Abstract
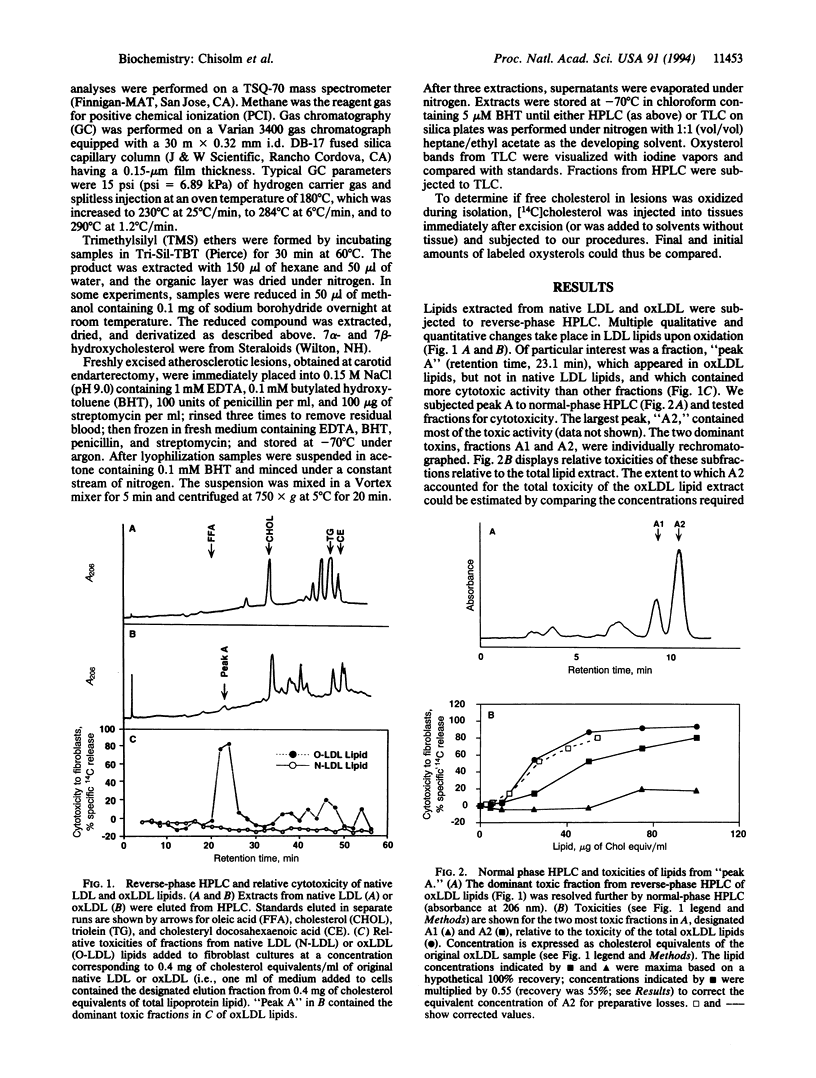
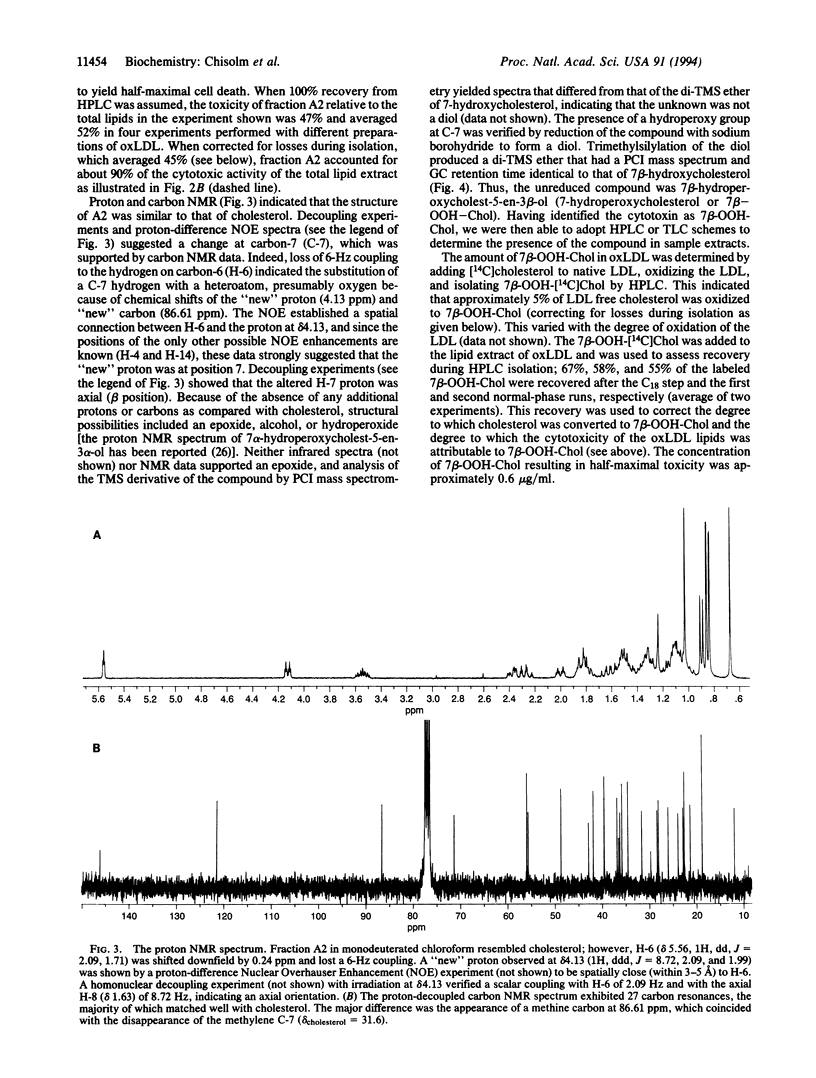
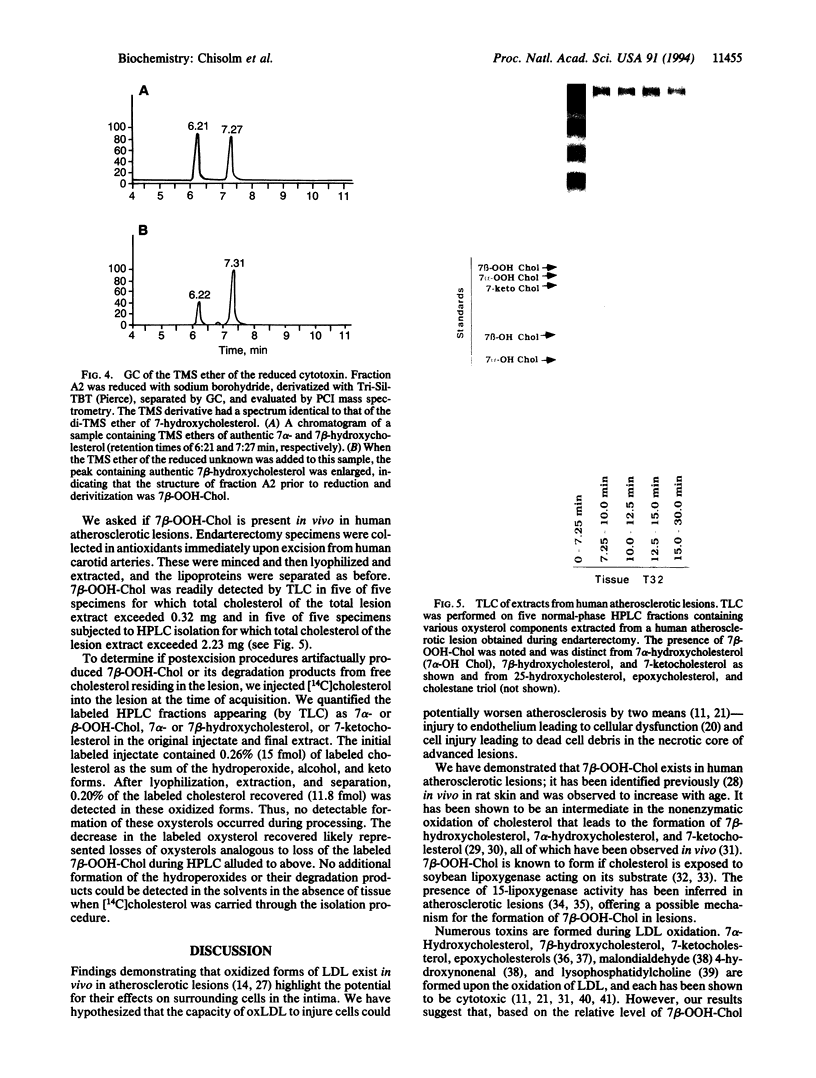
Modification of low density lipoprotein (LDL) by free radical oxidation renders this molecular complex cytotoxic. Oxidized lipoproteins exist in vivo in atherosclerotic lesions and in the plasma of diabetic animals, suggesting that lipoprotein-induced tissue damage may occur in certain diseases. We undertook purification and identification of the major cytotoxin in oxidized LDL. The lipid extract from oxidized LDL was subjected to multiple HPLC separations, and the fractions were assayed for cytotoxicity. Mass spectrometry and nuclear magnetic resonance identified the purified toxin as 7 beta-hydroperoxycholest-5-en-3 beta-ol (7 beta-OOH-Chol). This molecule accounted for approximately 90% of the cytotoxicity of the lipids of oxidized LDL. We also found 7 beta-OOH-Chol in human atherosclerotic lesions from endarterectomy specimens obtained immediately after excision. These results are consistent with the hypothesis that the oxidized LDL present in lesions has the capacity to induce cell and tissue injury, leading to progression of the disease and the generation of the necrotic core of the lesion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aringer L. Oxidation of 3-oxygenated delta 4 and delta 5 - C27 steroids by soybean lipoxygenase and rat liver microsomes. Lipids. 1980 Aug;15(8):563–571. doi: 10.1007/BF02534180. [DOI] [PubMed] [Google Scholar]
