Abstract
Objective
The aim of the study was to analyze the prevalence and distribution of ectopic eruption of the permanent maxillary first molar (EEM) in individuals scheduled for orthodontic treatment and to investigate the association of EEM with dental characteristics, maxillary skeletal features, crowding, and other dental anomalies.
Methods
A total of 1,317 individuals were included and randomly divided into two groups. The first 265 subjects were included as controls, while the remaining 1,052 subjects included the sample from which the final experimental EEM group was derived. The mesiodistal (M-D) crown width of the deciduous maxillary second molar and permanent maxillary first molar, maxillary arch length (A-PML), maxillomandibular transverse skeletal relationships (anterior and posterior transverse interarch discrepancies, ATID and PTID), maxillary and mandibular tooth crowding, and the presence of dental anomalies were recorded for each subject, and the statistical significance of differences in these parameters between the EEM and control groups was determined using independent sample t-tests. Chi-square tests were used to compare the prevalence of other dental anomalies between the two groups.
Results
The prevalence of maxillary EEM was 2.5%. The M-D crown widths, ATID and PTID, and tooth crowding were significantly greater, while A-PML was significantly smaller, in the EEM group than in the control group. Only two subjects showed an association between EEM and maxillary lateral incisor anomalies, which included agenesis in one and microdontia in the other.
Conclusions
EEM may be a risk factor for maxillary arch constriction and severe tooth crowding.
Keywords: Ectopic eruption, Tooth size, Crowding, Transverse maxillary deficiency
INTRODUCTION
Ectopic eruption of the permanent maxillary first molar (EEM) is a local eruption disturbance characterized by eruption that is mesial to the normal path.1,2 Data reported in the literature shows an EEM prevalence rate of 0.75-6%.3,4,5,6,7 The permanent molar is initially blocked from complete eruption by the deciduous second molar because of the close contact between the two teeth. This condition causes atypical resorption on the distal surface of the deciduous second molar,1 with a significant effect on malalignment of teeth, particularly the permanent teeth.8,9
Several etiological theories have been reported in the literature.1,4,10,11,12,13,14,15,16 Pulver4 found that EEM depended on a combination of factors, including macrodontia of the permanent maxillary teeth and first molars, maxillary hypoplasia, posterior position of the maxilla in relation to the cranial base, abnormal eruption angle of the permanent maxillary first molar, and delayed calcification of some affected permanent molars. Bjerklin and Kurol1 suggested the tendency toward a shorter maxilla and macrodontia of the permanent molars with a more pronounced mesial angle of eruption in children with irreversible ectopic eruption compared with those in children with normal eruption, while no significant differences were observed between children with reversible ectopic eruption and the controls. Studies by Yuen et al.10 and Canut and Raga11 reported an association of ectopic eruption with a short and posteriorly positioned maxilla, while other studies defined ectopic eruption as a multifactorial process.12,13,14
More recently, Bjerklin et al.15 analyzed the association between EEM and three other dental anomalies, defining the four conditions as different manifestations of a single syndrome with incomplete penetrance. Baccetti16 suggested the importance of local factors such as tooth size-arch length discrepancy in the etiology of this eruption anomaly.
Subsequently, Becktor et al.17 proposed that irreversible EEM can be an early indicator of lateral canine eruption, which leads to root resorption. Salbach et al.5 reported a significant association between EEM and other forms of malocclusion, such as crowding, lateral malocclusion, and mandibular prognathism.
Despite extensive analysis evaluating the association between EEM and maxillary arch length, no studies considered the association of EEM with maxillary transverse deficiency and maxillary and mandibular tooth crowding.
Therefore, this study was conducted to analyze the prevalence and distribution of EEM in a large cohort of individuals scheduled for orthodontic treatment and to investigate the association of EEM with dental characteristics, maxillary skeletal features, and crowding using a control group for comparison. The prevalence of other dental anomalies in the EEM group was also assessed.
MATERIALS AND METHODS
The parent sample for this study comprised 1,456 individuals in the early mixed dentition stage recruited from the Department of Orthodontics at the University of Rome "Tor Vergata." All subjects were observed prior to orthodontic treatment and at the prepuberal stage of skeletal growth using the cervical vertebral maturation method (CS1-CS2).18 In addition, dental casts and panoramic radiographs were examined for each subject. Subjects with craniofacial anomalies, cleft lip and/or palate, sequelae of traumatic injuries to the permanent teeth, odontomas, and/or cysts, were not included, as were subjects with Class II dental restorations, extensive caries, or premature loss of the deciduous maxillary second molars. Eventually, 1,317 subjects (628 boys and 689 girls) aged 7-10 years were included.
