Abstract
Brassica juncea is an important oilseed crop and drought stress is major abiotic stress that limits its growth and productivity. RH0116 (drought tolerant) and RH8812 (drought sensitive) genotypes were undertaken to study some of the physiological parameters and hsp gene expression related to stress tolerance under drought stress conditions. Differential response in terms of seed germination, electrolyte leakage, RWC, osmotic potential was observed in the selected genotypes. In vitro seed germination studies using PEG stress treatments indicated reduced seed germination with increasing levels of stress treatment. Electrolyte leakage increased, whereas, relative water content and osmotic potential decreased in stressed seedlings. Expression of hsp gene was found to be upregulated during drought stress as the transcripts were present only in the stressed plants and disappeared upon rehydration. The drought tolerant variety showed higher transcript accumulation as compared to the sensitive variety. The study showed that drought induced changes in gene expression in two contrasting genotypes were consistent with the physiological response.
Keywords: Brassica Juncea, Indian mustard, hsp gene, Physiological response, Drought stress
Introduction
Brassica juncea (Indian mustard), a member of Cruciferae family, is one of the major oilseed crops of India. It is an amphidiploid (2n = 36; AABB) of Brassica nigra (2n = 16 BB) and Brassica rapa (2n = 20 AA). In India, it occupies second largest position after groundnut with 6 million hectares of area under cultivation producing 5–6 million tones of seed annually, securing fourth position in world after European Union (34 %), China (23 %) and Canada (19 %). The global production of Indian mustard and its oil is around 38–42 and 12–14 MT, respectively. India contributes 28.3 and 19.8 % in world acreage and production (Shekhawat et al. 2012).
Abiotic stresses, such as drought, temperature extremes and salinity, are the major constraints on crop yield and quality in fields (Soda et al. 2015). Plant response to abiotic stress is dynamic and complex (Skirycz and Inze 2010; Cramer 2010), which is both elastic (reversible) and plastic (irreversible). The impact of climate change on agricultural crops is multidimensional and its magnitude may vary with the growth patterns and species (Chattopadhyay et al. 2011). Drought stress affects the growth and development of plant at all stages making it as one of the severe constraints for crop productivity particularly in arid and semi-arid areas of the world. The transcriptional responses to abiotic stress vary at cellular or tissue level in roots (Dinneny et al. 2008) and depend on duration of stress as well (Tattersall et al. 2007; Pinheiro and Chaves 2011).
Drought-induced crop losses have a significant economic impact and are predicted to increase with global climate change (Roychoudhury et al. 2013). As most of the abiotic stresses result in desiccation of the cell and osmotic imbalance, there is an overlap in the expression pattern of stress genes after cold, drought, high temperature, high salt and ABA treatment (Mahajan and Tuteja 2005). This suggests that various stress signals and ABA share common elements in their signaling pathways which cross talk with each other, to maintain cellular homeostasis.
The productivity of this crop is greatly influenced by abiotic stresses such as drought, salinity, frost and heat. Water stress causes heavy yield losses in Indian mustard and seed germination as well as seed development (flowering to pod formation) stage is understood more prone to drought and high temperature. So far, efforts have been done to improve economic traits such as yield, oil content and disease resistance and a few studies are undertaken on effect of abiotic stress in Indian mustard. Improving Indian mustard cultivars for drought tolerance is one of the major priorities for plant breeders in present times considering the challenges of climate change. Gene expression studies are prerequisite for identifying the genes which have role in stress tolerance. Brassica juncea cv. RH8812 is drought sensitive while RH0116 is drought tolerant genotype (Sharma et al. 2012), developed by CCS Haryana Agricultural University, Hisar. RH0116 is also tolerant to heat stress (Sharma and Sardana 2013). RH8812 was released in the year 1996, its average yield is 22q/ha, it gets matured at 142 days and possesses thick siliquae (www.hau.ernet.in). A comparative study of these genotypes provide an excellent source for understanding drought tolerance mechanism and isolation of stress induced genes which have key role in abiotic stress tolerance.
Heat shock proteins (HSP) are a group of proteins induced by heat shock which act as molecular chaperones in cells exposed to stressful stimuli (Stephanou and Latchman 2011) and play important role in protecting plants against environmental stresses including drought (Sato and Yokoya 2008). Most HSPs are constitutively expressed and perform essential function as molecular chaperones, facilitating the synthesis and folding of proteins throughout the cell. HSPs protect mainly lipid membranes, lipids, proteins, cytoskeletal components and nucleic acids in cells (Tkacova and Angelovicova 2012). In addition, HSPs have been shown to participate in protein assembly, secretion, trafficking, degradation and regulation of transcription factors and protein kinases (Stephanou and Latchman 2011). Park et al. (2007) classified HSPs into several classes based on their molecular weight, such as HSP90 (85–90 kDa), HSP70 (68–73 kDa), HSP60, HSP47 and small HSPs (12–43 kDa). The HSP70 family is necessary for protein synthesis, translocation, and folding. HSP60 family is important in stability of the protein. Many factors such as organic toxic substances and heavy metals, elevated temperature in cells responsive to the formation of proteins known as stress proteins. They are associated with the mitochondrial matrix and help in saving, production and transport of proteins into mitochondria (Richter-Landsberg and Goldbaum 2003). Protein family HSP90 is important in the formation of steroid receptor complexes (Pratt 1997).
The expression of HSPs has been investigated in a number of different plants and positive correlation is reported between high temperature and this protein. HSP101 is upregulated several fold under heat stress in Indian mustard but not under drought stress (Bhardwaj et al.2015). The plant Small HSPs are divided into five nuclear encoded gene families and class I and II sHSPs are developmentally regulated which suggest their distinct function in seed development (Wehmeyer and Vierling 2000). In the present investigation, we have studied HSP17.4 induction under drought stress conditions in two contrasting Indian mustard genotypes for drought tolerance. HSP17.4 is a member of the class I small heat-shock protein (sHSP) family, encoded by At3g46230 in Arabidopsis and accounts for the majority of sHSPs in maturing seeds (Yu et al. 2013). To gain additional insight as to the function of sHSPs in drought tolerance, we have examined sHSP gene transcript levels in a drought sensitive and drought tolerant genotype of Indian mustard.
The development of PCR to detect rare transcripts has revolutionized gene identification and sensitivity of gene expression analysis. PCR based gene expression strategies like semi-quantitative RT-PCR offers a rapid, versatile and sensitive way for studying gene expression under stress. The RT-PCR method can be used not only to detect specific mRNAs but also estimates their levels in different samples. It requires gene specific primers for various genes based on known DNA sequences of identified genes in other model plant species such as Arabidopsis and rice for differential gene expression (Chen et al. 2005). Hence, certain key genes involved in drought tolerance can be identified and further used in improving Indian mustard cultivars as characterization and over-expression of such genes can ultimately lead to enhanced productivity under drought stress conditions.