- Bhadra S., Arshad M. A., Rymaszewski Z., Norman E., Wherley R., Subbiah M. T. Oxidation of cholesterol moiety of low density lipoprotein in the presence of human endothelial cells or Cu+2 ions: identification of major products and their effects. Biochem Biophys Res Commun. 1991 Apr 15;176(1):431–440. doi: 10.1016/0006-291x(91)90942-z. [DOI] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Chisolm G. M. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991 Jan;32(1):63–70. [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Drüeke T., Hennessen U., Nabarra B., Ben Nasr L., Lucas P. A., Dang P., Thomasset M., Lacour B., Coudrier E., McCarron D. A. Ultrastructural and functional abnormalities of intestinal and renal epithelium in the SHR. Kidney Int. 1990 Jun;37(6):1438–1448. doi: 10.1038/ki.1990.134. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Hodis H. N., Kramsch D. M., Avogaro P., Bittolo-Bon G., Cazzolato G., Hwang J., Peterson H., Sevanian A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-). J Lipid Res. 1994 Apr;35(4):669–677. [PubMed] [Google Scholar]
- Jürgens G., Hoff H. F., Chisolm G. M., 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Morel D. W., DiCorleto P. E., Chisolm G. M. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987 Mar;130(3):311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- McNally A. K., Chisolm G. M., 3rd, Morel D. W., Cathcart M. K. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990 Jul 1;145(1):254–259. [PubMed] [Google Scholar]
- Morel D. W., Chisolm G. M. Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989 Dec;30(12):1827–1834. [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Murugesan G., Chisolm G. M., Fox P. L. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993 Feb;120(4):1011–1019. