Abstract
Henckelia incana is an endemic medicinal plant used for the treatment of fever and skin allergy. In the present study shoot regeneration was evaluated on Murashige and Skoog’s (MS) medium supplemented with auxins, Indole-3-acetic acid (IAA), Indole-3- butyric acid (IBA), 1-Naphthaleneacetic acid (NAA), 2, 4-Dichlorophenoxyacetic acid (2, 4-D) and cytokinins, 6-Benzylaminopurine (BAP) and Kinetin (Kn) at concentrations of 0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 mgl–1. MS medium with IBA (18.08), NAA (17.83) and IAA (17.58) at 0.5 mgl–1 concentrations showed efficient regeneration. Regenerated shoots were rooted on half-strength MS medium with and without 0.5 mgl–1 IBA or NAA. The plantlets were successfully hardened in rooting trays (peat, vermiculite and sand) and transferred to field mileu. The genetic fidelity of in vitro raised plants was assessed by using three different single primer amplification reaction (SPAR) markers namely random amplified polymorphic DNA (RAPD), inter-simple sequence repeat (ISSR) and direct amplification of mini-satellite DNA region (DAMD). The results consistently demonstrated true-to-true type propagation. This is the first report of in vitro propagation and establishment of true-to-true type genetic fidelity in H. incana.
Keywords: Henckelia incana, Auxin, SPAR, Genetic fidelity
Introduction
Henckelia incana (Vahl) Spreng., (Syn. Didymocarpus tomentosa Wight) of Gesneriaceae is a rare and endemic rosulate herb found in the peninsular hills of India. The plant is attractive with pleasant bluish-purple flowers in corymbs. It is an ethno-medicinal species, leaves are used in folk medicine for treating fever and skin allergy (Kottaimuthu 2008; Kholkute 2009; Sathyavathi and Janardhanan 2011). This species has been very less studied probably owing to its restricted availability in the rocky hills. It is available only for 3–4 months soon after the onset of monsoon.
Many Gesneriaceae plants are capable of forming a very high number of adventitious shoots from the epidermis of small organ fragments in vitro (Grunewaldt 1977). Among the members of Gesnericaeae, efficient regeneration protocols have been established in Achimenes (Vlahos et al. 1995), Saintpaulia (Lo 1997), Didymocarpus (Krishnarajah et al. 2002; Shaib et al. 2004), Kholeria (Kozak et al. 2007), Chirita (Tan et al. 2009), Aeschynanthus (Cui et al. 2009), Ramonda (Dontcheva et al. 2009), Streptocarpus (Wei et al. 2010), Metabriggsia (Ma et al. 2011), Titanotrichum (Takagi et al. 2011), Sinningia (Park et al. 2012), Primulina (Qin et al. 2013) and Lysionotus (Li et al. 2013).
The efficient micropropagation system calls for the genetic stability of in vitro regenerated plantlets. The regenerated plantlets though appear to be phenotypically similar to the mother plant need not necessarily possess the same genotype as various growth factors and culture conditions cause changes in their genotypes leading to somaclones. Therefore, determining the genetic fidelity of in vitro raised plants is prerequisite for accepting the regeneration protocol for mass propagation. The genetic fidelity of in vitro cultured plants had been assessed efficiently by using RAPD, ISSR and DAMD markers (Devi et al. 2014; Shilpha et al. 2014).
H. incana being a rare, endemic and a traditional medicinal plant is of greatest conservation interest. Several factors like habitat destruction or alteration, competition and ecological changes may in future destroy the very existence of this important species. Further, it is essential to note that, to date, in vitro propagation protocols are not established for H. incana. In order to conserve and to ensure sustainable usage, it is important to establish an efficient in vitro propagation protocols. The present study was undertaken to establish an efficient in vitro plant regeneration protocol and determine the clonal fidelity of in vitro raised plants using molecular markers.
Materials and methods
Plant material and explant sterilization
H. incana plant materials were collected from its natural habitat-Savandurga hills, about 50 Km west of Bangalore and were maintained in the greenhouse. The plant was identified using the Flora of the Palni Hills, South India (Matthew 1999) and further authentication was done by the National Ayurveda Dietetics Research Institute, Bangalore with voucher specimen number RRCBI/MCW/09.