Our results were also analyzed in the light of certain physiological parameters such as seed germination studies, relative water content, osmotic potential and electrolyte leakage. Polyethylene glycol (PEG) can be used to induce drought stress condition as it causes osmotic stress (Ashraf et al.1996; Turhan 1997). PEG-6000 is usually used to create the osmotic stress by most of the researchers (Hu and Jones 2004) for the development of water deficit environment in growth chamber studies. RWC represents a useful indicator of the state of water balance of a plant, essentially because it expresses the absolute amount of water, which the plant requires to reach artificial full saturation. Despite its simplicity, this technique needs to be adjusted for each plant material (Arjenaki et al. 2012). The electrolyte leakage data is an indicator of cell membrane stability and the cell membrane gets damaged during abiotic stress, the oxidative damage to lipids disrupts the membrane structural integrity, which in turn causes electrolyte leakage (Du et al. 2009).
Materials and methods
Plant material and drought stress treatments
Plants (RH0116 and RH8812) were grown in pots in nethouse. In each pot, equal quantity of sand and water was added to make them ready for sowing. Seeds started to germinate after 4–5 days. Plants were regularly watered with equal amounts of water (so as to keep the water level equal) and Hoagland Solution (50 ml each) to supply them adequate amounts of nutrients for growth. At the flowering stage, half of the plants of each genotype were separated as controls (in which watering was maintained) and to the other half of the plants, watering was withheld to create drought stress condition. The day wilting appeared in stressed plants, sampling of shoot and root tissue was done in control and stressed plants and samples were stored at −80 °C for RNA isolation. Samples (of control and stressed plants) were also taken for physiological parameters analysis such as osmotic potential, relative water content and electrolyte leakage.
After the sampling was done, the drought stressed plants were rehydrated by providing them equal amounts of water. The day wilting disappeared and the rehydrated plants appeared normal, samples were again taken for analysis.
In vitro seed germination under drought stress
The seeds of selected Indian mustard genotypes were germinated in vitro in MS medium (Murashige and Skoog 1962). The media was supplemented with PEG 6000 (4 %, 6 %, 8 % w/v) for various levels of stress treatments & physiological attributes such as seed germination, shoot and root length were recorded after 2 weeks. The medium was sterilized by autoclaving and was poured in glass petriplates. Medium was cut and removed from half of the plate so that seeds can be inoculated at the margin to clearly visualize the roots.
Physiological studies
The Brassica plants (stressed, control and rehydrated) grown in nethouse were used to analyse the following physiological parameters:
Relative water content
RWC was estimated by the standarad method (Yamasaki and Dillenburg 1999). For estimating RWC, two fully expanded leaves (in vivo grown plants) of each variety (RH0116 and RH8812) were cut into small pieces and weighed to record the fresh weight. Then the leaf samples were hydrated to full turgidity by floating on de-ionized water in a closed petridish for 4 h. After this, the samples were taken out of water and any surface moisture was removed quickly and lightly with filter paper and immediately weighed to obtain full turgid weight. Then the samples were dried in an oven at 70 °C for 24 h and weighed to determine the dry weight of the sample. The observations were recorded in triplicates and RWC was calculated by the following formula:
Osmotic potential
Osmotic Potential of leaf was determined by using Vapour pressure osmometer (Wescor INC., USA). The leaves were excised from the stressed, control and rehydrated plants and were sealed in syringes individually and quickly frozen at −20 °C. Before measuring the osmotic potential the samples were thawed for 60 min at 25 °C. The sap was then taken out from the syringe. Then a filter paper disc was dipped in the sap and immediately placed in the chamber of vapour pressure osmometer and chamber was sealed. After about 2 min., the osmotic potential readings were displayed on the digital meter automatically, which were recorded. The osmometer was calibrated by using standard solutions of NaCl (0.1 mM to 1 M).
Electrolyte leakage
The relative intactness of plasma memberane was measured as the leakage percentage of electrolytes, as described by Gong et al. (1998). Fresh leaves were cut into pieces and placed in test tubes. The test tubes were then incubated in a water bath at 52 °C for 1 h and then 10 ml of deionised water was added to it and kept overnight. Next day, the initial electrical conductivity of the medium EC1 was measured. The samples were autoclaved for 20 min. to release all the electrolytes, cooled and then, final electrical conductivity EC2 was measured. The leakage percentage of electrolytes was calculated as (1- EC1/EC2) X 100.
RNA isolation
RNA was isolated using Trizol method (Chomczynski 1993). The plant samples (shoot and root tissues) were crushed in liquid nitrogen in DEPC treated and autoclaved pestle and mortar. Trizol reagent (650 μl) was added to 0.7–0.8 g of ground tissue. It was vortexed vigorously and solution was incubated on ice for 10 min. Chloroform (200 μl) was added to sample solution, vortexed vigorously and incubated for 5 min on ice. The samples were centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatant was taken out carefully in a fresh eppendorff tube without disturbing the lower organic phase. To the supernatant, equal volume of isopropanol was added and the samples were incubated on ice for 10 min. The samples were centrifuged at 12,000 rpm for 10 min at 4 °C and the supernatant was discarded. The pellet was washed with 500 μl of 70 % ethanol and centrifuged at 11,000 rpm for 3 min. The pellet was dried at room temperature in laminar air flow. RNA pellet obtained was re-suspended in 20 μl of DEPC treated water and stored at −80 °C for future use.
Estimation of RNA
The RNA was quantified using Biophotometer (Eppendorf). One μl of RNA sample was added to 49 μl of DEPC treated water and absorbance was recorded at 260 nm. Blank was set using 50 μl of DEPC treated water. One O.D. unit corresponds to 40 μg of RNA/μl. Absorbance at 280 nm and 260 nm was also recorded to check the purity of RNA and the ratio of absorbance at 260/280 was calculated. The absorbance ratio in the range of 1.6–1.8 represented good quality RNA which was then used in RT-PCR for studying gene expression.
Semi-quantitative RT-PCR
The semi-quantitative RT-PCR was conducted using RNA samples isolated from shoot and root tissues of control, stressed and rehydrated plants. The total RNA was used in cDNA synthesis by reverse transcription. RT-PCR conditions were optimized by altering concentrations of oligo dT primer (0.1–0.5 μg), total RNA (2–7 μg), dNTPs mix (1–4 mM), reverse transcriptase enzyme (100–200 units) and RNase inhibitor (20–50 U).