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre-Salvayre A., Lopez M., Levade T., Pieraggi M. T., Dousset N., Douste-Blazy L., Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990 Aug 6;1045(3):224–232. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- Ozawa N., Yamazaki S., Chiba K., Aoyama H., Tomisawa H., Tateishi M., Watabe T. Occurrence of cholesterol 7 alpha- and 7 beta-hydroperoxides in rat skin as aging markers. Biochem Biophys Res Commun. 1991 Jul 15;178(1):242–247. doi: 10.1016/0006-291x(91)91805-m. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen K. S., Boberg K. M., Stabursvik A., Prydz H. Toxicity of oxygenated cholesterol derivatives toward cultured human umbilical vein endothelial cells. Arterioscler Thromb. 1991 Mar-Apr;11(2):423–428. doi: 10.1161/01.atv.11.2.423. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A., Berliner J., Peterson H. Uptake, metabolism, and cytotoxicity of isomeric cholesterol-5,6-epoxides in rabbit aortic endothelial cells. J Lipid Res. 1991 Jan;32(1):147–155. [PubMed] [Google Scholar]
- Shirhatti V., Krishna G. A simple and sensitive method for monitoring drug-induced cell injury in cultured cells. Anal Biochem. 1985 Jun;147(2):410–418. doi: 10.1016/0003-2697(85)90290-8. [DOI] [PubMed] [Google Scholar]
- Smith L. L., Johnson B. H. Biological activities of oxysterols. Free Radic Biol Med. 1989;7(3):285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. F., Basra H. J., Steinbrecher U. P. Effects of oxidatively modified LDL on cholesterol esterification in cultured macrophages. J Lipid Res. 1990 Aug;31(8):1361–1369. [PubMed] [Google Scholar]
- van Lier J. E., Kan G., Langlois R., Smith L. L. On the role of sterol hydroperoxides in steroid metabolism. Biochem Soc Symp. 1972;34:21–43. [PubMed] [Google Scholar]




