Abstract
Background
The spectrum of primary neuroendocrine tumors of the lungs ranges from typical carcinoid tumors, which are relatively benign, to highly aggressive small-cell carcinoma. In this review, we summarize the treatment of bronchopulmonary carcinoid, a disease with an incidence of 0.5 per 100 000 persons per year in Western countries.
Method
We selectively searched the PubMed database for scientific evidence on the treatment of bronchopulmonary carcinoid, considering only articles published up to February 2015. We also performed a survival analysis of 84 patients with this disease who underwent interdisciplinary treatment at the University of Freiburg Medical Center.
Results
Carcinoid tumors account for less than 1% of all lung tumors. They manifest themselves clinically with cough (35%), hemoptysis (25%), and/or bronchial obstruction (40%), depending on their location, size, and pattern of growth. 30% of patients are asymptomatic, and less than 1% have hormone-associated symptoms. Typical and atypical carcinoid tumors are distinguished on a histological basis; the histologic differential diagnosis also includes large-cell neuroendocrine tumors and small-cell carcinoma of the lung. 80% of patients who undergo resection of typical carcinoid tumors survive at least 10 years. Atypical carcinoid tumors recur more commonly than typical ones. If the mediastinal lymph nodes are involved, adjuvant treatment should be considered.
Conclusion
Because of their rarity, the treatment of bronchopulmonary carcinoid tumors presents an interdisciplinary challenge. Surgical resection, the treatment of choice for local carcinoid tumors, generally leads to long-term survival. The existing registers should be made more comprehensive so that the treatment of this disease can be better in the future.
Neuroendocrine tumors (NET) of the lung are rare neoplasms. NET arise from cells that have migrated into the organs from the embryonic neural crest (1). The term carcinoid was originally introduced for enteral tumors and was later also used for pulmonary NET (2). Because of the different clinical symptoms, NET was classified into well differentiated typical carcinoid tumors (TC) and intermediately differentiated atypical carcinoids (AC) in 1972. This classification was adopted by the World Health Organization in 2004 and 2015 (3, 4).
After the gastrointestinal tract, the lung is the second most common location for NET (10%). Less than 1% of all pulmonary tumors are carcinoid tumors (5). In Germany, no comprehensive analyses exist; the incidence of carcinoid tumors in Western countries is 0.5/100 000 (6– 9). Correspondingly, the number of new cases of bronchopulmonary carcinoid in Germany can be assumed to be 400–500. The estimated prevalence is more than 3000 cases (10). Interestingly, it has been observed that the incidence of bronchopulmonary NET is increasing (6, 9). Consumption of nicotine and known carcinogens do not seem to be of any relevance in the pathogenesis (11). Less than 10% of tumors have a genetic cause (12). The average age at diagnosis of a TC is 45 years—the same for both sexes—whereas patients with AC are usually 10 years older and notably more often develop lymph node metastases (50%) and distant metastases (20%) (9, 13– 15). Because of the few studies investigating carcinoid tumors, attempts are being made to integrate data collected on such tumors in databases, such as the German NET Registry (16– 18).
Method
We identified currently available data on the diagnostic evaluation and treatment of bronchopulmonary carcinoid tumors by conducting a selective literature search in PubMed. We searched for articles published up to February 2015. We identified merely individual randomized studies and no meta-analyses for these rare tumors. For this reason, we also included relevant case series.