PCR amplification of gene specific transcripts using specific primers
The primers of BjActin forward (5′TGGCATCACACTTTCTACAA3′) and reverse (5′CAACGGAATCTCTCAGCTCC3′) and hsp forward (5′ CGTGGCAGCGTTCACAAA3′) and reverse (5′CGTCCGCCTTGAACACATG 3′) were used for PCR analysis. The primers for sHSP gene were synthesized by using sequences from Arabidopsis (Zhang et al. 2008). PCR was performed to amplify the specific gene from cDNA in a 20 μl reaction volume containing 10X PCR buffer (containing 2.5 mM MgCl2), 200 μM of dNTPs, 0.6 μM of primers (forward and reverse), 1U Taq DNA Polymerase and template (cDNA; 20 ng). The optimized conditions were used in the gene expression experiments. PCR cycling conditions consisted of an initial denaturation at 94 °C for 2 min; 36 cycles of 92 °C for 1 min; 54.3 °C for actin, 56.4 °C for hsp for 1 min and 72 °C for 1 min; and a final extension at 72 °C for 10 min.
Results
Seed germination under PEG induced drought stress
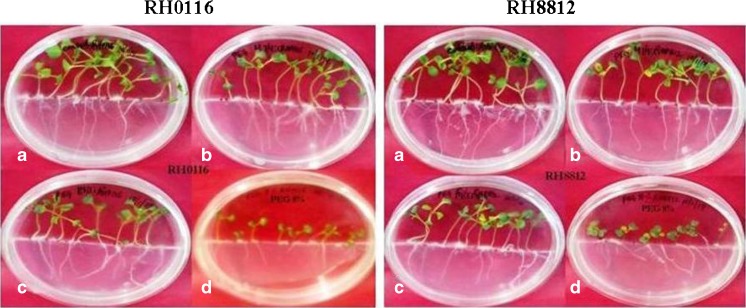
PEG 6000 was used at various concentrations (4 %, 6 %, 8 % w/v) and it was observed that seed germination was arrested at the initial stage only and reduction in seed germination was significant among treatments. The tolerant variety RH0116 showed 76.66, 66.66, 63.33 and 53.33 % seed germination, whereas the sensitive variety RH8812 showed 50.00, 38.66, 33.33 and 10.00 % seed germination in control, 4, 6 and 8 % PEG drought treatments respectively. Hence, the per cent seed germination in B. juncea under PEG induced drought stress treatment decreased with the increasing dose of the PEG treatment and the decrease was higher in the sensitive variety as compared to the tolerant one.
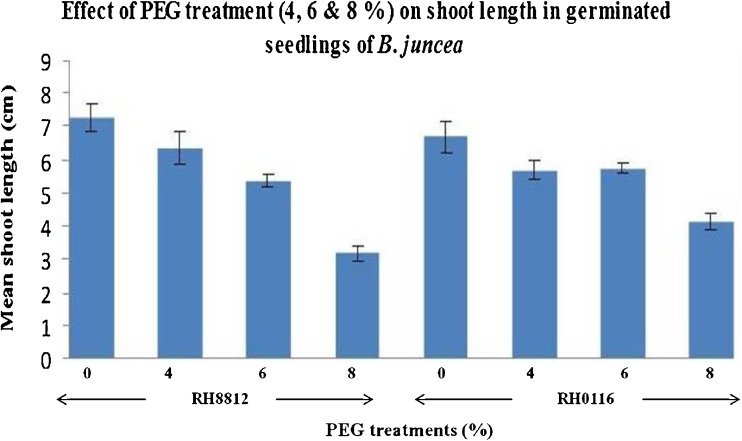
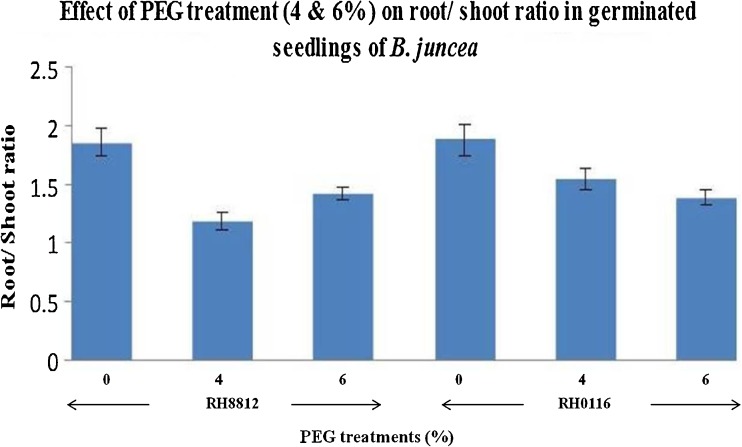
The average shoot and root lengths of the in vitro grown seedlings after PEG 6000 treatment was found to decrease with the dose of PEG in both the genotypes and the root/shoot ratio decreased in the stressed seedling as compared to the control. However, as the dose of PEG treatment increased, the root/shoot ratio increased, the tolerant genotype showed higher root/shoot ratio (Figs. 1, 2, 3 and 4). The percent decrease in RH0116 in shoot length was 12.77, 26.37 and 48.07 % and in root length was 46.34, 46.74 and 72.19 % and the percent decrease in RH8812 in shoot length was 14.17, 14.92 and 52.38 % and in root length was 29.09, 37.27 and 60.27 % with respect to 4, 6 and 8 % PEG 6000 respectively. Minimum shoot and root length was recorded when PEG 6000 (8 %) was used. The sensitive variety RH8812 recorded more reduction in shoot and root length.
Fig. 1.
In vitro seed germination response in Brassica juncea cvs. RH0116 and RH8812 subjected to various levels of PEG treatment (a = PEG 0 % i.e. control, b = PEG 4 %, c = PEG 6 %, d = PEG 8 %)
Fig. 2.
Effect of PEG treatment (4, 6 & 8 %) on shoot length in 2 weeks old in vitro germinated seedlings of B. juncea (The vertical lines above the bars represent the standard error)
Fig. 3.
Effect of PEG treatment (4, 6 & 8 %) on root length in 2 weeks old in vitro germinated seedlings of B. juncea (The vertical lines above the bars represent the standard error)
Fig. 4.
Effect of PEG treatment (4, 6 & 8 %) on root/shoot ratio in 2 weeks old in vitro germinated seedlings of B. juncea (The vertical lines above the bars represent the standard error)
Physiological response of Brassica juncea under drought stress
Nethouse grown Brassica plants (stressed, control and rehydrated) subjected to drought stress were used to estimate physiological response by parameters such as Relative water content, Osmotic potential and Electrolyte leakage.