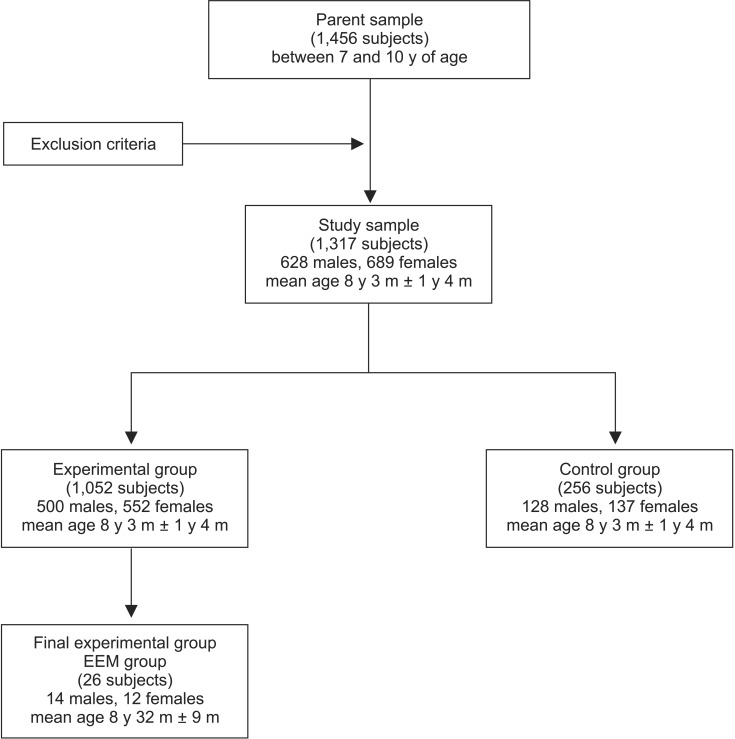
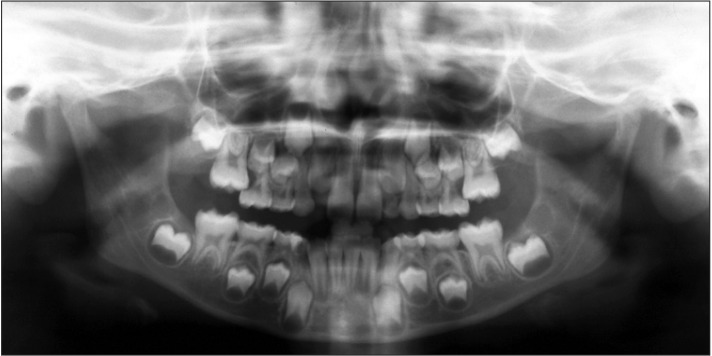
According to the methodology of previous studies,16,19 the study sample was randomly divided into two groups. The first 265 subjects, including 128 boys and 137 girls, were used as controls; the reference values for all examined parameters were calculated for this group. The remaining 1,052 subjects, including 500 boys and 552 girls with a mean age of 8 years and 3 months ± 1 year and 4 months, comprised the sample from which the final experimental group was derived; this group was investigated for the presence of EEM. In total, 26 subjects, including 14 boys and 12 girls with a mean age of 8 years and 2 months ± 9 months, were diagnosed with EEM in the experimental group and were identified as the EEM group (Figure 1). EEM was identified when the permanent first molar was initially blocked from complete eruption by the adjacent primary molar, which showed premature resorption on its distal surface (Figure 2). Two possible evolutions of EEM may follow: a reversible type, wherein the permanent molar frees itself and erupts to normal occlusion, and an irreversible type.2,19,20 These two forms were not distinguished in the present study for the early mean age of the sample. The unilateral or bilateral intraosseous ectopic position of the permanent first molar was evaluated on panoramic radiographs.
Figure 1. Study flow chart. EEM, Ectopic eruption of the permanent maxillary first molar; y, years; m, months.
Figure 2. Panoramic radiograph showing bilateral ectopic eruption of the permanent maxillary first molars in an 8-year-old subject.
