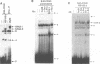Abstract
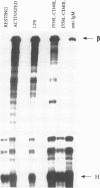
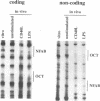
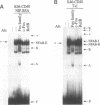
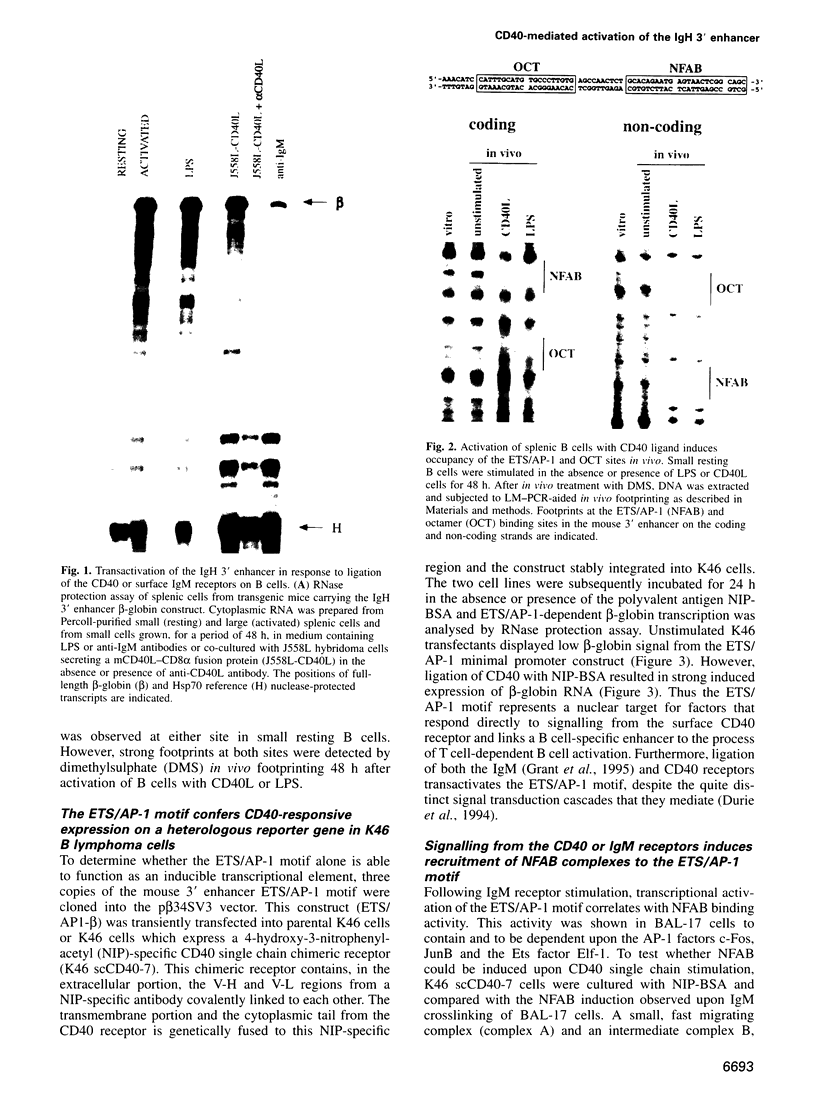
Immunoglobulin heavy chain (IgH) class switch recombination and regulation of IgH expression levels are processes suggested to be controlled by the IgH 3' enhancer. Here we demonstrate that CD40 or IgM receptor stimulation of primary B cells results in transactivation of this enhancer. 4-Hydroxy-3-nitrophenylacetyl (NIP)-BSA induction of a K46 B cell line expressing a chimeric NIP-specific CD40 single chain receptor results in a ligand receptor-dependent response of a 3' enhancer ETS/AP-1 minimal promoter construct. Gel retardation analysis and genomic footprinting experiments reveal that CD40 or IgM induction recruits NFAB (nuclear factors of activated B cells) to the ETS/AP-1 motif. While IgM signalling recruits c-Fos, JunB and Elf-1 (NFAB-I), only JunB and Elf-1 were observed following CD40 signalling (NFAB-II). CD40 signalling, however, induces a Fos family-related partner for JunB, which may account for the transcriptional activity observed by NFAB-II in K46 cells. We propose a model whereby CD40 and IgM receptor-mediated signalling converge in the process of 3' enhancer activation in B lymphocytes. Our data provide a putative molecular explanation as to why CD40L-deficient mice, and possibly patients with hyper-IgM syndrome, are unable to undergo T cell-dependent class switch recombination but respond properly upon lipopolysaccharide-induced switch recombination.