Relative water content under drought stress in Brassica juncea (cvs. RH8812 and RH0116)
In case of nethouse grown Indian mustard plants, the Relative water content (RWC) showed a rapid decline when plants were subjected to drought stress as compared to control plants (Fig. 5). The decline was higher in the sensitive RH8812 than RH0116 (i.e. from 81.67 to 36.51 %). The drought tolerant variety (RH0116) also showed higher recovery of RWC when stressed plants were rehydrated as compared to the sensitive one.
Fig. 5.
Relative water content (%) of Brassica juncea plants under drought stress under nethouse conditions (The vertical lines above the bars represent the standard error)
Osmotic potential under drought stress in Brassica juncea (cvs. RH8812 and RH0116)
The values of osmotic potential were more negative in both varieties subjected to drought stress as compared to the control plants (Fig. 6). The value of osmotic potential was more negative in RH8812 (i.e. -3.26 MPa) than RH0116 (i.e. -2.16 MPa). Also, osmotic potential recovered to some extent upon rehydration in both genotypes.
Fig. 6.
Osmotic potential (−MPa) in control, stressed and rehydrated plants under nethouse conditions (The vertical lines above the bars represent the standard error)
Electrolyte leakage under drought stress in Brassica juncea (cvs. RH8812 and RH0116)
An increase in electrolyte leakage was observed when the plants were subjected to the drought stress (Fig. 7). The increase was relatively more in the RH8812 variety (from 19.26 to 22.33 %) as compared to the RH0116 (from 18.46 to 20.16 %). The leakage of electrolytes decreased upon rehydration.
Fig. 7.
Electrolyte leakage (%) of Brassica juncea plants under drought stress (The vertical lines above the bars represent the standard error)
Expression of hsp in Brassica juncea cvs. RH8812 and RH0116
In the present study, the accumulation of hsp transcript was observed in stressed plants of Brassica juncea cvs. RH8812 and RH0116 (Fig. 8) and actin gene was used as control. The transcripts were missing in the control and rehydrated plants, which shows that the gene is upregulated under drought stress condition. The transcript was present in shoots as well as roots, however the expression was higher in the shoots as compared to the roots. The actin transcript was similar in control, stressed and rehydrated plants.
Fig. 8.
Electrophoretic pattern of hsp and actin transcripts in Brassica juncea genotypes under drought stress. Ladder (L), Negative control (NC), RH8812 control; shoot (2), RH8812 stressed; shoot (3), RH8812 rehydrated; shoot (4), RH0116 control; shoot (6), RH0116 stressed; shoot (7), RH0116 rehydrated; shoot (8), RH8812 control; root (11), RH8812 stressed; root (12), RH8812 rehydrated; root (13), RH0116 control; root (15), RH0116 stressed; root (16), RH0116 rehydrated; root (17)
Discussion
Plants, being sessile organisms are constantly challenged by a wide range of environmental stresses (e.g. drought, salinity, cold, heat, UV radiation, heavy metals, flooding etc.) which negatively influence plant growth, development, survival, biomass production and crop yield. It is estimated that these cause more than 50 % of crop yield losses worldwide (Rodziewicz et al. 2014). The molecular dissection of abiotic stress tolerance and understanding its mechanism is quite difficult as well as complex being a multigenic and quantitative trait. Particularly crop productivity is adversely impacted under rainfed conditions. Drought cannot be forecast unlike other stresses and plays a major role in destabilizing the productivity in crop plants (Amudha and Balasubramani 2011). A number of transcription factors, abiotic stress related genes and regulatory sequences in plant promoters have been studied and characterized that can substantially influence plant stress tolerance (Agarwal and Jha 2010).
Physiological studies in Brassica juncea under drought stress conditions
Combining physiological analysis with multiple gene expression during stress at different intensities and time regime can help in unraveling the limits and physiological mechanisms involved in stress tolerance. This knowledge is crucial to develop strategies for improving crop productivity under adverse environments. Studies have revealed that drought-induced gene expression in specific genotypes was consistent with the observed physiological responses (Hazen et al. 2005; Street et al. 2006).
In general, both environmental conditions and seed viability determine seed germination. Emergence of radicle leads to primary root formation which is followed by extension of hypocotyl bearing cotyledons and plumule. Development of stem from hypocotyl and root from radicle are adversely affected by drought stress. Seed germination studies under drought stress give a fair idea of stress tolerance in plant species. Therefore, radicle and hypocotyl growth under drought stress is considered as a primary criterion for stress tolerance (Zhu et al. 2006). In this study, seed germination, shoot and root length were recorded after 2 weeks of drought stress treatments using PEG 6000 (4 %, 6 %, 8 % w/v) under in vitro conditions. Seed germination was arrested at the initial stage only and reduction in seed germination was significant among PEG treatments and drought sensitive genotype (RH8812).
Abdoli and Saeidi (2012) demonstrated that the effects of drought stress at post-anthesis stage were significant on plumule length, radicle length, plumule weight, radicle weight, germination percent, mean germination time and seedling vigor index of seeds. Water stress resulted in reduced seed germination, plumule length and radicle length. We also observed that the average shoot and root lengths of the in vitro grown seedlings after PEG treatment decreased with increased dose of PEG in both the genotypes, however more reduction was recorded in RH8812 when PEG 6000 (8 %) was used. Drought stress induced reduction in the radicle length and percent germination was also reported by Jajarmi (2009) in safflower. Similar response of reduced seed germination was reported in Mongolian pine (Zhu et al. 2006), Cupressus sp. (Ahmadloo et al.2011) and Eremosparton songoricum (Litv.) Vass (Li et al. 2013).
Leaf relative water content (RWC) indicates the water status of the plant as it expresses the absolute amount of water which the plant requires to reach artificial full saturation. Physiological and biochemical changes in plants under abiotic stress conditions are related to altered gene expression (Saibo et al.2009) and in response to duration, intensity and rate of progression of imposed stress (Chaves et al.2009). However, depending upon plant species, certain stages such as germination, seedling or flowering could be the most critical stages for water stress. Seed germination is most sensitive and critical stage in plant life cycle (Ashraf and Mehmood 1990) and seeds exposed to unfavourable environmental conditions such as water stress may have to compromise the seedlings establishment (de Albuquerque and de Carvalho 2003). Significant decrease in relative water content is reported in Populus cathayana (Xu et al. 2008) Sorghum bicolor L. (Bhargava and Paranjpe 2004), Lycium nodosum Miers (Tezara et al. 2003), Hippophae rhamnoides L. (Li et al. 2004), Populus davidiana Dode (Zhang et al. 2004) and P. cathayana (Yin et al. 2004) in response to drought stress.