The EEM and control groups were matched in terms of origin, age, and gender distributions (Table 1). All subjects were Caucasian, and there was a possibility of the presence of EEM in the control group.
Table 1. Dermographic data for the EEM and control groups.
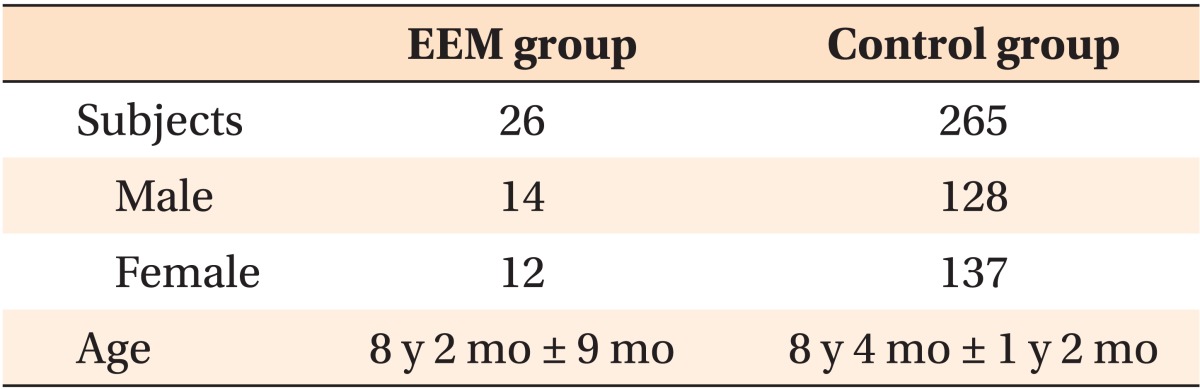
Values are presented as number or mean ± standard deviation.
EEM, Ectopic eruption of the permanent maxillary first molar; y, years; mo, months.
Initial dental casts were available for each subject. These casts were used to analyze the maxillary arch length, maxillary and mandibular arch diameters, tooth crowding, and crown widths using a tridimensional scanner (D800; 3Shape A/S, Copenhagen, Denmark; scan time, 25 s; resolution, two cameras, 5.0 megapixels; ultrahigh point accuracy, <15 microns). The virtual three-dimensional models were measured and analyzed using specific software (3Shape OrthoAnalyzer™ 2010; 3Shape A/S).
The following parameters were analyzed (Figure 3):
Figure 3. Linear measurements on digital models.
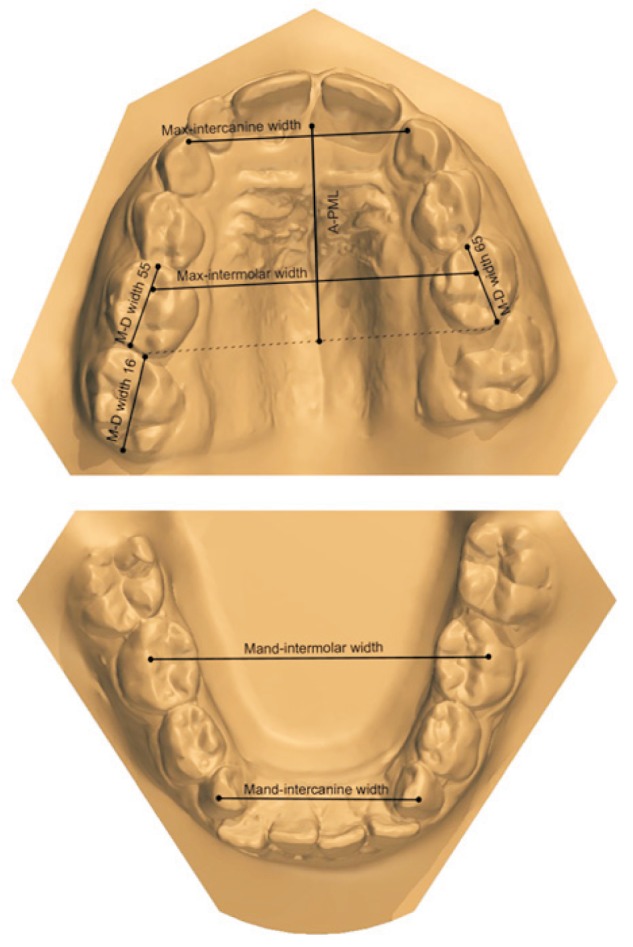
M-D crown widths, Mesiodistal crown widths; A-PML, anteroposterior maxillary length; Max-intercanine width, maxillary intercanine width; Max-intermolar width, maxillary intermolar width; Mand-intercanine width, mandibular intercanine width; Mand-intermolar width, mandibular intermolar width.
