Abstract
Background
Postoperative delirium is a frequent complication in elderly patients undergoing major abdominal surgery and is associated with a poor outcome. We compared postoperative delirium in elderly patients following laparoscopic gastrectomy (LG) versus open gastrectomy (OG).
Methods
In total, 130 patients aged ≥ 65 years with gastric cancer undergoing LG and OG were enrolled prospectively. Postoperative delirium and cognitive status were assessed daily using the Confusion Assessment Method (CAM) and Mini-Mental Status Examination (MMSE), respectively, for 3 days postoperatively. For CAM-positive patients, delirium severity was then assessed using the Delirium Index (DI).
Results
In total, 123 subjects (LG, n = 60; OG, n = 63) were included in the analysis. In both groups, the overall incidences of postoperative delirium were similar: 31.6% (19/60) in the LG group and 41.2% (26/63) in the OG group. When considering only those with delirium, the severity, expressed as the highest DI score, was similar between the groups. A decline in cognitive function (reduction in MMSE ≥ 2 points from baseline) during 3 days postoperatively was observed in 23 patients in the LG group (38.3%) and 27 patients in the OG group (42.9%) (P = 0.744). In both groups, postoperative cognitive decline was significantly associated with postoperative delirium (P < 0.001).
Conclusions
We found that, compared with traditional open gastrectomy, laparoscopic gastrectomy did not reduce either postoperative delirium or cognitive decline in elderly patients with gastric cancer.
Keywords: Aged, Delirium, Gastrectomy, Laparoscopy
Introduction
Together with the increased proportion of the elderly in the general population, the number of surgical procedures for elderly patients with gastric cancer has been increasing. An elderly patient with an already reduced cognitive reserve is at greater risk of delirium or cognitive decline in the postoperative period [1]. In particular, for abdominal surgery, its incidence ranges from 35 to 60% in elderly patients [2,3]. Postoperative delirium is associated with increased lengths of hospital stay, costs, morbidity, and mortality [4].
In addition to advanced age, there is growing evidence that systemic stress and the inflammatory response also play important roles in the pathogenesis of delirium [1,5,6]. Thus, reducing perioperative stress and inflammatory responses may minimize the occurrence of delirium. Laparoscopic gastrectomy, compared with open gastrectomy, causes less surgical trauma due to the minimal abdominal wall incision. It also offers other important advantages, such as reduced postoperative pain, reduced use of opioid analgesics, and faster functional recovery [7]. These advantages may lead to reduce postoperative delirium [1].
Thus, we hypothesized that laparoscopic gastrectomy would prevent or protect elderly patients from developing postoperative delirium more than open gastrectomy. To test this, we conducted a prospective, non-randomized, controlled study to compare laparoscopic versus open gastrectomy in patients aged ≥ 65 years with gastric cancer with respect to postoperative delirium (primary outcome) and postoperative cognitive decline (secondary outcome).
Materials and Methods
This study was approved by the Institutional Review Board at our hospital. All patients provided written informed consent before study entry. Between March 2013 and January 2014, 130 consecutive patients of American Society of Anesthesiologists physical status I or II, aged ≥ 65 years, scheduled to undergo elective laparoscopic gastrectomy (n = 65, LG group) or open gastrectomy (n = 65, OG group) for gastric cancer were enrolled.
Exclusion criteria were a preoperative score less than 24 on the Mini-Mental State Examination (MMSE) [8], neurological diseases, or diagnosed dementia before admission for surgery, and any other psychiatric illness. Patients unable to communicate due to severely impaired hearing were also excluded.
Anesthetic and operative procedures
Routine monitoring was used, and a BIS Quatro sensor (Covidien, Mansfield, MA, USA) was applied to the patient's forehead before inducing anesthesia. With no premedication, anesthesia was induced intravenously with lidocaine (30 mg), propofol (1.5-2.0 mg/kg), and rocuronium (0.5 mg/kg), and was maintained with sevoflurane and 50% oxygen in air. Anesthesia was supplemented with an intravenous (IV) infusion of remifentanil at a constant rate (0.05 µg/kg/min) until 5 min before the end of surgery. The depth of anesthesia was controlled by altering the inhaled sevoflurane concentration, based on the hemodynamic response and bispectral index (BIS) values (target values of 40-60). Muscle relaxation was supplemented with IV vecuronium (0.02 mg/kg) at regular intervals.
