1.5 | 80 | 75 | 3.11 ± 1.0E-H | 2.47 ± 0.81G-K | 3.03 ± 1.33GH | 1.67 ± 0.73R-T |
| 2 | 89 | 80 | 3.27 ± 1.2D-G | 2.88 ± 1.05E-I | 2.98 ± 1.25GH | 2.03 ± 0.82PO |
| 5 | 100 | 95 | 1.80 ± 0.5K-N | 2.00 ± 1.22I-N | 4.30 ± 1.18B | 2.01 ± 1.04PO |
| 10 | 100 | 100 | 1.65 ± 0.45LMN | 1.85 ± 0.48J-N | 2.85 ± 1.05H-J | 1.87 ± 0.85P-R |
| TDZ | ||||||
| 0.1 | 50 | 42 | 1.57 ± 1.2LMN | 1.69 ± 0.28K-N | 2.89 ± 0.22HI | 1.91 ± 0.09P-Q |
| 0.5 | 67 | 63 | 2.26 ± 0.95HpM | 1.61 ± 0.2LMN | 3.18 ± 1.0FG | 2.11 ± 0.57NO |
| 1 | 72 | 65 | 3.56 ± 1.19CDE | 2.50 ± 0.76F-L | 3.61 ± 1.2D | 2.26 ± 032MN |
| 1.5 | 78 | 67 | 3.66 ± 1.15CDE | 2.88 ± 1.08E-I | 3.77 ± 1.2CD | 2.75 ± 0.87IJ |
| 2 | 83 | 75 | 4.77 ± 1.16B | 3.11 ± 1.17E-H | 4.82 ± 0.97A | 2.90 ± 0.66HI |
| 5 | 98 | 92 | 3.10 ± 1.2E-H | 1.94 ± 0.85I-N | 3.40 ± 1.11E | 2.75 ± 1.15IJ |
| 10 | 100 | 100 | 2.30 ± 0.6H-M | 1.85 ± 0.60J-N | 2.90 ± 0.81HI | 2.09 ± 1.3NP |
Means with the same letter for each parameter are not significantly different at the 5 % level according to DMRT
The Table 2 shows shoot regeneration of S. avromanica explants in response to different concentrations of BA/IBA and TDZ/2,4-D combinations. In all cases, presence of IBA and 2,4-D auxins in conjunction with BA, BAP and TDZ cytokinins, decreased percentage of explants with direct shoot regeneration, while most cultured explant exhibited callogenesis (see following). Commonly, the auxin/cytokinin proportion determine the fate of explant morphogenesis (Hwang et al. 2012). Individual application of BA, BAP and TDZ were in favor of S. avromanica direct shoot regeneration whereas treatment media with combination of IBA and BA as well as 2,4-D and TDZ resulted in callogenesis in most explants. As shown in the Fig. 2g, we could obtain plantlet bearing flowers which produced viable seeds. This is worthy when acclimatization process is difficult for species with herbaceous leaf and stem.
Table 2.
Effect of various BA/IBA and 2,4-D/TDZ combination on shoot regeneration parameters from leaf disc explants of Satureja avoromanica after 30 days of culture on MS and WPM media containing 0.7 % agar and 3 % commercial sugar
| Concentrations (mgl−1) | Frequency of response (%) | Axillary Shoot number | Main shoot length | ||||
|---|---|---|---|---|---|---|---|
| Cytokinin | Auxin | MS | WPM | MS | WPM | MS | WPM |
| 0 | 0 | 15 | 10 | 1.00 ± 0.00G | 1.33 ± 1.00E-G | 0.98Q | 0.98Q |
| BA | IBA | ||||||
| 1 | 0.1 | 35 | 15 | 1.71 ± 0.37B-G | 1.33 ± 0.2E-G | 2.31 ± 1.2H-K | 2.40 ± 1.2H-I |
| 0.5 | 65 | 5 | 1.53 ± 0.88C-G | 1.88 ± 0.00A-G | 1.74 ± 1.2L-P | 1.83 ± 0.00L-O | |
| 1 | 70 | 0 | 1.31 ± 0.42E-G | – | 1.59 ± 1.2N-P | – | |
| 2 | 0.1 | 90 | 25 | 2.00 ± 0.86A-G | 1.80 ± 0.3B-G | 2.33 ± 0.2H-K | 2.54 ± 0.62GI |
| 0.5 | 85 | 0 | 1.47 ± 0.75C-G | – | 2.27 ± 0.56I-K | – | |
| 1 | 80 | 0 | 1.31 ± 0.82E-G | – | 1.96 ± 0.45KN | – | |
| 5 | 0.1 | 100 | 35 | 2.26 ± 1.2A-E | 2.28 ± 1.00A-E | 2.94 ± 0.30DF | 3.09 ± 0.78CD |
| 0.5 | 90 | 0 | 2.00 ± 1.2A-G | – | 2.56 ± 0.50FI | – | |
| 1 | 90 | 0 | 1.58 ± 1.2C-G | – | 2.00 ± 0.85K-M | – | |