1) Mesiodistal (M-D) crown width of the deciduous second molar, as calculated on digital models by recording the distance between the central point of the mesial marginal crest and the central point of the distal marginal crest21
2) M-D crown width of the permanent first molar, as calculated on digital models by recording the distance between the central point of the mesial marginal crest and the central point of the distal marginal crest.21 In the EEM group, measurements were obtained after extraction of the deciduous second molars in subjects with permanent first molars that were locked distal to the deciduous se-cond molars1
3) Maxillary and mandibular tooth crowding, as evaluated on digital models using the space analysis method of Tweed21; the necessary space in subjects with several unerupted permanent teeth was calculated using the prediction tables of Moyers22
4) Maxillary arch length (A-PML), as calculated on digital models from the central point of the incisive papilla to the tangent of the most distal point of the deciduous maxillary second molars on the right and left sides, using the median palatal raphe as the midsagittal arch plane10
5) Maxillomandibular transverse skeletal relationships, as calculated on digital models by recording the intercanine and intermolar distances for the deciduous teeth
The maxillary intercanine width was measured as the distance between the most mesial points on the palatal surfaces of the maxillary deciduous canines. The maxillary intermolar width was evaluated as the distance between the central fossae of the deciduous maxillary right and left second molars. The mandibular intercanine width was measured as the distance between the cusp tips of the deciduous mandibular canines. The mandibular intermolar width was evaluated as the distance between the tips of the distobuccal cusps of the deciduous mandibular right and left second molars.23 The anterior transverse interarch discrepancy (ATID) was calculated as the difference between the maxillary and mandibular intercanine widths. In subjects with normal occlusion, the cusp tips of the deciduous mandibular canines occlude with the most mesial points on the palatal surface of the deciduous maxillary canines; consequently, the maxillary and mandibular intercanine widths are equal in these subjects. The posterior transverse interarch discrepancy (PTID) was calculated as the difference between the maxillary and mandibular intermolar widths. In subjects with normal occlusion, the distobuccal cusp of the deciduous mandibular second molar occludes with the central fossa of the deciduous maxillary second molar; consequently, the maxillary and mandibular intermolar widths are equal in these subjects. A smaller maxillary width compared with the mandibular width indicates a transverse discrepancy between the dental arches.
All measurements were performed with the investigator (MM) blinded to the group investigated.
Furthermore, the presence of other associated dental anomalies was evaluated in both groups using panoramic radiographs.
Statistical analysis
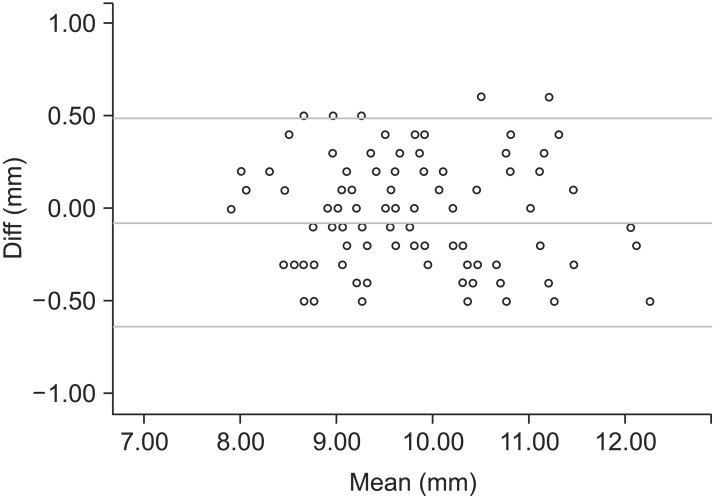
The reproducibility of EEM diagnosis was found to be 100% when the records of 100 subjects were reexamined 5 months after the first examination. Reproducibility of the measurements on radiographs and digital models was also estimated at this time by repeating all measurements and assessments for the abovementioned 100 subjects. Statistical analysis was completed using the Statistical Package for Social Sciences (version 13.0; SPSS Inc., Chicago, IL, USA). The two sets of coordinates were compared using paired t-tests, evaluated using Bland-Altman plots,24 and confirmed by Pearson and linear regression analyses (Figure 4). No significant systematic error was found between the measurement sessions (p > 0.05), and the method error was 0.3 mm for the digital model measurements. The Shapiro-Wilk test was used as a normality test; all the measured values followed a normal distribution. The statistical significance of differences between the EEM and control groups in the M-D crown widths, A-PML, ATID and PTID, and maxillary and mandibular tooth crowding was tested using independent sample t-tests (p < 0.01). Considering the large variance and small sample size, the Kruskal-Wallis test was used to confirm the results of the t-tests. Chi-square tests with Yates' correction were performed to compare the prevalence of other dental anomalies between the two groups.