Full text
PDF



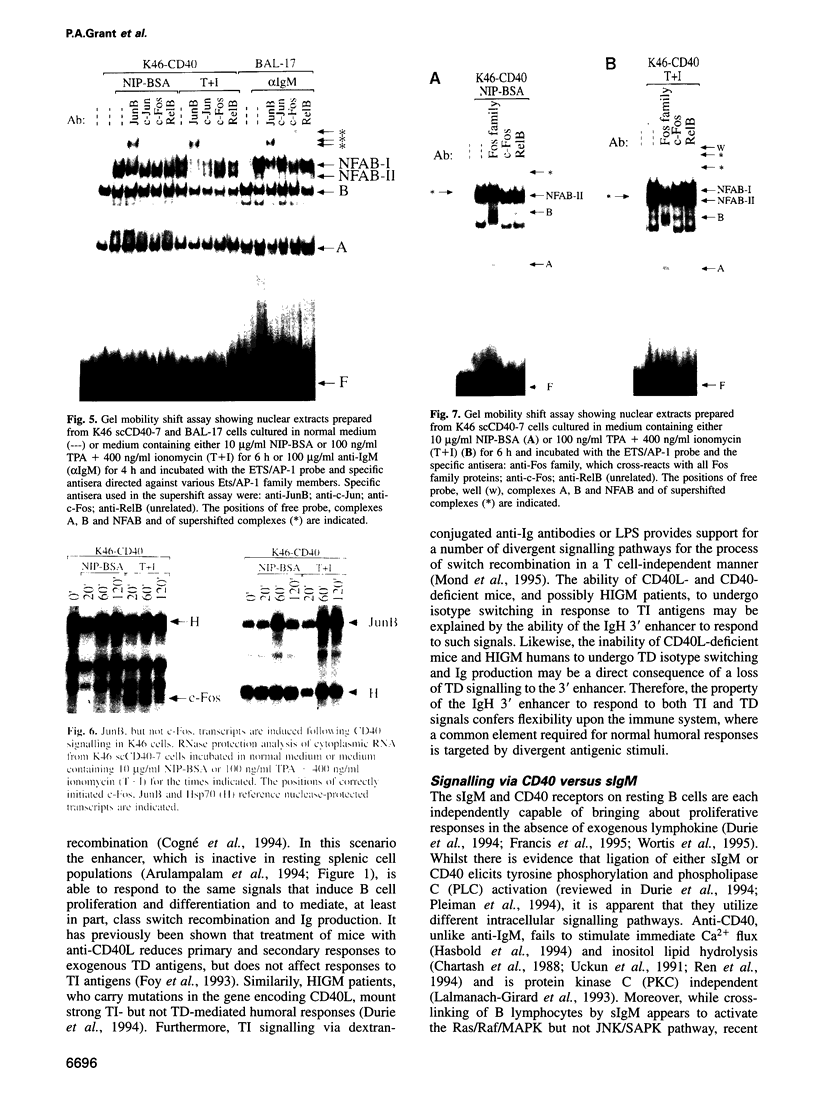




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Arulampalam V., Furebring C., Samuelsson A., Lendahl U., Borrebaeck C., Lundkvist I., Pettersson S. Elevated expression levels of an Ig transgene in mice links the IgH 3' enhancer to the regulation of IgH expression. Int Immunol. 1996 Jul;8(7):1149–1157. doi: 10.1093/intimm/8.7.1149. [DOI] [PubMed] [Google Scholar]
- Arulampalam V., Grant P. A., Samuelsson A., Lendahl U., Pettersson S. Lipopolysaccharide-dependent transactivation of the temporally regulated immunoglobulin heavy chain 3' enhancer. Eur J Immunol. 1994 Jul;24(7):1671–1677. doi: 10.1002/eji.1830240732. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Bassuk A. G., Leiden J. M. A direct physical association between ETS and AP-1 transcription factors in normal human T cells. Immunity. 1995 Aug;3(2):223–237. doi: 10.1016/1074-7613(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Berberich I., Shu G., Siebelt F., Woodgett J. R., Kyriakis J. M., Clark E. A. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO J. 1996 Jan 2;15(1):92–101. [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Graninger P., Braselmann S., Wrighton C., Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol. 1995 Jul;15(7):3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian A. A. Stimulation of B-cell proliferation by membrane-associated molecules from activated T cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):564–568. doi: 10.1073/pnas.85.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S. J., Sassone-Corsi P. Fos, Jun and CREB basic-domain peptides have intrinsic DNA-binding activity enhanced by a novel stabilizing factor. Oncogene. 1990 Oct;5(10):1549–1556. [PubMed] [Google Scholar]
- Castigli E., Alt F. W., Davidson L., Bottaro A., Mizoguchi E., Bhan A. K., Geha R. S. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartash E. K., Imai A., Gershengorn M. C., Crow M. K., Friedman S. M. Direct human T helper cell-induced B cell activation is not mediated by inositol lipid hydrolysis. J Immunol. 1988 Mar 15;140(6):1974–1981. [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Cogné M., Lansford R., Bottaro A., Zhang J., Gorman J., Young F., Cheng H. L., Alt F. W. A class switch control region at the 3' end of the immunoglobulin heavy chain locus. Cell. 1994 Jun 3;77(5):737–747. doi: 10.1016/0092-8674(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Dariavach P., Williams G. T., Campbell K., Pettersson S., Neuberger M. S. The mouse IgH 3'-enhancer. Eur J Immunol. 1991 Jun;21(6):1499–1504. doi: 10.1002/eji.1830210625. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Bonnefoy J. Y., Gauchat J. F., Fischer A., de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- Durie F. H., Foy T. M., Masters S. R., Laman J. D., Noelle R. J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994 Sep;15(9):406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Foy T. M., Laman J. D., Ledbetter J. A., Aruffo A., Claassen E., Noelle R. J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994 Jul 1;180(1):157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T. M., Shepherd D. M., Durie F. H., Aruffo A., Ledbetter J. A., Noelle R. J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993 Nov 1;178(5):1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. A., Karras J. G., Ke X. Y., Sen R., Rothstein T. L. Induction of the transcription factors NF-kappa B, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995 Feb;7(2):151–161. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Loh R., Jabara H., Rosen R. S., Chatila T., Fu S. M., Stamenkovic I., Geha R. S. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grant P. A., Arulampalam V., Ahrlund-Richter L., Pettersson S. Identification of Ets-like lymphoid specific elements within the immunoglobulin heavy chain 3' enhancer. Nucleic Acids Res. 1992 Sep 11;20(17):4401–4408. doi: 10.1093/nar/20.17.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. A., Thompson C. B., Pettersson S. IgM receptor-mediated transactivation of the IgH 3' enhancer couples a novel Elf-1-AP-1 protein complex to the developmental control of enhancer function. EMBO J. 1995 Sep 15;14(18):4501–4513. doi: 10.1002/j.1460-2075.1995.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hasbold J., Johnson-Léger C., Atkins C. J., Clark E. A., Klaus G. G. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol. 1994 Aug;24(8):1835–1842. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hirohata S., Jelinek D. F., Lipsky P. E. T cell-dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J Immunol. 1988 Jun 1;140(11):3736–3744. [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994 Jun;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992 Nov;12(11):5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991 May;11(5):2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalmanach-Girard A. C., Chiles T. C., Parker D. C., Rothstein T. L. T cell-dependent induction of NF-kappa B in B cells. J Exp Med. 1993 Apr 1;177(4):1215–1219. doi: 10.1084/jem.177.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lane P., Brocker T., Hubele S., Padovan E., Lanzavecchia A., McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993 Apr 1;177(4):1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Parodi B., Celada F. Activation of human B lymphocytes: frequency of antigen-specific B cells triggered by alloreactive or by antigen-specific T cell clones. Eur J Immunol. 1983 Sep;13(9):733–738. doi: 10.1002/eji.1830130908. [DOI] [PubMed] [Google Scholar]
- Lieberson R., Giannini S. L., Birshtein B. K., Eckhardt L. A. An enhancer at the 3' end of the mouse immunoglobulin heavy chain locus. Nucleic Acids Res. 1991 Feb 25;19(4):933–937. doi: 10.1093/nar/19.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberson R., Ong J., Shi X., Eckhardt L. A. Immunoglobulin gene transcription ceases upon deletion of a distant enhancer. EMBO J. 1995 Dec 15;14(24):6229–6238. doi: 10.1002/j.1460-2075.1995.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Vos Q., Lees A., Snapper C. M. T cell independent antigens. Curr Opin Immunol. 1995 Jun;7(3):349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Neuberg M., Schuermann M., Hunter J. B., Müller R. Two functionally different regions in Fos are required for the sequence-specific DNA interaction of the Fos/Jun protein complex. Nature. 1989 Apr 13;338(6216):589–590. doi: 10.1038/338589a0. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Max E. E., Strober W. Pax5 (BSAP) regulates the murine immunoglobulin 3' alpha enhancer by suppressing binding of NF-alpha P, a protein that controls heavy chain transcription. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5336–5340. doi: 10.1073/pnas.92.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- Pettersson S., Cook G. P., Brüggemann M., Williams G. T., Neuberger M. S. A second B cell-specific enhancer 3' of the immunoglobulin heavy-chain locus. Nature. 1990 Mar 8;344(6262):165–168. doi: 10.1038/344165a0. [DOI] [PubMed] [Google Scholar]