Throughout surgery, end-tidal concentrations of sevoflurane were recorded at 5 min intervals using a pre-calibrated gas monitor (Datex-Ohmeda Airway module for Aestiva/5 M-CAiOVX-S5, Helsinki, Finland). In each patient, the anesthetic exposure was also calculated as the minimum alveolar concentration (MAC)-h (average MAC × length of exposure). In addition, at the end of each case, BIS data were collected from the internal memory of the BIS monitor and stored on a disk for further offline analysis. The BIS reading was taken as the average of four readings at 15 s intervals over a 1 min period with the smoothing ratio set at 15 s. Then, we calculated the time-averaged BIS values during the surgery. The amount of time when the BIS was < 40 (as an indicator of deep hypnosis) was noted. At the end of surgery, the residual neuromuscular blockade was reversed with IV glycopyrrolate (8 µg/kg) and pyridostigmine (0.2 mg/kg).
Gastric resection and determination of the dissection area of the lymph node stations were performed based on the 2010 Japanese gastric cancer treatment guidelines [9]. Laparoscopic gastrectomy procedures included both laparoscopy-assisted gastrectomy and totally laparoscopic gastrectomy. At our institution, the surgical indication for both laparoscopic procedures was the same: preoperative stage T1-2N0-1. While a 25-30 cm-long upper median skin incision was made for the open gastrectomy, a 4-5 cm midline incision in the epigastrium (laparoscopy-assisted gastrectomy) or vertical incision in the infraumbilical port site (totally laparoscopic gastrectomy) was made for laparoscopic gastrectomy. The three participating surgeons were all familiar with both open and laparoscopic surgery and had performed each procedure more than 100 times.
After surgery, all patients were managed using the following standardized postoperative protocol: (1) IV patient-controlled analgesia (PCA) using 120 mg of ketorolac and 1,200 µg of fentanyl in 100 ml of saline (the basal infusion rate, bolus dose, and lockout interval were 0.5 ml/h, 0.5 ml, and 15 min, respectively), and IV injection of rescue analgesics (25 mg of meperidine) at the patient's request, (2) removal of the nasogastric tube and Foley catheter on postoperative day 1, and encouragement of early ambulation, (3) clear liquid diet after the first flatus, and (4) discharge after tolerance of a soft diet for 2 more days if no surgical or systemic complications occurred.
Outcome measures
The presence of delirium was assessed once a day for up to 3 days postoperatively with the Confusion Assessment Method (CAM), which has been validated in the elderly with high sensitivity and specificity [10,11]. For CAM-positive patients, the severity of delirium was then assessed using the Delirium Index (DI) [12]. The DI includes seven neurocognitive symptom domains (disorders of attention, thought, consciousness, orientation, memory, perception, and psychomotor activity), each scored on a scale from 0 (absent) to 3 (present and severe). Thus, the total DI score ranges from 0 to 21, with a higher score indicating greater severity.
Cognitive function of the patients was assessed using the MMSE (score range of 0-30) the day before surgery, which was then repeated on days 1, 2, and 3 postoperatively. A decrease in MMSE score ≥ 2 points from baseline was considered to indicate a decline in cognitive function [13]. All tests were conducted independently by a trained research nurse blinded to the group assignment.
Pain score at rest and coughing on 11-point numerical rating scale (NRS), cumulative IV PCA consumption, and cumulative rescue analgesic consumption were recorded on days 1, 2 and 3 postoperatively. Postoperative recovery profiles (time to first time out of bed, time to first ward ambulation, time to pass flatus, and length of hospital stay) were also documented.
Statistical analysis
The primary outcome was the overall incidence of postoperative delirium within 3 days after surgery. We projected this incidence to be 35% in the OG group, based on the findings of several previous studies [2,3]. We considered a 2/3 reduction in the incidence in the LG group would be clinically significant. Assuming a statistical power of 80% at an alpha level of 0.05, we estimated that 59 patients would be required per group. To account for potential for drop-outs or incomplete follow-up for some subjects, we planned to enroll 130 patients.