Figure 4. Results of Bland-Altman difference plot analyses. Example for M-D crown widths. Diff, difference.
RESULTS
The prevalence of maxillary EEM was 2.5% (26 of 1,052 subjects), with six and 20 subjects showing unilateral and bilateral EEM, respectively (1:5). Twenty boys and six girls showed EEM, indicating an approximate M:F ratio of 5:1.
The results of descriptive statistics for all measurements in both groups are shown in Table 2.
Table 2. Statistical analysis of the EEM and control groups*.
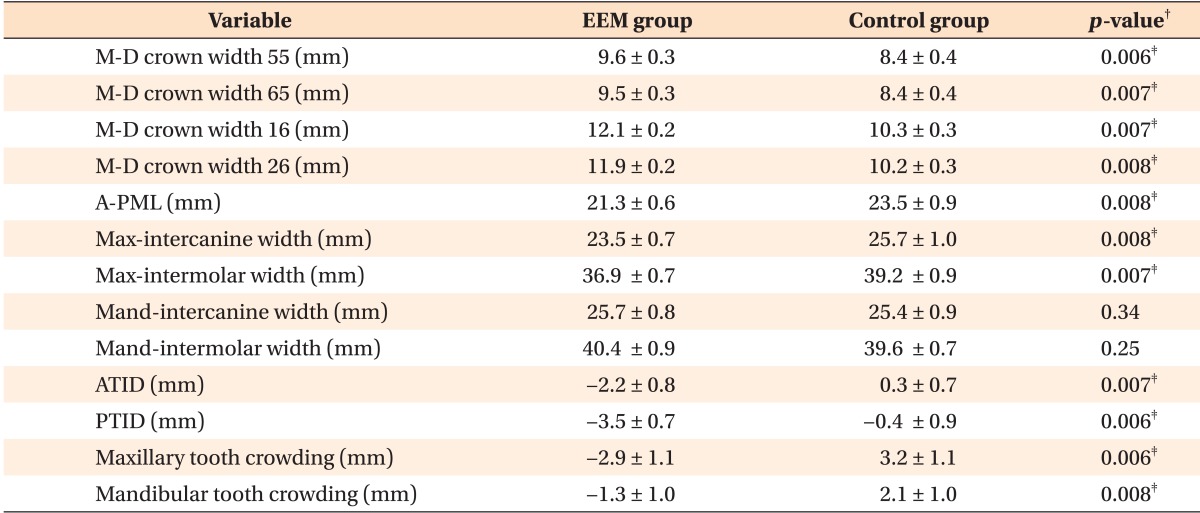
*Descriptive statistics and statistical comparisons of dental characteristics, maxillomandibular transverse skeletal relationships, and tooth crowding between the EEM and control groups.
Values are presented as mean ± standard deviation.
EEM, Ectopic eruption of the permanent maxillary first molar; M-D crown widths, mesiodistal crown widths; A-PML, anteroposterior maxillary length; Max-intercanine width, maxillary intercanine width; Max-intermolar width, maxillary intermolar width; Mand-intercanine width, mandibular intercanine width; Mand-intermolar width, mandibular intermolar width; ATID, anterior transverse interarch discrepancy; PTID, posterior transverse interarch discrepancy.
†Independent t-test; ‡p < 0.01.