Healthy leaves were excised and thoroughly washed in tap water for 5 min, surface sterilized in 2 % Bavistin (along with 2–3 drops of Tween-20) for 30 min, washed and treated with 70 % ethanol for 45 s, followed by 2–3 times rinsing in sterile water for 5 min each (Siva et al. 2009, 2012). It was further sterilized using 0.1 % HgCl2 for 6 min in LAF and rinsed three times with sterilized double distilled water. The explants were trimmed and cut into 6 cm2 size and inoculated with the abaxial side of leaf in contact with the medium.
Nutrient medium and plant growth regulators (PGRs)
The leaf explants were cultured onto MS medium supplemented individually with IAA, IBA, NAA, 2, 4-D, BAP and Kn (0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 mgl–1) to test the efficiency of shoot regeneration.
Cultures were incubated under fluorescent lights at 25 °C, the humidity was maintained at 85 % under a photoperiod of 16 h light (25 μmol s−1 m−1) and 8 h dark. After 4 weeks of incubation, percentage of shoot regeneration and number of shoots per explants were assessed.
Growth and multiplication
After shoot induction the cultures were allowed to grow on the same media or on MS media. Huge clusters of shoots were formed which were excised into smaller clusters and sub cultured.
Rooting and acclimatization
Healthy shoots with minimum 4–6 leaves were transferred on to half-strength and full strength MS media either alone or supplemented with 0.5 mgl–1 IBA or 0.5 mgl–1 NAA for rooting. The percentage of rooting was recorded after 3 weeks of culture. Further, plantlets were allowed to grow for another 2 weeks on the same media and well developed plantlets were transferred to seed trays containing a mixture of peat, vermiculite and sand in equal proportion and covered with plastic bags to maintain high humidity. The plastic bags were partially opened after 2 weeks and further opening was gradual so as to allow the plants to acclimatize slowly to the ambient conditions. After 4 weeks of transfer, plantlets were evaluated for their survival capacity.
Clonal fidelity
Genomic DNA was extracted from the micropropagated as well as the mother plant using the CTAB (Cetyl trimethyl ammonium bromide) method as modified by Murray and Thomson (1980). The DNA was dissolved in 1 ml of 1X TE buffer and stored at −20 °C. In vitro amplification using PCR was performed in a BioRad thermocycler with primers in a total volume of 20 μl each, containing 2.0 μl of 50 ng template DNA, 2.0 μl of 10 X PCR buffer containing 15 mM MgCl2, 2.0 μl of 10 mM dNTPs, 2.0 μl of 100 pM primer, 0.5 μl of Taq DNA polymerase and 11.5 μl of sterile water. Amplification was performed using the temperature profile as initial DNA denaturation of one cycle (at 94 °C for 5 min) followed by 40 cycles of denaturation (at 94 °C for 45 s), primer annealing (at varying temperature) and elongation (at 72 °C for 2 min) and one cycle of the final extension (at 72 °C for 7 min). Ten RAPD primers (OPD and OPE series), 20 ISSR primers and 18 DAMD primers were used in the present study.
Statistical analyses
Data were subjected to one-way ANOVA and means were separated using Duncan’s multiple range test at a significance level of P ≤ 0.05.
Results and discussion
Effects of PGR concentrations on shoot proliferation
The leaf explants of H. incana cultured on growth regulator-free MS medium and on MS medium supplemented with different PGRs gave significant responses for frequency of plant regeneration (%) and number of shoots per explant.
Effect of auxins
The effect of IAA, IBA, NAA and 2, 4- D on adventitious shoot formation on the leaf explants of H. incana are presented in Table 1. Direct adventitious shoot proliferation was stimulated by all the auxins simultaneously at the fourth week except 2, 4-D (3–5 mgl–1) and NAA (2–5 mgl–1). Among the four auxin treatments, all the six concentrations of IBA and IAA (0.5, 1–5 mgl–1) and two concentrations of NAA (0.5 and 1 mgl–1) were productive both in terms of frequency of plant regeneration and total number of shoots per explant. The frequency of plant regeneration was high and varied from 100 to 91.7 %. Likewise, the number of shoots per explant was high and varied from 18 to 13.25. The highest regeneration and multiple shoots were observed in 0.5 mgl–1 IBA. Equally significant results were observed in 0.5 mgl–1 NAA and IAA. These results suggest that low concentration of auxins favors higher shoot proliferation in H. incana. In the same way, induction of shoot formation by auxins has been reported in other gesneriads (Appelgren and Helde Ola 1972; Godo et al. 2010; Ma et al. 2011).
Table 1.