We also found a rapid decline in Relative water content (RWC) when plants were subjected to drought stress. The decline was higher in sensitive check than RH0116. Further, the drought tolerant variety (RH0116) also showed higher recovery of RWC when stressed plants were rehydrated. In vitro studies also suggest the similar response (Personal data). Temperature tolerant Indian mustard genotypes were identified on the basis of RWC by Kumar et al. (2013). The late sown Indian mustard genotypes showed more decrease in RWC as compared to the early sown. The tolerant genotypes like Proagro, NDR 8801 and CS-52 showed lower decline in RWC while sensitive genotypes such as Pusa Agrani, EJ-15 and Pusa Tarak showed comparatively higher decline in RWC. These observations are further substantiated in woody species as well (Saura-Mas and Lloret 2007). They assessed RWC in 30 woody species of a coastal shrubland, with different post-fire regenerative strategies (seeding, resprouting or both). They suggested that the resprouters had more efficient mechanisms to reduce water losses and maintain water supply between seasons. Rahimi et al. (2010) also observed that as stress intensified, RWC significantly decreased in Plantago. This is in agreement with our findings as increased duration of air drying from 15 min to 2 h in Brassica seedlings reduced the RWC progressively (Personal data).
In our study, the osmotic potential values were more negative in plants subjected to drought stress as compared to the control plants. The sensitive variety showed more decline in osmotic potential as compared to the tolerant variety. Also, osmotic potential recovered to some extent upon rehydration in both the genotypes. Rahimi et al. (2010) also reported in Plantago species that the leaf water potential was constant within 5 days after irrigation stopped and afterwards, it decreased to −1.6 and −2.0 MPa for P. psyllium and P. ovata at eighth day and −2.3 and −2.8 MPa on tenth day, respectively. We also found that as the drought stress treatment increased, the seedlings showed more negative osmotic potential as well as genotypic variation. In rapeseed, reduced relative water contents, osmotic potential were observed under water stress (Alikhan et al. 2010).
The electrolyte leakage data indicates the stability of cell membrane and the cell membrane gets damaged during heat stress, the oxidative damage to lipids disrupts the membrane structural integrity, which in turn results in electrolyte leakage (Du et al. 2009). Wilson et al. (2014) evaluated Brassica juncea seedlings for heat stress tolerance in terms of biochemical components. Electrolyte leakage was significantly higher in susceptible genotypes than the tolerant ones with respect to control seedlings. We also observed that electrolyte leakage was increased when the plants were subjected to drought stress as compared to control plants and the increase was relatively more in sensitive variety (RH8812) as compared to the tolerant variety (RH0116). This is also supported by Sayar et al. (2008) as they reported that the sensitive varieties showed significantly higher leakage than the tolerant ones indicating conserved membrane integrity for tolerant than sensitive varieties and electrolyte leakage increased as the dehydration time increased. We also found progressive increase in the values of electrolyte leakage with increased drought stress treatment. Wilson and Jacobs (2004) described the use of electrolyte leakage (EL) from stem tissue as a potential method for assessing cold hardiness of hardwood seedlings and found that higher EL values at lower temperatures and longer duration represented an increase in cell damage and loss of hardiness. This suggests that electrolyte leakage parameter is an indicator for assessing drought, heat or cold stress in crop plants.
The physiological changes are culminated due to differential gene expression. These genes are turned on in response to drought/ heat/ cold stress as these genes share common network involved in stress tolerance. Previously we have found that cor gene which is cold inducible is activated under drought stress in Indian mustard (Sharma 2010). In the present study, we analysed the hsp gene induction in response to drought stress in two important cultivars of Brassica juncea RH8812 and RH0116, which are drought sensitive and drought tolerant genotypes respectively using semi-quantitative RT-PCR approach. We used actin, a constitutive or house keeping gene, i.e. it is expressed at all stages as positive control in our experiments. The actin transcript was found to be induced in roots as well as shoot tissues irrespective of the treatment, i.e. it was present in control, stressed and rehydrated plants. Also the expression was similar in control, stressed and rehydrated plants.
Expression of hsp in Brassica juncea under drought stress
Plants must cope with stress for survival, so they develop different mechanisms such as maintenance of cell membrane stability, synthesis of antioxidants, capturing ROS, osmoregulation of osmoticum, induction of transcription factor and accumulation of HSPs (Hossain et al. 2013). HSPs are required not only for quick adaptation to temperature changes, but also for rapid recovery after heat or cold release (Yang et al. 2010). Heat-shock proteins (HSPs) and heat-shock transcription factors (HSFs) are central components of the heat-shock regulatory network and are involved in cellular responses to various forms of stresses (Lee et al. 2010). In Indian mustard plants, drought stress mainly affects seed germination and seed set stages. The sHSPs are highly conserved and most abundant stress-induced proteins with an α-crystallin domain (ACD) in the C-terminal. Plants contain a wide array of sHSPs that are divided into six classes based on their sequence alignments and immunological cross-reactivity. The sHSPs might act as an electrolyte leakage lowering factor during seed desiccation and also might participate in preserving membrane integrity during thermal fluctuations in winter. The cytosolic sHSPs (class I) function as molecular chaperones, protect cellular components during seed desiccation and prevent the thermal aggregation of substrate proteins (Alamillo et al. 1995; Wehmeyer et al. 1996), we considered it worthwhile to understand its role in drought tolerance in Indian mustard. The most highly expressed sHSP gene during seed development in Arabidopsis is AtHSP17.4 and this protein has a similar role during seed development, i.e. preventing the irreversible aggregation of other proteins during desiccation and/or assisting in the refolding of denatured proteins during imbibition (Wehmeyer and Vierling 2000). Therefore, we targeted this gene for our study. The function for sHSPs such as HSP17.4 in desiccation tolerance is further supported by analysis of seed development mutants (Vernon and Meinke 1995; Meinke et al. 1994).
In the present study, the accumulation of hsp transcript was observed in stressed plants of Brassica juncea cvs. RH8812 and RH0116. The transcripts were missing in the control and rehydrated plants, which shows that the gene is upregulated under drought stress conditions. The transcript was present in shoots as well as roots, however, the transcript abundance was higher in shoots as compared to roots. Changes in sHSPs expression are similar to those in LEA proteins at the time of dehydration and crucial developmental stages in Arabidopsis plants transformed with LimHSP16.45 (Mu et al. 2013). Its strong expression in the guard cells, indicated that LimHSP16.45 probably regulated stomatal movement during times of drought. Therefore, many sHSPs are known to function in either the presence or absence of abiotic stress. For example, an Arabidopsis cytoplasmic sHSP, AtHSP17.6, is expressed in heat-shocked leaves but not in untreated control leaves of Arabidopsis.