Dental characteristics
The permanent maxillary first molars were significantly larger in the EEM group (12.1 and 11.9 mm for the right and left molars, respectively) than in the control group (10.3 and 10.2 mm for the right and left molars, respectively; p < 0.01). The mean size of the deciduous second molar was greater in the EEM group (9.6 mm and 9.5 mm for the right and left molars, respectively) than in the control group (8.4 mm for both the right and left molars).
Maxillary skeletal features
A-PML was significantly smaller in the EEM group (21.3 mm) than in the control group (23.5 mm; p < 0.01).
ATID and PTID was significantly greater in the EEM group (-2.2 mm and -3.5 mm, respectively) than in the control group (0.3 mm and -0.4 mm, respectively; p < 0.01). This result was associated with significantly smaller maxillary intermolar and intercanine widths in the EEM group (36.9 mm and 23.5 mm, respectively) compared with those in the control group (39.2 mm and 25.7 mm, respectively; p < 0.01). There were no significant differences in the mandibular intermolar and intercanine widths between the two groups.
Tooth crowding
The EEM group showed a significantly greater degree of tooth crowding in the maxillary and mandibular arches (-2.9 mm and -1.3 mm, respectively) compared with the control group (3.2 mm and 2.1 mm, respectively; p < 0.01).
Additional dental anomalies
There was no significant association between EEM and the presence of other dental anomalies (Table 3). Only two subjects in the EEM group showed maxillary lateral incisor anomalies, which included agenesis in one and localized microdontia in the other.
Table 3. Prevalence and distribution study of dental anomalies in the EEM and control groups.
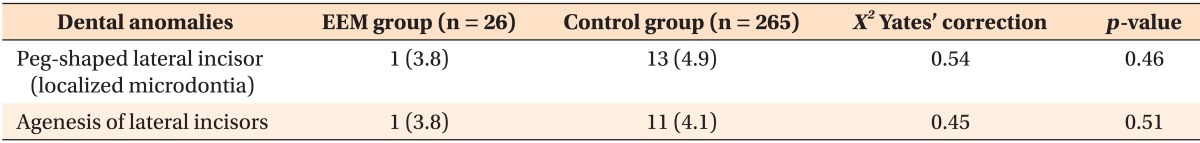
Values are presented as number (prevalence, %).
EEM, Ectopic eruption of the permanent maxillary first molar.
p < 0.05 is significant.
DISCUSSION
The prevalence of EEM in the examined sample in this study was 2.5% (26 of 1,052 subjects). This rate reflects the occurrence of this eruption anomaly in an orthodontic population and does not indicate its absolute prevalence in the general population. Data reported in the literature shows an EEM prevalence rate of 0.75-6%.3,4,5,6,7 This variation may be related to the number of children included.2
The present study analyzed the dental characteristics associated with ectopic disorders of the permanent maxillary first molar and found increased dimensions of the deciduous second molars and permanent first molars in the EEM group compared with those in the control group (p < 0.01). Similar results were obtained by Bjerklin and Kurol.1 The authors reported that the molar on the side with normal eruption was wider in subjects with unilateral ectopic eruption than in those with bilateral normal eruption. In contrast, Pulver4 suggested that the mean size of the molar on the side with normal eruption was similar in children with unilateral EEM and children with bilateral normal eruption.
Previous studies1,4,7,10 analyzed the eruption angle of the permanent first molars in subjects with EEM. The results showed an increased mesial angle of eruption in the EEM group compared with that in the control group. However, the eruption angle of the permanent maxillary first molar needs prospective investigation; therefore, this parameter was not considered in the present study.
With regard to maxillary skeletal features, previous authors suggested that the length of the maxilla or poor posterior growth of the maxilla were associated with EEM.4,10 Canut and Raga11 mentioned that EEM results in a posteriorly positioned maxilla. In the present study, this parameter was assessed by measuring the distance from the incisive papilla to the tangent of the most distal point of the deciduous maxillary second molar on the right and left sides; the arch length was smaller in the EEM group than in the control group (p < 0.01).
With regard to transverse arch discrepancies, the results of this study suggested a significant maxillomandibular discrepancy in the posterior (-3.5 mm) and anterior (-2.2 mm) regions and a decreased maxillary anterior (23.5 mm) and posterior (36.9 mm) transverse diameter in the EEM group compared with those in the control group.
The findings for ATID and PTID indicated a decreased maxillary arch length and increased anterior and posterior maxillomandibular discrepancies associated with small maxillary intercanine and intermolar widths in the EEM group, suggesting that EEM is associated with severe maxillary hypoplasia.