Categorical variables (e.g., the presence of delirium or cognitive decline) were compared using the Pearson's χ2-test with a continuity correction or Fisher's exact test, as applicable. Continuous variables (e.g., MAC-hours, pain NRS score, cumulative IV PCA or rescue analgesic consumption, and postoperative recovery profiles) were tested for normality using the Kolmogorov-Smirnov test. Non-normally distributed continuous variables were analyzed using the Mann-Whitney U-test. Normally distributed variables were analyzed using the unpaired t-test. In both groups, further sub-group analysis was performed to examine the association between postoperative delirium and cognitive decline. P < 0.05 indicated statistical significance.
Results
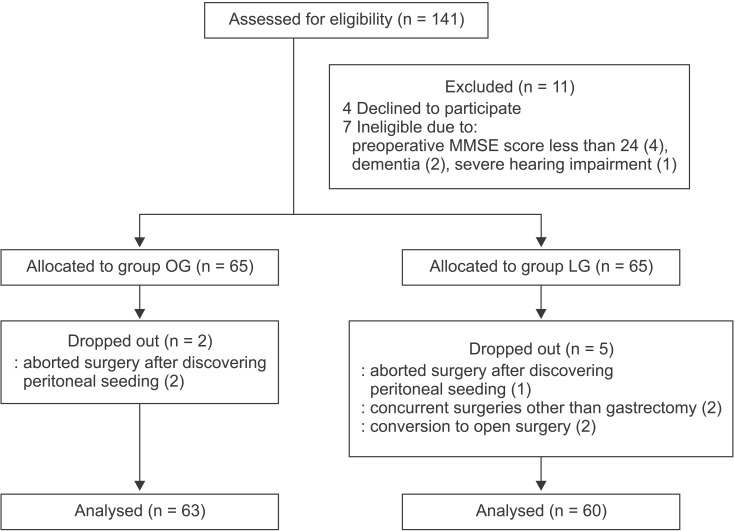
Of the 130 subjects enrolled, two in the OG group and five in the LG group were excluded (Fig. 1). Finally, 63 subjects in the OG group and 60 subjects in the LG group were included in the analysis.
Fig. 1. CONSORT flow diagram. MMSE: Mini-Mental State Examination.
While 48 cases of subtotal gastrectomy and 15 cases of total gastrectomy were performed in the OG group, 38, 21, and 1 case in the LG group were laparoscopy-assisted distal gastrectomy, totally laparoscopic distal gastrectomy, and totally laparoscopic total gastrectomy, respectively. Except for estimated blood loss, there was no significant difference between the groups in demographic, anesthetic, and surgical data (Tables 1 and 2). In both groups, preoperative baseline MMSE values were similar: 27.0 ± 1.7 in the LG group and 26.7 ± 1.7 in the OG group.
Table 1. Demographic Data in the Open Gastrectomy (OG) and Laparoscopic Gastrectomy (LG) Groups.
| OG group (n = 63) |
LG Group (n = 60) |
P value | |
|---|---|---|---|
| Gender (F/M) | 23/40 | 22/38 | 1.000 |
| Age (yr) | 72.2 ± 4.6 | 71.6 ± 5.0 | 0.484 |
| BMI (kg/m2) | 23.7 ± 3.2 | 24.6 ± 8.0 | 0.374 |
| ASA physical status (I/II) | 14/49 | 23/37 | 0.080 |
| Education level (less than high school/ high school/college graduate) | 34/23/6 | 29/20/11 | 0.391 |
| Heavy drinker* (yes/no) | 7/56 | 5/55 | 0.830 |
| Any tobacco exposure (yes/no) | 26/37 | 27/33 | 0.814 |
| Preoperative baseline MMSE (0-30) | 26.7 ± 1.7 | 27.0 ± 1.7 | 0.338 |
Data are expressed as the means ± SD or number. BMI: body mass index, ASA: American Society of Anesthesiologists, MMSE: Mini-Mental State Examination. *Defined as current intake of alcohol, on average, 3-4 drinks per day at least four times per week.
Table 2. Anesthetic and Surgical Data in the Open Gastrectomy (OG) and Laparoscopic Gastrectomy (LG) Groups.
| OG group (n = 63) |
LG Group (n = 60) |
P value | |
|---|---|---|---|
| Duration of surgery (min) | 146.3 ± 34.9 | 141.3 ± 37.8 | 0.446 |
| Duration of anesthesia (min) | 183.4 ± 35.2 | 179.2 ± 44.8 | 0.556 |
| EBL (ml) | 207.1 ± 178.2 | 111.2 ± 58.9 | < 0.001* |
| Total time when BIS < 40 (min) | 19.8 ± 26.3 | 22.6 ± 32.7 | 0.601 |
| MAC-hours | 2.6 ± 0.8 | 2.4 ± 0.8 | 0.187 |
Data are expressed as the means ± SD or number. EBL: estimated blood loss, BIS: bispectral index, MAC: minimum alveolar concentration. *Statistically significant difference (P < 0.05).