Bhardwaj et al. (2015) used a combined approach of next generation sequencing and de-novo assembly to discover Brassica juncea transcriptome associated with drought and high temperature stresses. They used Varuna, a drought sensitive variety in the study. It was found that maximum number of differentially regulated transcription factors in drought stress and high temperature were MYB factors. MYB TFs are also induced under drought stress in RH0116 (drought tolerant) and CS52 (salt tolerant) genotypes of Indian mustard (Sharma 2010). The identified transcripts can be helpful in engineering abiotic stress tolerance in Brassica juncea and related crop species. Yang et al. (2014) cloned nine sHSP genes from Tamarix hispida. The expression patterns of Triticum durum cultivars in response to various abiotic stresses showed differences in HSP transcripts accumulation with contrasting thermotolerance indicating that induction of HSP gene expression has a role in the acquisition of thermotolerance. Further, the accumulation of mitochondrial HSP transcripts appeared to be related to the acquisition of thermotolerance (Patrizia et al. 2009). Liu et al. (2013) reported that over-expression of OsHsfA7 gene increased tolerance to salt and drought stresses in rice seedlings. They observed that transgenic plants exhibited less, shorter lateral roots and root hairs while the transgenic rice seedlings restored normal growth unlike the wild type plants.
Enhanced expression of heat shock proteins (HSPs) was observed in response to heat shock, heat acclimation and SA in four genotypes of Brassica by Kaur et al. (2009). The pre-treatments helped seedlings recover from heat stress by reduced electrolyte leakage, increasing seedling length and conferring membrane protection. It was observed that Brassica seedlings protect themselves against heat stress by expressing different HSPs. This supports our observation that physiological traits and transcript changes may be linked to enhanced tolerance to drought stress damage and could be used as a marker for screening against drought stress in Indian mustard genotypes.
HSP 90 plays an important role in the drought tolerance of Brassica napus (rapeseed) (Mohammadi et al. 2012). Expression levels of hsp genes may serve as markers for easier and faster HTCL (heat-tolerant cabbage lines) selection in cabbage breeding as strong or constitutive expression of several heat stress-related genes may contribute to proper cabbage head formation at high- temperature stresses (Park et al. 2013). Small HSPs (sHSPs) represent the major family of heat induced HSPs in plants (Yang et al. 2010) but Sato and Yokoya (2008) reported that high temperature exposure to rice seedlings resulted in significant increase in drought tolerance. Rice seedlings overexpressing rice sHSP17.7 also had enhanced tolerance to drought stress, which suggests that the observed drought-tolerance acquisition after heat shock is associated with the accumulation of sHSP proteins.
Genomics assisted breeding strategies have picked momentum and breeding for abiotic stress tolerant cultivars is a priority research area under climate change. Interestingly, there are reports that water saving traits co-map with major terminal drought tolerance QTL (Kholova et al. 2012). Development of drought tolerant crops is necessity of hour and there is an urgent need to look into the molecular networks involved in abiotic stress tolerance since they share a common pathway. Our study does not characterize the HSP protein categories and further analysis is needed to understand the nature of hsp gene products induced under drought stress. Knowledge on the molecular mechanism of stress tolerance in Brassica crops have not advanced much, but information available on Arabidopsis thaliana, a member of family Brassicaceae, can be directly applied which can certainly shed some light on the mechanisms underlining drought and salt tolerance in Brassica crops directly contributing to agronomic-trait improvement (Zhang et al. 2014).
References
- Abdoli M, Saeidi M. Effects of water deficiency stress during seed growth on yield and its components, germination and seedling growth parameters of some wheat cultivars. Int J Agric Crop Sci. 2012;4(15):1110–1118. [Google Scholar]
- Agarwal PK, Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol Plant. 2010;54:201–212. doi: 10.1007/s10535-010-0038-7. [DOI] [Google Scholar]
- Ahmadloo F, Tabari M, Behtari B. Effect of drought stress on the germination parameters of Cupressus seeds. Int J For Soil Eros. 2011;1(1):11–17. [Google Scholar]
- Alamillo J, Almogura C, Bartels D, Jordano J. Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum. Plant Mol Biol. 1995;29:1093–1099. doi: 10.1007/BF00014981. [DOI] [PubMed] [Google Scholar]
- Alikhan M, Ashraf MY, Mujtaba SM, Shirazi MU, Khan MA, Shereen A, Mumtaz S, Siddiqui MA, Kaleri GM. Evaluation of high yielding canola type Brassica genotypes/mutants for drought tolerance using physiological indices as screening tool. Pak J Bot. 2010;42:3807–3816. [Google Scholar]
- Amudha J, Balasubramani G. Recent molecular advances to combat abiotic stress tolerance in crop plants. Biotechnol Mol Biol Rev. 2011;6(2):31–58. [Google Scholar]
- Arjenaki FG, Jabbari R, Morshedi A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int J Agric Crop Sci. 2012;4(11):726–729. [Google Scholar]
- Ashraf M, Mehmood S. Response of four Brassica species to drought stress. Environ Exp Bot. 1990;30:93–100. doi: 10.1016/0098-8472(90)90013-T. [DOI] [Google Scholar]
- Ashraf MY, Naqvi MH, Khan AH. Evaluation of four screening techniques for drought tolerance in wheat (Triticum aestivum L.) Acta Agron Hung. 1996;44:213–220. [Google Scholar]