In the present study, significant maxillary and mandibular tooth crowding was observed in the EEM group compared with that in the control group (p < 0.01). This result is in agreement with that of a previous study5 that analyzed the association between EEM and dental crowding with no distinction between the upper and lower arches. We hypothesize that the combination of maxillary macrodontia and hypoplasia plays a role in dental crowding in individuals with EEM, supporting the involvement of local dentoskeletal factors in the etiology of EEM.
The findings of our study indicate an association between EEM and severe maxillary tooth crowding. Other studies8,9 suggested that the premature loss of the deciduous canine or first or second molars were predictors of crowding. Similarly, in individuals with EEM, the permanent first molar compresses the distal root surface of the deciduous second molar, leading to resorption and premature exfoliation of the latter.
In our study, no significant association was found between EEM and the presence of other dental anomalies, suggesting that local dentoskeletal factors play a role in the ethiopathogenesis of EEM and opposing the belief that there may be an underlying genetic mechanism. These findings also confirm the hypotheses of some authors that EEM is a manifestation of local dentoskeletal factors.4,10 Baccetti16 investigated the existence of significant reciprocal associations among different types of dental anomalies in a large sample of human subjects in the developmental stages and reported a significant association between EEM and only two of seven dental anomalies analyzed; microdontia of lateral incisors and infraocclusion of the deciduous molars. The author highlighted the lack of any significant association between EEM and other dental anomalies, suggesting an important role of local dentoskeletal factors in the etiology of the anomaly. On the contrary, Bjerklin et al.15 suggested that genetic factors are involved in the etiology of tooth eruption disturbances. They investigated the associations among four different eruption disturbances, namely EEM, infraocclusion of the deciduous molars, ectopic eruption of the maxillary canines, and aplasia of premolars, and indicated that EEM was associated with an increased prevalence of deciduous molar infraocclusion and ectopic eruption of the maxillary canines; this led them to assume a common hereditary etiology. Therefore, the four conditions were considered to be different manifestations of a single syndrome with incomplete penetrance.15 Becktor et al.17 hypothesized a biological association between EEM and ectopic canine eruption that led to pathological root resorption of the lateral incisors because of general defects in the periodontal ligament. The limitations of this study were related to the selection of the subjects. The sampled consisted of a population scheduled for orthodontic treatment and the small number of individuals included in the study could influence the results.
CONCLUSION
In conclusion, the prevalence of EEM in the present study was 2.5%. EEM was significantly associated with increased dimensions of the deciduous maxillary second molars and permanent maxillary first molars, maxillary hypoplasia, and dental crowding. These findings suggest that EEM is a risk factor for maxillary arch constriction and severe crowding.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Bjerklin K, Kurol J. Ectopic eruption of the maxillary first permanent molar: etiologic factors. Am J Orthod. 1983;84:147–155. doi: 10.1016/0002-9416(83)90179-3. [DOI] [PubMed] [Google Scholar]
- 2.Kurol J, Bjerklin K. Ectopic eruption of maxillary first permanent molars: a review. ASDC J Dent Child. 1986;53:209–214. [PubMed] [Google Scholar]
- 3.Bjerklin K, Kurol J. Prevalence of ectopic eruption of the maxillary first permanent molar. Swed Dent J. 1981;5:29–34. [PubMed] [Google Scholar]
- 4.Pulver F. The etiology and prevalence of ectopic eruption of the maxillary first permanent molar. ASDC J Dent Child. 1968;35:138–146. [PubMed] [Google Scholar]