All NRS pain scores at rest and coughing in the LG group were significantly lower than in the OG group at 24, 48, and 72 h postoperatively. Significantly less of both IV PCA and rescue analgesic were required in the LG group than in the OG group (Table 3).
Table 3. Postoperative Data for the Open Gastrectomy (OG) and Laparoscopic Gastrectomy (LG) Groups.
| OG group (n = 63) | LG Group (n = 60) | P value | |
|---|---|---|---|
| Pain NRS score at rest (0-10) | |||
| 24 h | 5.1 ± 1.9 | 4.2 ± 1.8 | 0.007* |
| 48 h | 4.5 ± 1.8 | 3.5 ± 1.5 | 0.001* |
| 72 h | 3.4 ± 1.6 | 2.4 ± 1.1 | < 0.001* |
| Pain NRS score at coughing (0-10) | |||
| 24 h | 7.1 ± 1.7 | 6.3 ± 1.7 | 0.007* |
| 48 h | 6.7 ± 1.6 | 5.5 ± 1.6 | < 0.001* |
| 72 h | 5.5 ± 1.9 | 4.2 ± 1.6 | < 0.001* |
| Cumulative IV PCA consumption at 72 h (ml) | 52.6 ± 9.9 | 47.8 ± 11.9 | 0.017* |
| Cumulative rescue meperidine consumption (mg) | 183.7 ± 104.1 | 112.5 ± 114.1 | < 0.001* |
| Time to the first out of bed (h) | 25.8 ± 8.7 | 22.3 ± 4.7 | 0.007* |
| Time to first ward ambulation (h) | 27.3 ± 11.1 | 22.8 ± 5.4 | 0.006* |
| Time to pass flatus (days) | 4.4 ± 0.9 | 3.9 ± 0.7 | 0.002* |
| Length of hospital stay (days) | 8.4 ± 1.1 | 7.8 ± 1.5 | 0.018* |
Data are expressed as the means ± SD. NRS: numerical rating scale, IV PCA: intravenous patient-controlled analgesia. *Statistically significant difference (P < 0.05).
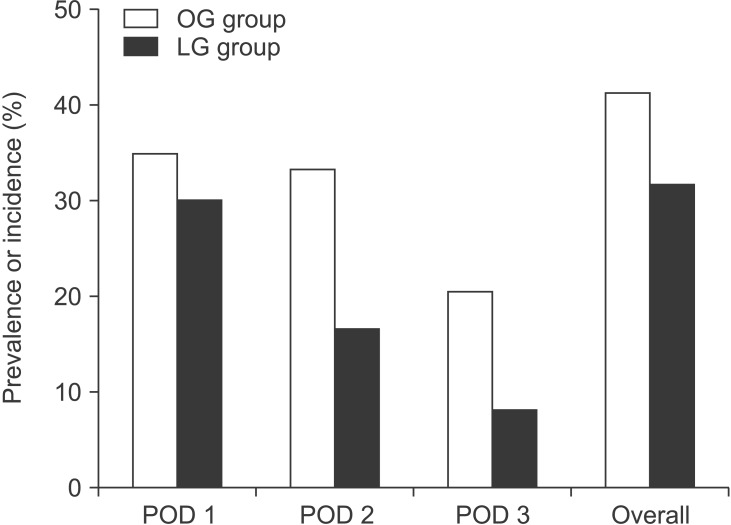
In both groups, the overall incidence of postoperative delirium was similar: 31.6% (19/60) in the LG group and 41.2% (26/63) in the OG group (P = 0.359). Regarding the individual prevalence of delirium on each of the 3 days after surgery, there was no significant difference between the groups (Fig. 2). In both groups, most delirium was observed on postoperative day 1 (18/19 delirious patients in the LG group and 22/26 in the OG group). No patient in either group was newly diagnosed with delirium on postoperative day 3. When considering only those in whom delirium occurred, the severities of postoperative delirium, expressed as the highest DI score, were similar between the LG and OG groups (5.4 ± 1.7 vs. 6.0 ± 3.4; respectively) (P = 0.477). In both groups, no pharmacological intervention occurred.