| 10 | 0.1 | 95 | 30 | 2.44 ± 0.6A-D | 2.16 ± 1.10A-F | 3.37 ± 0.40C | 3.22 ± 0.90CD |
| 0.5 | 90 | 0 | 1.94 ± 0.62A-G | – | 2.46 ± 1.15HI | – | |
| 1 | 75 | 0 | 1.60 ± 0.15C-G | – | 1.50 ± 0.45OP | – | |
| TDZ | 2,4-D | ||||||
| 1 | 0.1 | 15 | 25 | 1.66 ± 0.50B-G | 1.80 ± 0.20B-G | 1.63 ± 0.15M-P | 3.02 ± 1.05CE |
| 0.5 | 0 | 45 | – | 1.20 ± 0.25F-G | – | 2.92 ± 0.65DG | |
| 1 | 0 | 50 | – | 1.45 ± 0.25D-G | – | 2.67 ± 0.60EG | |
| 2 | 0.1 | 20 | 90 | 2.25 ± 1.00A-E | 2.66 ± 0.35AB | 1.72 ± 0.17L-P | 3.20 ± 0.35CD |
| 0.5 | 0 | 80 | – | 1.72 ± 0.11B-G | – | 2.86 ± 0.65DG | |
| 1 | 0 | 80 | – | 1.61 ± 0.15C-G | – | 2.03 ± 0.55JL | |
| 5 | 0.1 | 35 | 100 | 2.14 ± 0.75A-F | 2.85 ± 0.56A | 2.53 ± 0.29GI | 4.28 ± 1.25A |
| 0.5 | 0 | 85 | – | 2.23 ± 0.28A-E | – | 2.97 ± 0.75DE | |
| 1 | 0 | 80 | – | 1.82 ± 0.13B-G | – | 2.68 ± 1.2E-H | |
| 10 | 0.1 | 30 | 95 | 2.50 ± 0.75A-C | 2.50 ± 0.20A-C | 3.88 ± 0.45B | 4.34 ± 1.1A |
| 0.5 | 0 | 80 | – | 1.18 ± 0.30F-G | – | 2.68 ± 0.85GH | |
| 1 | 0 | 70 | – | 1.10 ± 0.15G | – | 1.39 ± 0.70P | |
Means with the same letter for each parameter are not significantly different at the 5 % level according to DMRT
Fig. 2.
a Initial plant material were obtained from individual wild plant of S. avromanica grown on rocks (Belbar village, Avraman region, Kurdistan province, Iran). b-c Regenerative soft, friable and yellowish callus formed on leaf explant grown on WPM fortified with 5 mgl−1 TDZ and 1 mgl−1 2, 4-D. d Indirect shoot regeneration from callus grown on 5 mgl−1 TDZ and 1 mgl−1 2, 4-D e Multiple shoot formation on MS medium supplemented with 2 mgl−1 BA and 3 % sucrose. f Rooting well-developed shoots on MS medium containing 0.1 mgl−1 IBA. g Flowering in vitro originated plantlet of S. avromanica
Callus induction
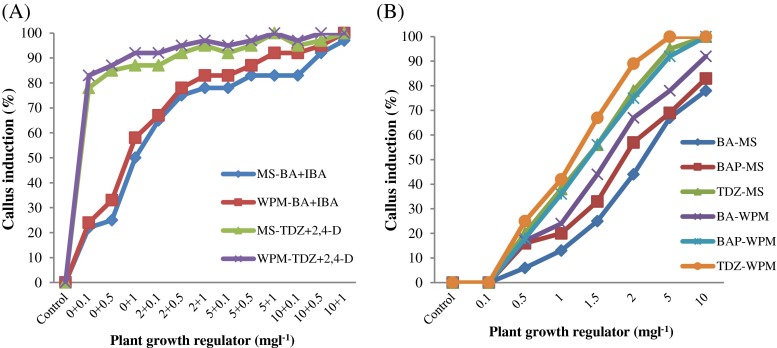
Callogenesis was efficiently occurred by application of high concentrations of BA, BAP and TDZ individually or BA/IBA and TDZ/2,4-D combinations (Fig. 1). As it was expected, no callus formation took place with leaf explants cultured on MS medium without the plant growth regulator (control) as well as in media containing 0.1 mgl−1 of BA, BAP and TDZ (Fig. 1). The highest mean frequency of callus initiation (100 %) was achieved on the WPM medium containing 5 and 10 mgl−1 TDZ and 1 mgl−1 2,4-D. Like shoot direct regeneration, the frequency of callus formation was also increased progressively when BA, BAP and TDZ concentration improved (Fig. 1a). On the other side, low frequency of callus occurrence on MS and WMP media was observed when BA, BAP and TDZ were used separately at low concentrations. The appearance, size and color of the obtained calli varied depending on the type of the applied cytokinin and based on the auxin:cytokinin ratio. Calli originated from leaf explants on both MS and WPM media fortified with TDZ and 2,4-D were friable with light yellow color (Fig. 2b and c) which could regenerate successfully (D). In contrast, the calli developed on the other media were hard or semi-hard with milky color which failed to regenerate. In many studies, 2,4-D as an effective synthetic auxin could induce and maintain highly regenerative callus growth and it have been used individually or in combination with several cytokinins such as BA, BAP, and TDZ in Nerium odorum (Rashmi and Trivedi 2014), Chamaecytisus purpureus and Chamaecytisus austriacus (Greinwald and Czygan 2014) and Dendrobium huoshanensei (Lee and Chen 2014).