- 5.Salbach A, Schremmer B, Grabowski R, Stahl de Castrillon F. Correlation between the frequency of eruption disorders for first permanent molars and the occurrence of malocclusions in early mixed dentition. J Orofac Orthop. 2012;73:298–306. doi: 10.1007/s00056-012-0083-2. [DOI] [PubMed] [Google Scholar]
- 6.Barberia-Leache E, Suarez-Clúa MC, Saavedra-Ontiveros D. Ectopic eruption of the maxillary first permanent molar: characteristics and occurrence in growing children. Angle Orthod. 2005;75:610–615. doi: 10.1043/0003-3219(2005)75[610:EEOTMF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Chintakanon K, Boonpinon P. Ectopic eruption of the first permanent molars: prevalence and etiologic factors. Angle Orthod. 1998;68:153–160. doi: 10.1043/0003-3219(1998)068<0153:EEOTFP>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Martins-Júnior PA, Marques LS. Clinical implications of early loss of a lower deciduous canine. Int J Orthod Milwaukee. 2012;23:23–27. [PubMed] [Google Scholar]
- 9.Miyamoto W, Chung CS, Yee PK. Effect of premature loss of deciduous canines and molars on malocclusion of the permanent dentition. J Dent Res. 1976;55:584–590. doi: 10.1177/00220345760550040601. [DOI] [PubMed] [Google Scholar]
- 10.Yuen S, Chan J, Tay F. Ectopic eruption of the maxillary permanent first molar: the effect of increased mesial angulation on arch length. J Am Dent Assoc. 1985;111:447–451. doi: 10.14219/jada.archive.1985.0135. [DOI] [PubMed] [Google Scholar]
- 11.Canut JA, Raga C. Morphological analysis of cases with ectopic eruption of the maxillary first permanent molar. Eur J Orthod. 1983;5:249–253. doi: 10.1093/ejo/5.3.249. [DOI] [PubMed] [Google Scholar]
- 12.Raghoebar GM, Boering G, Vissink A, Stegenga B. Eruption disturbances of permanent molars: a review. J Oral Pathol Med. 1991;20:159–166. doi: 10.1111/j.1600-0714.1991.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 13.Mooney GC, Morgan AG, Rodd HD, North S. Ectopic eruption of first permanent molars: presenting features and associations. Eur Arch Paediatr Dent. 2007;8:153–157. doi: 10.1007/BF03262586. [DOI] [PubMed] [Google Scholar]
- 14.Valmaseda-Castellón E, De-la-Rosa-Gay C, Gay-Escoda C. Eruption disturbances of the first and second permanent molars: results of treatment in 43 cases. Am J Orthod Dentofacial Orthop. 1999;116:651–658. doi: 10.1016/s0889-5406(99)70200-3. [DOI] [PubMed] [Google Scholar]
- 15.Bjerklin K, Kurol J, Valentin J. Ectopic eruption of maxillary first permanent molars and association with other tooth and developmental disturbances. Eur J Orthod. 1992;14:369–375. doi: 10.1093/ejo/14.5.369. [DOI] [PubMed] [Google Scholar]
- 16.Baccetti T. A controlled study of associated dental anomalies. Angle Orthod. 1998;68:267–274. doi: 10.1043/0003-3219(1998)068<0267:ACSOAD>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Becktor KB, Steiniche K, Kjaer I. Association between ectopic eruption of maxillary canines and first molars. Eur J Orthod. 2005;27:186–189. doi: 10.1093/ejo/cjh075. [DOI] [PubMed] [Google Scholar]
- 18.Baccetti T, Franchi L, Mc Namara JA., Jr The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod. 2005;11:119–129. [Google Scholar]
- 19.Mucedero M, Ricchiuti MR, Cozza P, Baccetti T. Prevalence rate and dentoskeletal features associated with buccally displaced maxillary canines. Eur J Orthod. 2013;35:305–309. doi: 10.1093/ejo/cjr133. [DOI] [PubMed] [Google Scholar]
- 20.Kurol J, Bjerklin K. Ectopic eruption of maxillary first permanent molars: familial tendencies. ASDC J Dent Child. 1982;49:35–38. [PubMed] [Google Scholar]
- 21.Tweed CH. Clinical orthodontics. 3th ed. St. Louis: The CV Mosby Company; 1966. [Google Scholar]
- 22.Moyers RE. Handbook of orthodontics. 4th ed. Chicago: Year Book Medical; 1988. [Google Scholar]
- 23.Tollaro I, Baccetti T, Franchi L, Tanasescu CD. Role of posterior transverse interarch discrepancy in Class II, Division 1 malocclusion during the mixed dentition phase. Am J Orthod Dentofacial Orthop. 1996;110:417–422. doi: 10.1016/s0889-5406(96)70045-8. [DOI] [PubMed] [Google Scholar]
- 24.Donatelli RE, Lee SJ. How to report reliability in orthodontic research: Part 1. Am J Orthod Dentofacial Orthop. 2013;144:156–161. doi: 10.1016/j.ajodo.2013.03.014. [DOI] [PubMed] [Google Scholar]