Effect of auxins on adventitious shoot proliferation in leaf explants of H. incana a
| Plant Growth regulators (PGRs) | Concentration (mg l−1) | Percentage response (%) | Mean number of shoots per explantb |
|---|---|---|---|
| Control | 0.0 | 100 | 15.67de |
| IBA | 0.5 | 100 | 18.08a |
| 1.0 | 91.66 | 17.50abc | |
| 2.0 | 95.68 | 17.08abcd | |
| 3.0 | 95.68 | 16.17cde | |
| 4.0 | 91.66 | 15.75de | |
| 5.0 | 91.66 | 15.67de | |
| IAA | 0.5 | 95.68 | 17.58abc |
| 1.0 | 95.68 | 17.58abc | |
| 2.0 | 95.68 | 16.92abcd | |
| 3.0 | 95.68 | 16.50bcde | |
| 4.0 | 91.66 | 16.00de | |
| 5.0 | 91.66 | 15.25e | |
| NAA | 0.5 | 95.68 | 17.83ab |
| 1.0 | 91.66 | 13.25f | |
| 2.0 | 14.60 | 4.08g | |
| 3.0 | 45.76 | 3.17gh | |
| 4.0 | 33.28 | 1.42ij | |
| 5.0 | 41.60 | 1.25ij | |
| 2, 4-D | 0.5 | 37.44 | 2.67hi |
| 1.0 | 37.44 | 1.25ij | |
| 2.0 | 14.60 | 1.17ij | |
| 3.0 | 0 | 0.00k | |
| 4.0 | 0 | 0.00k | |
| 5.0 | 0 | 0.00k |
a Data were collected after 4 weeks of culture
bMean values followed by the same letter are not significantly different according to Duncan’s multiple range test at P ≤ 0.05
In addition, direct shoot regeneration was observed in lower concentration of 2, 4-D (0.5–2 mgl–1). The frequency of plant regeneration and total number of shoots per explants were not significant. Besides the time taken by these explants for shoot induction was almost double (7–8 weeks) as against other auxins.
The production of shoots in large numbers directly on the leaf explants was observed without active callusing. This indicates its high in vitro regeneration capacity. The development of shoots directly from the surface of leaves without callus formation has also been reported in a number of Gesneriaceae plants (Vazquez et al. 1977; Wuttisit and Kanchanapoom 1996; Ma et al. 2011; Li et al. 2013).
In case of other concentrations of 2, 4-D (3–5 mgl–1) and NAA (2–5 mgl–1), direct shoot proliferation was observed after a brief phase of callogenesis and rhizogenesis respectively. Both these phases were observed for short duration (2–3 weeks) and proliferation of adventitious shoots was observed immediately all over the explants. The frequency of plant regeneration and total number of shoots per explants was comparatively far lesser than that observed in other auxin treatments. Therefore shoot proliferation in all the concentrations of 2, 4-D and 2–5 mgl–1 of NAA were considered to be low. Similar callus formation before shoot proliferation has been reported in some Gesneriaceaea species (Godo et al. 2010; Takagi et al.2011). The roots that occurred on the explants were short and hairy, which corroborates the earlier reports from other Gesneriaceae species (Appelgren and Helde Ola 1972; Lo 1997; Tóth et al. 2004).
Effect of cytokinins
Leaf explants on MS media supplemented with various concentrations of cytokinins, did not show regeneration till the fourth week as was the case in auxin treatments. As the incubation time progressed to 7–8 week shoots were proliferated on few explants which slightly increased over a period of time (Table 2). Moreover, shoot proliferation in control was more significant than all the concentrations of cytokinins used, which demonstrates that explants have high levels of endogenous cytokinins sufficient enough for shoot regeneration. These observations are in conformity with some gesneriads like Achimenes longiflora (Vlahos 1989) and Streptocarpus (Appelgren and Helde Ola 1972).
Table 2.
Effect of cytokinins on adventitious shoot proliferation in leaf explants of H. incana a
| Plant Growth regulators (PGRs) | Concentration (mg l−1) | Percentage response (%) | Mean number of shoots per explantb |
|---|---|---|---|
| Control | 0.0 | 100 | 15.92a |
| BAP | 0.5 | 95.68 | 7.42d |
| 1.0 | 95.68 | 8.25d | |
| 2.0 | 95.68 | 8.50d | |
| 3.0 | 91.66 | 9.59d | |
| 4.0 | 91.66 | 11.66b | |
| 5.0 | 91.66 | 10.12c | |
| Kn | 0.5 | 81.36 | 7.92d |
| 1.0 | 91.66 | 7.67d | |
| 2.0 | 91.66 | 8.17d | |
| 3.0 | 91.66 | 7.83d | |
| 4.0 | 91.66 | 2.75e | |
| 5.0 | 79.17 | 2.17e |
a Data were collected after 7 weeks of culture
bMean values followed by the same letter are not significantly different according to Duncan’s multiple range test at P ≤ 0.05
The response of cytokinins on shoot regeneration in general was slow and less when compared to both the control and most of the auxin treatments. Leaf explants showed direct shoot organogenesis without passing through callus phase. The frequency of plant regeneration varied from 95.8 to 79.2 %, while the average number of shoots per explant varied from 11.6 to 2.2. The highest number of shoots per explant among the cytokinins was recorded on 4 mgl–1 BAP with an average of 11.7 shoots.