Fig. 1.
Callus induction from leaf explants grown on MS and WPM media affected by a Different concentration of BA, BAP and TDZ and b Different concentration of BA/BAP and TDZ/2,4-D combinations
Root induction
The obtained microshoot cuttings were cultured on MS and WPM media supplemented with 0, 0.1, 0.2, 0.5 and 1 mgl−1 IAA. Table 3 summarizes the effect of different concentrations of IBA on rooting parameters including frequency of rooting (%), main root length (cm) and lateral root number. The maximum frequency of rooting (93 %) was achieved at 0.1 mgl−1 IBA with WPM medium, while shoots cultured on MS medium without IBA (control) exhibited only 46 % rhizogenesis. The highest value of root length (1.83) and root number (2.92) were obtained by using MS and WPM containing 0.1 mgl−1 IBA, respectively. Results also revealed a slight inhibitory effect of high IBA level on rooting, which has been proposed to be a general phenomenon (Swarna and Ravindhran 2012). Optimal rhizogenesis using IBA has been shown for other plant species, including Viola pilosa (Soni and Kaur 2014), Talinum triangulare (Swarna and Ravindhran 2012) and Teucrium stocksianum (Bouhouche and Ksiksi 2007).
Table 3.
Effect of different IBA concentration on rooting of in vitro originated microshoots of Satureja avromanica on MS and WPM media containing 0.7 % agar and 3 % commercial sugar
| IBA (mgl−1) | Frequency of rooting (%) | Main root Length (cm) | Lateral root Number | |||
|---|---|---|---|---|---|---|
| MS | WPM | MS | WPM | MS | WPM | |
| 0 | 27 | 46 | 1.49 ± 0.43A | 1.49 ± 0.28A | 2.37 ± 0.43C | 2.37 ± 0.50B |
| 0.1 | 56 | 93 | 1.83 ± 0.05B | 1.52 ± 0.25A | 2.71 ± 0.83A | 2.92 ± 0.24A |
| 0.5 | 50 | 83 | 1.74 ± 0.22B | 0.91 ± 0.25B | 2.49 ± 0.61B | 2.43 ± 0.43AB |
| 1 | 44 | 75 | 1.69 ± 0.21B | 0.87 ± 0.24B | 2.47 ± 0.68B | 1.36 ± 0.29C |
| 1.5 | 38 | 64 | 1.12 ± 0.24C | 0.81 ± 0.23B | 2.11 ± 0.43C | 1.61 ± 0.26C |
Means with the same letter for each parameter are not significantly different at the 5 % level according to DMRT
Acclimatization of in vitro-derived plants
The in vitro-derived plantlets with fully expanded leaves and well-developed roots were removed from the medium after 45 days of culture and treated with fungicide and washed in sterile double distilled water. The rooted plantlets were successfully acclimatized and eventually established in plastic pots containing peat moss, coco peat and perlite in the ratio of 1:1:1 under greenhouse condition. Of 100 plants transferred to greenhouse, 60 plants survived showing a survival rate of 60 % which was lower than Isodon wightii with 70 % (Thirugnanasampandan et al. 2010), Teucrium stocksianum with 75–80 % (Bouhouche and Ksiksi 2007), and Justicia gendarussa with 90 % of survival rate (Dennis Thomas and Yoichiro 2010).