Fig. 2. Individual prevalence of delirium on postoperative days (POD) 1, 2, and 3, and overall incidence of delirium in the 3 days postoperatively in the open gastrectomy (OG) and laparoscopic gastrectomy (LG) groups. There was no significant difference between the groups in the individual prevalence of delirium on POD 1, 2 or 3 (P = 0.697, P = 0.055, and P = 0.094, respectively). There was no significant difference between the groups in the overall incidence of delirium during the 3 days postoperatively (P = 0.359).
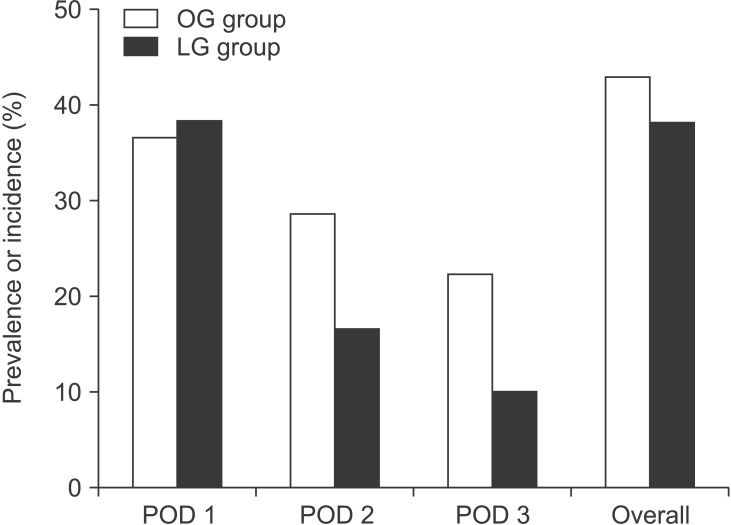
Declines in cognitive function during the 3 days postoperatively were observed in 23/60 patients of the LG group (38.3%) and 27/63 patients of the OG group (42.9%) (P = 0.744). Regarding the individual prevalence of cognitive decline on each of the 3 days after surgery, there was no significant difference between the groups (Fig. 3). Postoperative cognitive decline started most often on day 1 after surgery (23/23 in the LG group and 23/27 in the OG group). On postoperative day 3, only 6 patients in the LG group and 14 in the OG group exhibited persistent declines in cognitive function. For those with postoperative cognitive decline, 14/23 (60.9%) in the LG group and 23/27 (85.2%) in the OG group experienced postoperative delirium. In both groups, postoperative cognitive decline was significantly associated with postoperative delirium (P < 0.001 in both).
Fig. 3. Individual prevalence of cognitive decline on postoperative days (POD) 1, 2, and 3, and overall incidence of cognitive in the 3 days postoperatively in the open gastrectomy (OG) and laparoscopic gastrectomy (LG) groups. There was no significant difference between the groups in the individual prevalence of cognitive decline on POD 1, 2, or 3 (P = 0.982, P = 0.174, and P = 0.111, respectively). There was no significant difference between the groups in the overall incidence of cognitive decline during 3 days postoperatively (P = 0.744).
All the functional recovery outcomes (time to first time out of bed, time to first ward ambulation, and time to pass flatus) were significantly more rapid in the LG group than in the OG group. As a result, these differences in function recovery resulted in a shorter hospital stay in the LG group versus the OG group (Table 3). However, further subgroup analysis showed that delirious patients had a similar length of hospital stay to non-delirious patients in both groups.
Discussion
Postoperative cognitive deterioration is one of the most common complications in elderly surgical patients. Contrasting postoperative cognitive decline or delirium, postoperative cognitive dysfunction typically develops over weeks to months, and the diagnosis requires sensitive pre- and post-operative neuropsychiatric testing. However, because of similarities in risk factors, postoperative cognitive decline, delirium, and cognitive dysfunction are generally considered to be part of the same continuum. Among the various risk factors, surgical stress itself plays a major role in the pathogenesis of delirium [1]. Previous experimental [5] and clinical [6] studies have suggested that surgical trauma results in increased levels of inflammatory cytokines and cortisol in the peripheral and central nervous system and thus impairs cognitive function.