Growth and multiplication
Shoot proliferation was observed in most of the treatments, though the time, frequency of regeneration and number of shoots per explants varied. Shoot buds developed from the cut ends of leaf explants at fourth week and subsequently after a period of 3–4 weeks multiple shoots started to appear all over the explants (Fig. 1a). By 9–10 weeks of culture multiple shoots were well developed to an extent which covered the entire explant to form clusters (Fig. 1b). As the culture time progressed, new shoots were continuously formed and a combination of micro-shoots and well developed macro-shoots were observed within each cluster. Some of the leaves in the culture which came in contact with the media also started producing new shoots. As a result, the number of shoots per cluster increased phenomenally. Thereafter huge clusters were cut into smaller ones and sub cultured onto the same media or on growth regulator-free MS medium. By the end of 15–18 weeks most of the shoots showed further development and were considered for rooting (Fig. 1c). Therefore, in vitro regeneration of shoots was successfully established and was a continuous process in this plant.
Fig. 1.
Shoot proliferation, development and plantlet establishment from leaf explants of H. incana (a) Shoot proliferation from leaf explants after 4 weeks of culture (b) Large clusters of multiple shoots on leaf explants after 10 weeks of culture (c) Fully developed cluster after 15 weeks of culture (d) Rooted shoots after 5 weeks of transfer to rooting media (e) Plantlets growing in seed tray containing equal portion of peat, vermicompost and sand after 3 weeks of transfer (f) Plantlet acclimatized in outside environment
Rooting and acclimatization
The regenerated shoots successfully started to produce roots from the third week onwards. A fastest root development with 100 % response occurred on half MS medium, with or without 0.5 mgl–1 IBA or 0.5 mgl–1 NAA (Table 3). Park et al. (2012) previously reported similar results that half MS without growth regulators induced rooting in gloxinia after 3 weeks with more than 95 % of shoots producing roots. Likewise, Godo et al. (2010) reported that in Lysionotus pauciflorus the regenerated shoots exhibited good rooting on growth regulator-free half MS medium.
Table 3.
Influence of MS and half MS medium on rooting of regenerated shootsa
| Culture media | Percentage of rooted shootsb |
|---|---|
| ½ MS | 100a |
| ½ MS + 0.5 mg l−1 NAA | 100a |
| ½ MS + 0.5 mg l−1 IBA | 100a |
| MS | 10.33d |
| MS + 0.5 mg l−1 NAA | 18.33c |
| MS + 0.5 mg l−1 IBA | 25.00b |
a Data were collected at 3rd week of culture
bMean values followed by the same letter are not significantly different according to Duncan’s multiple range test at P ≤ 0.05
Full strength MS medium alone and that with 0.5 mgl–1 of IBA or 0.5 mgl–1 NAA too induced rooting initially, although with less frequency (10.3 to 25.0 %) and shortly by the next couple of weeks all the shoots successfully rooted. Roots were robust and healthy, cylindrical and long without much lateral roots (Fig. 1d). Therefore, rooting was induced in all the treatments between 3 and 5 weeks. The plantlets were further maintained in the same medium for another 2–3 weeks. Thereafter, rooted plants were washed thoroughly in tap water to remove the traces of agar and transplanted onto seedling trays filled with equal proportion of peat, vermicompost and sand (Fig. 1e). The plants were covered with plastic bag to maintain high humidity and were given 16 h photoperiod. The cover was removed completely after 4 weeks to allow the plantlets to acclimatize to the ambient conditions. The regenerated plants survived at a rate of 70 % with normal growth and morphological characteristics (Fig. 1f).