Assessment of genetic stability
From three studied primers (OPS1, OPS2 and OPS3), 45 distinguishable amplified bands were generated. Each primer generated a unique set of amplification products ranging in size from 250 to 3000 bp. Based on RAPD analysis, there was no polymorphism band between mother plant and in-vitro regenerated plantlets. (Fig. 3). There are numerous reports on confirmation of the genetic fidelity of in vitro raised plantlets using RAPD fingerprints (Devi et al. 2013; Rawat et al. 2013; Sharma et al. 2009).
Fig. 3.
RAPD amplification profiles with primers OPS1, OPS2, and OPS3. M 1 kb molecular weight marker; SAM S. avromanica mother plant; SA1-SA3 in-vitro raised hardened plants. As shown, there is no polymorphism band between S. avromanica mother plant and in-vitro grown counterparts
Conclusion
As a matter of fact, our protocol for in vitro propagation of S. avromanica can not only be helpful in supplying a continuous source of plant material for S. avromanica conservation, but also provide an alternative to environmentally and economically unwise random harvesting of plants from the wild. In the course of S. avoromanica domestication process, obtained plants can be further grown in the glasshouse or field to produce seed which then may be dispersed to establish new populations in the habitat nature S. avromanica. Although, further study also is needed to evaluate S. avromanica active bio-compounds and phytochemicals of ex situ and in vitro grown populations.
Abbreviations
- 2,4-D
2,4-Dichlorophenoxyacetic acid
- BA
6-Benzyladenine
- BAP
6-Benzylaminopurine
- IBA
Indole-3-Butyric acid
- MS
Murashig and Skoog
- TDZ
Thidiazuron
- WPM
Woody plant medium
References
- Aremu AO, Gruz J, Subrtova M, Szucova L, Dolezal K, Bairu MW, Finnie JF, Van Staden J. Antioxidant and phenolic acid profiles of tissue cultured and acclimatized Merwilla plumbea plantlets in relation to the applied cytokinins. J Plant Physiol. 2013;170:1303–8. doi: 10.1016/j.jplph.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Arrebola ML, Socorro O, BarcelÒ-Muńoz A, SimÒn-Pérez E, Pliego-Alfaro F. Micropropagation of Satureja obovata Lag. Hortic Sci. 1997;32:1278–1280. [Google Scholar]
- Bouhouche N, Ksiksi T. An efficient in vitro plant regeneration system for the medicinal plant Teucrium stocksianum Boiss. Plant Biol Rep. 2007;1:179–184. [Google Scholar]
- Canter PH, Thomas T, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2006;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Charles DJ (2013) Savory. In: Charles DJ (ed) Antioxidant Properties of Spices, Herbs and Other Sources, , Springer Science + Business Media, New York, pp 531–536
- Debnath M, Malik CP, Bisen PS. Micropropagation: a tool for the production of high quality plant-based medicines. Curr Pharm Biotechnol. 2006;7(1):33–49. doi: 10.2174/138920106775789638. [DOI] [PubMed] [Google Scholar]
- Devi SP, Kumaria S, Rao SA, Tandon P. In vitro propagation and assessment of clonal fidelity of Nepentheskhasiana Hook. f.: a medicinal insectivorous plant of India. Acta Physiol Plant. 2013;35:2813–2820. doi: 10.1007/s11738-013-1314-x. [DOI] [Google Scholar]
- Francis SV, Senapati SK, Rout GR. Rapid clonal propagation of Curculigo orchioides Gaertn., an endangered medicinal plant. In Vitro Cell Dev Biol. 2007;43:140–143. doi: 10.1007/s11627-007-9041-x. [DOI] [Google Scholar]
- Greinwald R, Czygan FC. Regeneration of Plantlets from Callus Cultures of Chamaecytisus purpureus and Chamaecytisus austriacus (Leguminosae) Plant Biol. 2014;104:64–67. [Google Scholar]