- Bhardwaj AR, Joshi G, Kukreja B, Malik V, Arora P, Pandey R, Shukla RN, Bankar KG, Katiyar-Agarwal S, Goel S, Jagannath A, Kumar A, Agarwal M. Global insights into high temperature and drought stress regulated genes by RNA-Seq in economically important oilseed crop Brassica juncea. BMC Plant Biol. 2015;15:9. doi: 10.1186/s12870-014-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S, Paranjpe S. Genotypic variation in the photosynthetic competence of Sorghum bicolor seedlings subjected to polyethylene glycol-mediated drought stress. J Plant Physiol. 2004;161:125–129. doi: 10.1078/0176-1617-01126. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay C, Bhattacharya BK, Kumar V, Kumar A, Meena PD. Impact of climate change on pests and diseases of oilseeds Brassica- the scenario unfolding in India. J Oilseed Brassica. 2011;2(2):48–55. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103(4):551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Ni Z, Nie X, Qin Y, Dong G, Sun Q. Isolation and characterization of genes encoding Myb transcription factor in wheat (Triticum aestivum L.) Plant Sci. 2005;169:1146–1154. doi: 10.1016/j.plantsci.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15(3):532–537. [PubMed] [Google Scholar]
- Cramer GR. Abiotic stress & plant responses from the whole vine to the genes. Aust J Grape Wine Res. 2010;16:86–93. doi: 10.1111/j.1755-0238.2009.00058.x. [DOI] [Google Scholar]
- de Albuquerque FMC, de Carvalho NM. Effect of type of environmental stress on the emergence of sunflower (Helianthus annuus L.), soyabean (Glycine max (L.) Merril) and maize (Zea mays L.) seeds with different levels of vigor. Seed Sci Technol. 2003;31(2):465–479. doi: 10.15258/sst.2003.31.2.23. [DOI] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320(5878):942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- Du H, Wang Z, Huang B (2009) Differential responses of warm- season and cool- season turfgrass species to heat stress associated with antioxidant enzyme activity. J Am Soc Hort Sci 134:417–422
- Gong M, Van der Luit A, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 1998;116:429–437. doi: 10.1104/pp.116.1.429. [DOI] [Google Scholar]
- Hazen SP, Pathan MS, Sanchez A, Baxter I, Dunn M. Expression profiling of rice segregating for drought tolerance QTLs using a rice genome array. Funct Integr Genomics. 2005;5:104–116. doi: 10.1007/s10142-004-0126-x. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Mostofa MG, Fujita M. Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed. 2013;4(7):50–70. [Google Scholar]
- Hu FD, Jones RJ. Effects of plant extracts of Bothriochloa pertusa and Urochloa mosambicensis on seed germination and seedling growth of Stylosanthes hamata cv. Verano and Stylosanthes scabra cv. Seca. Aust J Agric Res. 2004;48:1257–1264. doi: 10.1071/A97036. [DOI] [Google Scholar]
- Jajarmi V. Effect of water stress on germination indices in seven safflower cultivars (Carthamus tinctorius L.). World Academy of Science. Eng Technol. 2009;3:79–80. [Google Scholar]
- Kaur P, Ghai N, Sangha MK. Induction of thermotolerance through heat acclimation and salicylic acid in Brassica species. Afr J Biotechnol. 2009;8(4):619–625. [Google Scholar]
- Kholova J, Nepoleon T, Hash CT, Ambavat S, Rajaram V, Senthivel S, Kakkera A, Yadav R, Vadez V. Water saving traits co-map with a major terminal drought tolerance quqntitative trait locus in pearl millet [Pennisetum glaucum (L.) R. Br.] Mol Breed. 2012;30(3):1337–1353. doi: 10.1007/s11032-012-9720-0. [DOI] [Google Scholar]
- Kumar S, Sairam RK, Prabhu KV. Physiological traits for high temperature stress tolerance in Brassica juncea. Indian J Plant Physiol. 2013;18(1):89–93. doi: 10.1007/s40502-013-0015-1. [DOI] [Google Scholar]
- Lee J, Song H, Han C-T, Lim Y-P, Chung S-M, Hur Y (2010) Expression characteristics of heat shock protein genes in two comparable inbred lines of Chinese cabbage, Chiifu and Kenshin. Genes Genom 32(3):247–257
- Li C, Ren J, Luo J, Lu R. Sex-specific physiological and growth responses to water stress in Hippophae rhamnoides L. populations. Acta Physiol Plant. 2004;26:123–129. doi: 10.1007/s11738-004-0001-3. [DOI] [Google Scholar]
- Li H, Li X, Zhang D, Liu H, Guan K. Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (fabaceae) EXCLI J. 2013;12:89–101. [PMC free article] [PubMed] [Google Scholar]
- Liu A-L, Zou J, Liu C-F, Zhou X-Y, Zhang X-W, Luo G-Y, Chen X-B. Over-expression of OsHsfA7 enhanced salt and drought tolerance in transgenic rice. BMB Rep. 2013;46(1):31–36. doi: 10.5483/BMBRep.2013.46.1.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Meinke D, Franzmann L, Nickle T, Yeung E. Leafy cotyledon mutants of Arabidopsis. Plant Cell. 1994;6:1049–1064. doi: 10.1105/tpc.6.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi PP, Moieni A, Komatsu S. Comparative proteome analysis of drought-sensitive and drought-tolerant rapeseed roots and their hybrid F1 line under drought stress. Amino Acids. 2012;43(5):2137–2152. doi: 10.1007/s00726-012-1299-6. [DOI] [PubMed] [Google Scholar]
- Mu C, Zhang S, Yu G, Chen N, Li X, et al. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0082264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Park H, Ahn IY, Lee HE. Expression of Hsp70 in the thermally stressed Antarctic clam Laternula elliptica. Cell Stres Chaperons. 2007;12:275–282. doi: 10.1379/CSC-271.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Jung WY, Lee SS, Song JH, Kwon S-Y, Kim HR, Kim CW, Ahn JC, Cho HS. Use of heat stress responsive gene expression levels for early selection of heat tolerant cabbage (Brassica oleracea L.) Int J Mol Sci. 2013;14:11871–11894. doi: 10.3390/ijms140611871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizia R, Giovanni M, Stefano P, Mariarosaria DP, Natale DF, Carla P. Acquisition of thermotolerance and HSP gene expression in durum wheat (Triticum durum Desf.) cultivars. Environ Exp Bot. 2009;66(2):257–264. doi: 10.1016/j.envexpbot.2009.04.001. [DOI] [Google Scholar]
- Pinheiro C, Chaves MM. Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot. 2011;62(3):869–882. doi: 10.1093/jxb/erq340. [DOI] [PubMed] [Google Scholar]
- Pratt WB. The role of the hsp90 based chaperone system in signal transduction by nuclear receptors and receptor signalling via map dinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Hosseini SM, Pooryoosef M, Fateh I. Variation of leaf water potential, relative water content and SPAD under gradual drought stress and stress recovery in two medicinal species of Plantago ovata and P. psyllium. Plant Ecophysiol. 2010;2:53–60. [Google Scholar]
- Richter-Landsberg C, Goldbaum O. Stress proteins in neural cells: functional roles in health and disease. Cell Mol Life Sci. 2003;60:337–349. doi: 10.1007/s000180300028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M. Influence of abiotic stresses on plant proteome and metabolome Changes. Acta Physiol Plant. 2014;36:1–19. doi: 10.1007/s11738-013-1402-y. [DOI] [Google Scholar]