Clonal fidelity
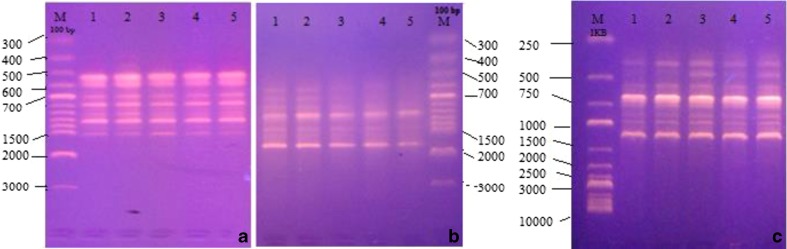
To confirm the genetic stability, DNA was isolated from both micropropagated and mother plants. Three PCR-based primers RAPD, ISSR and DAMD were used to test the clonal fidelity. Out of 10 RAPD, 20 ISSR and 18 DAMD primers screened, only 4 RAPD, 3 ISSR and 3 DAMD primers produced clear and distinct bands. The RAPD, ISSR and DAMD profiles generated by one primer each is shown in Fig. 2(a-c). All banding profiles were found to be monomorphic and the regenerated plants were similar to that of the mother plant, confirming the true-to-true type nature of the in vitro-raised plants. Therefore the present micropropagation method established for H. incana can be used as a reliable protocol for mass production without any risk of genetic instability, which also helps in the conservation of this type species.
Fig. 2.
DNA finger printing pattern of H. incana: mother plant (lane 1) and micropropagated plants (lane 2–5) obtained by (a) OPD-20, (b) UBC-856, (c) URP4R
Acknowledgments
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Appelgren M, Helde Ola M. Regeneration in Streptocarpus leaf disks and its regulation by temperature and growth substances. Physiol Plant. 1972;27:417–423. doi: 10.1111/j.1399-3054.1972.tb03637.x. [DOI] [Google Scholar]
- Cui J, Chen J, Henny RJ. Regeneration of Aeschynanthus radicans via direct somatic embryogenesis and analysis of regenerants with flow cytometry. In vitro Cell Dev Biol Plant. 2009;45:34–43. doi: 10.1007/s11627-008-9147-9. [DOI] [Google Scholar]
- Devi SP, Kumaria S, Rao SR, Tandon P. Single primer amplification reaction (SPAR) methods reveal subsequent increase in genetic variations in micropropagated plants of Nepenthes khasiana Hook. f. maintained for three consecutive regenerations. Gene. 2014;538:23–29. doi: 10.1016/j.gene.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Dontcheva S, Daskalova E, Yahubyan G, Denev I, Minkov I, Toneva V. Conservation of the protected resurrection species Ramonda serbica panč.-habitat Montana district, Bulgaria as in vitro plants through a modified micropropagation system. Biotechnol Biotechnol. 2009;23:369–372. doi: 10.1080/13102818.2009.10818441. [DOI] [Google Scholar]
- Godo T, Lu Y, Mii M. Micropropagation of Lysionotus pauciflorus Maxim. (Gesneriaceae) Method Mol Biol. 2010;587:127–139. doi: 10.1007/978-1-60327-114-1_13. [DOI] [PubMed] [Google Scholar]
- Grunewaldt J. Adventivknospenbildung und Pflanzenregeneration bei Gesneriaceae in vitro. Gartenbauwissenschaft. 1977;42:171–175. [Google Scholar]
- Kholkute SD. Database on ethnomedicinal plants of Western Ghats. Final report. Belgaum: Regional Medical Research Centre (ICMR); 2009. [Google Scholar]
- Kottaimuthu R. Ethnobotany of the Valaiyans of Karandamalai, Dindigul District, Tamil Nadu, India. Ethnobot Leaflets. 2008;12:195–203. [Google Scholar]
- Kozak D, Hetman J, Witek M. The influence of the mineral composition of the medium on in vitro propagation of Kohleria amabilis (Planch. ET Linden) Fritsch shoots. Acta Agrobot. 2007;60:95–99. doi: 10.5586/aa.2007.011. [DOI] [Google Scholar]
- Krishnarajah SA, Dhanasekera DMUB, Ratnayake RHPPM, Ratnayake RHBPM. Utilization of wild flora to develop the floriculture industry. Ann Sri Lanka Dept Agric. 2002;4:151–159. [Google Scholar]
- Li Q, Deng M, Zhang J, Zhao W, Song Y, Li Q, Huang Q. Shoot organogenesis and plant regeneration from leaf explants of Lysionotus serratus. D. Don. Sci World J. 2013;2013:1–7. doi: 10.1155/2013/280384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KH. Factors affecting shoot organogenesis in leaf disc culture of African violet. Sci Hortic. 1997;72:49–57. doi: 10.1016/S0304-4238(97)00116-7. [DOI] [Google Scholar]