- Gupta AK, Harish Rai MK, Phulwaria M, Agarwal T, Shekhawat NS. In vitro propagation, encapsulation, and genetic fidelity analysis of Terminalia arjuna: a cardioprotective medicinal tree. Appl Biochem Biotechnol. 2014;173(6):1481–94. doi: 10.1007/s12010-014-0920-4. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annu Rev Plant Biol. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- Karimi N, Ghasmpour HR, Yari M. Effect of different growth regulators on callus induction and plant regeneration of Satureja species. Ann Res Rev Biol. 2014;16:2646–2654. doi: 10.9734/ARRB/2014/7938. [DOI] [Google Scholar]
- Lee PL, Chen JT. Plant regeneration via callus culture and subsequent in vitro flowering of Dendrobium huoshanense. Acta Physiol Plant. 2014;36:2619–2625. doi: 10.1007/s11738-014-1632-7. [DOI] [Google Scholar]
- Lloyd G, McCown BH. Commercially-feasible micropropagation of Mountain Laurel, Kalmia latifolia, by shoot tip culture. Proc Int Plant Prop Soc. 1981;30:421–427. [Google Scholar]
- Maroofi H. Two new plant species from Kurdistan province, West of Iran. Iran J Bot. 2010;16(1):76–80. [Google Scholar]
- Monemi MB, Kazemitabar SK, Khaniki GB, Yasari E, Sohrevardi F, Pourbagher R. Tissue culture study of the medicinal plant leek (Allium Ampeloprasum L) Int J Mol Cell Med. 2014;3:118–25. [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Rashmi R, Trivedi MP. Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl Biochem Biotechnol. 2014;172:2562–70. doi: 10.1007/s12010-013-0693-1. [DOI] [PubMed] [Google Scholar]
- Rawat JM, Rawat B, Agnihotri RK, Chandra C, Nautiyal C. In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: a threatened medicinal herb. Acta Physiol Plant. 2013;35:2589–2599. doi: 10.1007/s11738-013-1294-x. [DOI] [Google Scholar]
- Rechinger KH (1982) Satureja. Flora Desiranischen Hoclandes and der Umrahmenden Gebirge, vol. 150. Akademische Druku Verlags Antalt Graz, Austria, pp.495–504
- Sefidkon F, Jamzad Z. Chemical composition of the essential oil of three Iranian Satureja species (S. mutica, S. macrantha and S. intermedia) Food Chem. 2005;91:1–4. doi: 10.1016/j.foodchem.2004.01.027. [DOI] [Google Scholar]
- Sharma M, Rai SK, Purshottam DK, Jain M, Chakrabarty D, Awasthi A, Nair KN, Sharma AK. In vitro clonal propagation of Clerodendrum serratum (Linn.) Moon (barangi): a rare and threatened medicinal plant. Acta Physiol Plant. 2009;31:379–383. doi: 10.1007/s11738-008-0245-4. [DOI] [Google Scholar]
- Siddique I, Anis M, Aref IM. In vitro adventitious shoot regeneration via indirect organogenesis from petiole explants of Cassia angustifolia Vahl. -A potential medicinal plant. Appl Biochem Biotechnol. 2010;162:2067–74. doi: 10.1007/s12010-010-8982-4. [DOI] [PubMed] [Google Scholar]
- Soni M, Kaur R. Rapid in vitro propagation, conservation and analysis of genetic stability of Viola pilosa. Phys Mol Biol Plants. 2014;20:95–101. doi: 10.1007/s12298-013-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarna J, Ravindhran R. In vitro propagation and assessment of genetic integrity of Talinum triangulare (Jacq.) Willd: a valuable medicinal herb. Acta Physiol Plant. 2012;34:1987–1996. doi: 10.1007/s11738-012-0999-6. [DOI] [Google Scholar]
- Thirugnanasampandan R, Mahendran G, Narmatha Bai V. High frequency in vitro propagation of Isodon wightii (Bentham) H. Hara. Acta Physiol Plant. 2010;32:405–109. doi: 10.1007/s11738-009-0401-5. [DOI] [Google Scholar]
- Thomas TD, Yoichiro H. In vitro propagation for the conservation of a rare medicinal plant Justicia gendarussa Burm. f. by nodal explants and shoot regeneration from callus. Acta Physiol Plant. 2010;32:943–950. doi: 10.1007/s11738-010-0482-1. [DOI] [Google Scholar]
- Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LG, Wu JY. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat Prod Rep. 2006;23(5):789–810. doi: 10.1039/b610767b. [DOI] [PubMed] [Google Scholar]