- Pettersson S., Sharpe M. J., Gilmore D. R., Surani M. A., Neuberger M. S. Cellular selection leads to age-dependent and reversible down-regulation of transgenic immunoglobulin light chain genes. Int Immunol. 1989;1(5):509–516. doi: 10.1093/intimm/1.5.509. [DOI] [PubMed] [Google Scholar]
- Pleiman C. M., D'Ambrosio D., Cambier J. C. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994 Sep;15(9):393–399. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Preston G. A., Lyon T. T., Yin Y., Lang J. E., Solomon G., Annab L., Srinivasan D. G., Alcorta D. A., Barrett J. C. Induction of apoptosis by c-Fos protein. Mol Cell Biol. 1996 Jan;16(1):211–218. doi: 10.1128/mcb.16.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C. L., Morio T., Fu S. M., Geha R. S. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase C gamma 2. J Exp Med. 1994 Feb 1;179(2):673–680. doi: 10.1084/jem.179.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw B. R., Fanslow W. C., 3rd, Armitage R. J., Campbell K. A., Liggitt D., Wright B., Davison B. L., Maliszewski C. R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994 Nov 1;180(5):1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen F. S. X-linked immunodeficiency: B-cell diseases. Curr Biol. 1993 May 1;3(5):312–314. doi: 10.1016/0960-9822(93)90189-u. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Rüther U., Müller W., Sumida T., Tokuhisa T., Rajewsky K., Wagner E. F. c-fos expression interferes with thymus development in transgenic mice. Cell. 1988 Jun 17;53(6):847–856. doi: 10.1016/s0092-8674(88)90289-9. [DOI] [PubMed] [Google Scholar]
- Sakata N., Patel H. R., Terada N., Aruffo A., Johnson G. L., Gelfand E. W. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J Biol Chem. 1995 Dec 22;270(51):30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Schuermann M., Neuberg M., Hunter J. B., Jenuwein T., Ryseck R. P., Bravo R., Müller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989 Feb 10;56(3):507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Dibirdik I., Chandan-Langlie M., Tuel-Ahlgren L., Ledbetter J. A. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the Pan-B-cell receptor CD40/Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991 Sep 15;266(26):17478–17485. [PubMed] [Google Scholar]
- Wakatsuki Y., Neurath M. F., Max E. E., Strober W. The B cell-specific transcription factor BSAP regulates B cell proliferation. J Exp Med. 1994 Apr 1;179(4):1099–1108. doi: 10.1084/jem.179.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- Wisdon R., Verma I. M. Transformation by Fos proteins requires a C-terminal transactivation domain. Mol Cell Biol. 1993 Dec;13(12):7429–7438. doi: 10.1128/mcb.13.12.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Teutsch M., Higer M., Zheng J., Parker D. C. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3348–3352. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. Mice deficient for the CD40 ligand. Immunity. 1994 Aug;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]