In light of this pathogenesis, we hypothesized that laparoscopic gastrectomy, compared with open gastrectomy, would result in favorable cognitive outcome because it causes minimal abdominal wall incision. However, in contrast to our expectations, there was no significant difference in either the incidence or severity of postoperative delirium between laparoscopic and open gastrectomy.
It is clear that laparoscopic gastrectomy causes less surgical trauma than conventional open surgery that requires a large abdominal incision (25-30 cm). A previous randomized study [14] confirmed the minimally invasive nature of laparoscopic gastrectomy with regard to inflammation and postoperative recovery. Thus, a reasonable explanation for our result is that the difference in the degree of surgical trauma and subsequent inflammation was not large enough to see a difference in the development of delirium between the groups. This assumption is supported by a recent prospective study [15], in which the incidence of postoperative delirium was similar between elderly patients undergoing open versus laparoscopic colectomy. Unlike our study, that study evaluated serum levels of inflammatory markers, and found that the interleukine-6 levels were higher in the open group than in the laparoscopic group. Similarly, another retrospective study [16] suggested that there was no relevant relationship between the surgical approach and the incidence of postoperative delirium in elderly patients undergoing colon surgery. Although the neuro-inflammatory hypothesis of delirium seems to be persuasive, the extent of surgical trauma is not the sole determining factor for the development of postoperative delirium. However, because we did not attempt to measure the serum levels of inflammatory parameters or proinflammatory cytokines, further studies are needed to clarify our assumption.
In this study, we confirmed that laparoscopic gastrectomy resulted in reduced postoperative pain and opioid analgesics (fentanyl as IV PCA and meperidine as rescue analgesic) consumption, and a more rapid return to physical activities, compared with open gastrectomy. However, these advantages in laparoscopic gastrectomy did not lead to reduced postoperative delirium. Although postoperative pain is suggested to be associated with postoperative delirium [1,17], its relative importance in postoperative delirium remains unclear. Especially, in this study, the difference in the NRS pain scores between the groups was not very large during the postoperative 3 days, although it was statistically significant. Furthermore, there is controversy as to whether postoperative pain or opioid analgesia (opioid administration per se or the amount of opioid consumed) is an independent risk factor for postoperative delirium [18].
Postoperative delirium is the result of a complex interplay of predisposing (patient vulnerability) and precipitating (anesthetic, operative, and postoperative) factors. In the presence of major predisposing factors, even trivial precipitating factors may trigger delirium, whereas in patients with only minor predisposing factors, a major precipitating insult is necessary to trigger delirium [17]. A recent study [19] confirmed these interactions between pre-existing predisposing factors and acute precipitants. That study found that the effect of postoperative pain and opioid doses on the incidence of postoperative delirium was not same for all patients; patients with high baseline risk factors are more vulnerable to postoperative pain interventions. When considering the high incidence of postoperative delirium in the LG and OG groups, it is possible that the vulnerability for developing delirium among patients at high preoperative risk (e.g., advanced age and upper abdominal surgery) was sufficiently high that the additive effect of postoperative pain or opioid doses might not be detectable in our study.
In this study, the first-line analgesics were fentanyl and ketolorac, which were administered by IV PCA mode. In addition, IV meperidine (a routine IV analgesic in our surgical wards) was administered as a rescue analgesic. Many studies have suggested a strong association between the use of meperidine and delirium [1,17]. In this study, however, the reduced meperidine consumption in the LG group did not decrease the risk of postoperative delirium compared to the OG group. Consequently, the most likely explanation for this is that even the reduced extent of meperidine consumption in the LG group might have been large enough to trigger delirium in most cases.
Generally, anesthetic technique is known to have no effect on postoperative delirium. However, a growing body of evidence suggests that a long period of deep hypnosis (usually defined as BIS < 40) may increase the risk of postoperative delirium [20]. Thus, in this study, anesthetic technique was standardized as BIS-guided sevoflurane anesthesia with constant infusion of supplemental remifentanil. As a result, the total duration of deep hypnosis was comparable between the groups.
This study had several limitations. First, it was non-randomized. Thus, there was a selection bias. The superiority of laparoscopic gastrectomy is relatively obvious for early gastric cancer, but its suitability for advanced gastric cancer remains controversial. Thus, it would be unethical to decide surgical approach in a truly randomized manner.