- Roychoudhury A, Paul S, Basu S. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013;32:985–1006. doi: 10.1007/s00299-013-1414-5. [DOI] [PubMed] [Google Scholar]
- Saibo NJ, Lourenco T, Oliveira MM. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann Bot. 2009;103:609–623. doi: 10.1093/aob/mcn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Yokoya S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 2008;27:329–334. doi: 10.1007/s00299-007-0470-0. [DOI] [PubMed] [Google Scholar]
- Saura-Mas S, Lloret F. Leaf and shoot water content and leaf dry matter content of mediterranean woody species with different post-fire regenerative strategies. Ann Bot. 2007;99:545–554. doi: 10.1093/aob/mcl284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayar R, Khemira H, Kameli A, Mosbahi M. Physiological tests as predictive appreciation for drought tolerance in durum wheat (Triticum durum Desf.) Agron Res. 2008;6(1):79–90. [Google Scholar]
- Sharma P (2010) Studies on transcription factor and related gene expression in Brassica juncea under drought and salinity stress, PhD Thesis, CCS Haryana Agricultural University
- Sharma P, Sardana V. Screening of Indian mustard (Brassica juncea) for thermo tolerance at seedling and terminal stages. J Oilseed Brassica. 2013;4(2):61–67. [Google Scholar]
- Sharma P, Pradeep, Supriya, Aneja B, Saini P, Yadav NR, Singh D, Yadav RC. Induction of myb gene under drought stress in Brassica juncea, B. carinata and B. tournefortii. J Oilseed Brassica. 2012;3((2):121. [Google Scholar]
- Shekhawat K, Rathore SS, Premi OP, Kandpal BK, Chauhan JS. Advances in agronomic management of Indian Mustard (Brassica juncea (L.) Czernj. Cosson): an overview. Int J Agron. 2012 [Google Scholar]
- Skirycz A, Inze D. More from less: plant growth under limited water. Curr Opin Biotechnol. 2010;21(2):197–203. doi: 10.1016/j.copbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Soda N, Wallace S, Karan R. Omics study for abiotic stress responses in plants. Adv Plant Agric Res. 2015;2(1):00037. [Google Scholar]
- Stephanou A, Latchman DS. Transcriptional modulation of heat-shock protein gene expression. Biochem Res Int. 2011 doi: 10.1155/2011/238601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street NR, Skogstrom O, Tucker J, Rodriguez-Acosta M, Nilsson P, Jansson S, Taylor G. The genetics and genomics of the drought response in populus. Plant J. 2006;48:321–341. doi: 10.1111/j.1365-313X.2006.02864.x. [DOI] [PubMed] [Google Scholar]
- Tattersall EA, Grimplet J, Deluc L, Wheatley MD, Vincent D, Osborne C, Ergul A, Lomen E, Blank RR, Schlauch KA, Cushman JC, Cramer GR. Transcript abundance profiles reveal larger and more complex responses of grapevine to chilling compared to osmotic and salinity stress. Funct Integr Genomics. 2007;7(4):317–333. doi: 10.1007/s10142-007-0051-x. [DOI] [PubMed] [Google Scholar]
- Tezara W, Martínez D, Rengifo E, Herrera A. Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann Bot. 2003;92:757–765. doi: 10.1093/aob/mcg199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacova J, Angelovicova M. Heat shock proteins (HSPs): a review. Anim Sci Biotechnol. 2012;45(1):349–353. [Google Scholar]
- Turhan H (1997) Salinity studies in potato (Solanum tuberosum L.). Ph.D. Thesis, p. 255. The University of Reading, Reading, UK
- Vernon D, Meinke D. Late embryo-defective mutants of Arabidopsis. Dev Genet. 1995;16:311–320. doi: 10.1002/dvg.1020160404. [DOI] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol. 2000;122:1099–1108. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Hernandez L, Finkelstein R, Vierling E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996;112:757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BC, Jacobs DF (2004) Using electrolyte leakage for evaluating hardwood seedling cold hardiness. USDA Forest Service Proceedings RMRS-P-33
- Wilson RA, Sangha MK, Banga SS, Atwal AK, Gupta S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J Environ Biol. 2014;35:383–387. [PubMed] [Google Scholar]
- Xu X, Peng G, Wu C, Korpelainen H, Li C. Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiol. 2008;28:1751–1759. doi: 10.1093/treephys/28.11.1751. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Dillenburg LR. Measurements of leaf relative water content in Araucaria angustifolia. Rev Bras de Fisiol Veg. 1999;11(2):69–75. [Google Scholar]
- Yang S, Vanderbeld B, Wan J, Huang Y. Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol Plant. 2010;3(3):469–490. doi: 10.1093/mp/ssq016. [DOI] [PubMed] [Google Scholar]
- Yang G, Wang Y, Zhang K, Gao C. Expression analysis of nine small heat shock protein genes from Tamarix hispida in response to different abiotic stresses and abscisic acid treatment. Mol Biol Rep. 2014;41(3):1279–1289. doi: 10.1007/s11033-013-2973-9. [DOI] [PubMed] [Google Scholar]
- Yin C, Duan B, Wang X, Li C. Morphological and physiological responses of two contrasting poplar species to drought stress and exogenous abscisic acid application. Plant Sci. 2004;167:1091–1097. doi: 10.1016/j.plantsci.2004.06.005. [DOI] [Google Scholar]
- Yu X, Yang J, Li X, Liu X, Sun C, Wu F, He Y. Global analysis of cis-natural antisense transcripts and their heat-responsive nat-siRNAs in Brassica rapa. BMC Plant Biol. 2013;13:208. doi: 10.1186/1471-2229-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zang R, Li C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004;166:791–797. doi: 10.1016/j.plantsci.2003.11.016. [DOI] [Google Scholar]
- Zhang X, Wollenweber B, Jiang D, Liu F, Zhao J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis constitutively expressing ABP9, a ZIP transcription factor. J Exp Bot. 2008;59(4):839–848. doi: 10.1093/jxb/erm364. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lu G, Long W, Zou X, Li F, Nishio T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed Sci. 2014;64(1):60–73. doi: 10.1270/jsbbs.64.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Kang H, Tan H, Xu M. Effects of drought stresses induced by polyethylene glycol on germination of Pinus sylvestris var. mongolica seeds from natural and plantation forests on sandy land. J For Res. 2006;11:319–328. doi: 10.1007/s10310-006-0214-y. [DOI] [Google Scholar]