- Ma G, Jaime A, da Silva T, Lü J, Zhang X, Zhao J. Shoot organogenesis and plant regeneration in Metabriggsia ovalifolia. Plant Cell Tiss Org. 2011;105:355–361. doi: 10.1007/s11240-010-9875-5. [DOI] [Google Scholar]
- Matthew KM. The Flora of the Palni Hills, South India. Tiruchirapalli: The Rapinat Herbarium; 1999. pp. 909–910. [Google Scholar]
- Murray MG, Thomson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E-HO, Bae H, Park WT, Kim YB, Chae SC, Park SU. Improved shoot organogenesis of gloxinia (Sinningia speciosa) using silver nitrate and putrescine treatment. Plant Omics J. 2012;5:6–9. [Google Scholar]
- Qin G, Qin W, Wen F, Huang R, Yang Q. Aseptic seeding, tissue culture and rapid propagation of Primulina hochiensis. Heilongjiang Agric Sci. 2013;8:14–17. [Google Scholar]
- Sathyavathi R, Janardhanan KJ. Folklore medicinal practices of Badaga community in Nilgiri biosphere reserve, Tamil Nadu, India. Int J Pharm Res Dev. 2011;3:50–63. [Google Scholar]
- Shaib JM, Hapsah MG, Zulhazmi S. Application of in vitro techniques in species conservation and micropropagation of Didymocarpus platypus. J Trop Agric Food Sci. 2004;32:81–84. [Google Scholar]
- Shilpha J, Silambarasan T, Largia MJV, Ramesh M. Improved in vitro propagation, solasodine accumulation and assessment of clonal fidelity in regenerants of Solanum trilobatum L. by flow cytometry and SPAR methods. Plant Cell Tiss Org. 2014;117:125–129. doi: 10.1007/s11240-013-0420-1. [DOI] [Google Scholar]
- Siva R, Rajasekaran C, Mudgal G. Induction of somatic embryogenesis and organogenesis in Oldenlandia umbellata L., a dye-yielding medicinal plant. Plant Cell Tiss Organ Cult. 2009;98:205–211. doi: 10.1007/s11240-009-9553-7. [DOI] [Google Scholar]
- Siva R, Mayes S, Behera SK, Rajasekaran C. Anthraquinones dye production using root cultures of Oldenlandia umbellata L. Ind Crop Prod. 2012;37:415–419. doi: 10.1016/j.indcrop.2011.12.027. [DOI] [Google Scholar]
- Takagi H, Sugawara S, Saito T, Tasaki H, Yuanxue L, Kaiyun G, Han DS, Godo T, Nakano M. Plant regeneration via direct and indirect adventitious shoot formation and chromosome-doubled somaclonal variation in Titanotrichum oldhamii (Hemsl.) Solereder. Plant Biotechnol Rep. 2011;5:187–195. doi: 10.1007/s11816-011-0172-5. [DOI] [Google Scholar]
- Tan X, Deng J, Xiao-yi Hu, Wu Y, Bao M (2009) Tissue Culture and Plant Regeneration in Chirita langshanica. Nonwood For Res 3
- Tóth S, Scott P, Sorvari S, Toldi O. Effective and reproducible protocols for in vitro culturing and plant regeneration of the physiological model plant Ramonda myconi (L.) Rchb. Plant Sci. 2004;166:1027–1034. doi: 10.1016/j.plantsci.2003.12.020. [DOI] [Google Scholar]
- Vazquez AM, Davey MR, Short KC. Organogenesis in cultures of Saintpaulia ionantha. Acta Hortic. 1977;78:249–258. [Google Scholar]
- Vlahos JC. Regeneration of two cultivars of Achimenes longiflora DC. In vitro. Acta Hortic. 1989;251:255–273. [Google Scholar]
- Vlahos JC, Dragassaki M, Vasilaki A, Assargiotaki I. Microproagtion of Achimenes hybrids for winter production. HortSci. 1995;30:757. [Google Scholar]
- Wei W, Hao Q, Zhi-gang C. Studies on tissue culture of Streptocarpus wendlanddii. J Jilin Agric Univ. 2010;32:51–53. [Google Scholar]
- Wuttisit M, Kanchanapoom K. Tissue culture propagation of Gloxinia. Suranaree J Sci Technol. 1996;3:63–67. [Google Scholar]