Second, patients were assessed for delirium for only 3 days postoperatively. It has been shown that delirium occurs predominantly within the first 2-3 days after surgery [1,17]. Thus, our study may well reflect the actual incidence of postoperative delirium.
Third, only one cognitive function test (MMSE) was used to evaluate postoperative cognitive decline. However, the MMSE gives a clear impression of overall cognitive deficits and facilitates monitoring of the development and resolution of delirium in elderly patients [8]. Although the MMSE is not well suited for the more selective and smaller deficits of postoperative cognitive decline, elderly patients may not be capable of proper compliance during a complex cognitive function test situation, especially in the early postoperative period.
Lastly, in both groups, the majority of delirium cases were mild in severity. In this study, we quantified the severity of delirium using the DI because it is a reliable, valid, responsive measure of the severity of delirium [12]. Although how much severe delirium is clinically significant remains an open subject, a potential dose-response relationship between postoperative delirium and long-term negative outcomes has been consistently suggested [21]. Fully developed, prolonged delirium is associated with worse outcomes than mild and/or short-lived delirium. However, our evaluation of outcome measures for only 3 postoperative days cannot allow insights into any long-term associations of delirium with cognition.
In conclusion, we found that, compared with traditional open gastrectomy, laparoscopic gastrectomy did not reduce either the incidence or severity of postoperative delirium in elderly patients with gastric cancer. In terms of postoperative cognitive decline, the two surgical approaches showed similar incidences. Given the negative effect of postoperative delirium on patient outcomes and the few available therapeutic measures, there is a continuing need for strategies to reduce postoperative delirium in elderly patients, regardless of surgical approach.
References
- 1.van der Mast RC. Postoperative delirium. Dement Geriatr Cogn Disord. 1999;10:401–405. doi: 10.1159/000017178. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, et al. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg Today. 2002;32:310–314. doi: 10.1007/s005950200044. [DOI] [PubMed] [Google Scholar]
- 3.Ganai S, Lee KF, Merrill A, Lee MH, Bellantonio S, Brennan M, et al. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg. 2007;142:1072–1078. doi: 10.1001/archsurg.142.11.1072. [DOI] [PubMed] [Google Scholar]
- 4.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meat-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 5.Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawins JN, Bannerman DM, et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2012;33:603–616. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 7.Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.O'Keeffe ST, Mulkerrin EC, Nayeem K, Varughese M, Pillay I. Use of serial Mini-Mental State Examinations to diagnose and monitor delirium in elderly hospital patients. J Am Geriatr Soc. 2005;53:867–870. doi: 10.1111/j.1532-5415.2005.53266.x. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 11.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCusker J, Cole M, Bellavance F, Primeau F. Reliability and validity of a new measure of severity of delirium. Int Psychogeriatr. 1998;10:421–433. doi: 10.1017/s1041610298005493. [DOI] [PubMed] [Google Scholar]
- 13.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101:740–747. doi: 10.1213/01.ane.0000166974.96219.cd. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 15.Tan CB, Ng J, Jeganathan R, Kawai F, Pan CX, Pollock S, et al. Cognitive changes after surgery in the elderly: does minimally invasive surgery influence the incidence of postoperative cognitive changes compared to open colon surgery? Dement Geriatr Cogn Disord. 2015;39:125–131. doi: 10.1159/000357804. [DOI] [PubMed] [Google Scholar]
- 16.Tei M, Ikeda M, Haraguchi N, Takemasa I, Mizushima T, Ishii H, et al. Risk factors for postoperative delirium in elderly patients with colorectal cancer. Surg Endosc. 2010;24:2135–2139. doi: 10.1007/s00464-010-0911-7. [DOI] [PubMed] [Google Scholar]
- 17.Steiner LA. Postoperative delirium Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–636. doi: 10.1097/EJA.0b013e328349b7f5. [DOI] [PubMed] [Google Scholar]
- 18.Sieber FE, Mears S, Lee H, Gottschalk A. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256–2262. doi: 10.1111/j.1532-5415.2011.03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21:946–956. doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CG, Brummel NE, Vasilevskis EE, Girard TD, Pandharipande PP. Future directions of delirium research and management. Best Pract Res Clin Anaesthesiol. 2012;26:395–405. doi: 10.1016/j.bpa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]