Clinical symptoms
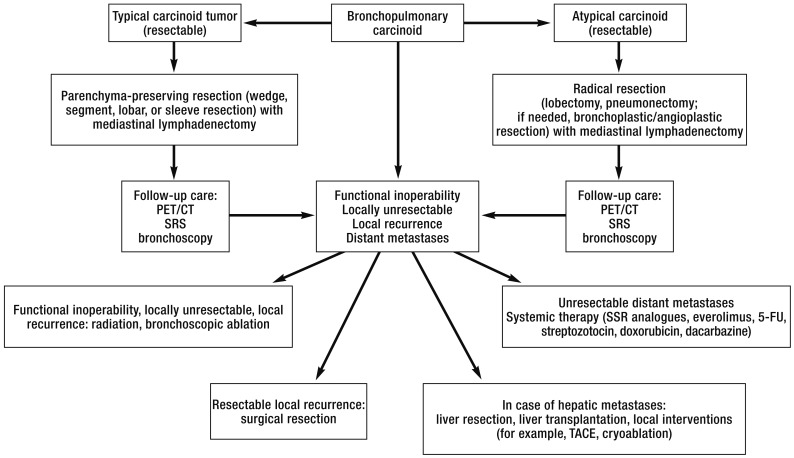
Three quarters of bronchopulmonary carcinoid tumors are located in the central airways. Airway obstruction or hemorrhages from these characteristically hypervascularized tumors are among the symptoms (Figure 1) (7). The remaining 25%, especially AC, are peripheral, mostly incidentally detected, solitary pulmonary nodules (19). Even when symptoms are present the diagnosis is often delayed. Many patients go through years of diagnostic evaluation, for example, in recurrent occurrences of pneumonia. 30% of patients are asymptomatic, 40% have bronchial obstruction, 35% have a cough, and 25% experience hemoptysis (11). Imaging techniques can be used to show that 75% of carcinoid tumors result in atelectasis owing to bronchial obstruction, which is visible in the thoracic radiograph (20). Except for CT and bronchoscopy, carcinoid patients do not require any further special investigations before the resection (Figure 2) (21).
Figure 1.
Atypical carcinoid tumor (AC) in a 70-year-old patient with chronic cough. The computed tomogram shows a stenosing tumor (a; arrows) in the right lower lobe bronchus. Bronchoscopy showed a strongly vascularized tumor (b). After complete surgical resection, histological analysis confirmed AC without lymph node involvement (Union internationale contre le cancer [UICC] stage IB). Further therapy was not indicated.
Figure 2.
Algorithm of the University Medical Center Freiburg for the purpose of interdisciplinary treatment of bronchopulmonary carcinoids
PET, positron emission tomography; SRS, somatostatin receptor scintigraphy; SSR, somatostatin receptor; TACE, transarterial chemoembolization; 5-FU, 5-fluorouracil
In contrast to gastroenteropancreatic (GEP) NET (10%), carcinoid syndrome is very rare in bronchopulmonary carcinoid (<1%); however, again differently to GEP-NET, the carcinoid syndrome can occur even in the absence of hepatic metastases (22, 23). Rarely, hormonally active bronchopulmonary carcinoid tumors are also the cause of Cushing’217;s syndrome or acromegaly (24, 25).
Risk factors
Smoking is not considered etiologically relevant in carcinoid tumors, quite in contrast to large-cell-neuroendocrine tumors and small-cell lung cancers (11, 15). Although most of the tumors arise sporadically, 1.4–9.5%—depending on the study—of patients with multiple endocrine neoplasia (MEN)-1 develop bronchopulmonary carcinoid tumors, which usually take an indolent course. MEN-associated carcinoid tumors of the thymus are, however, more aggressive (12, 16). Independently of the MEN-syndrome, familial carcinoid tumors have also been described (27). Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH), which is characterized by a generalized proliferation of neuroendocrine cells, is a rare predisposing factor (28). Cell proliferations that break through the basal membrane are known as tumorlets. Surgical resection of the larger lesions is recommended. If the patient’217;s condition is stable, follow-up examinations should be undertaken (29).
Differential diagnoses
Lung cancers, other lung tumors, metastases, and benign disorders such as asthma or aspirated foreign bodies range among the differential diagnoses. In case of solitary pulmonary nodules, granulomata, hamartoma, arteriovenous malformations, pneumoconiosis, abscesses, septic embolism, fungal infections, or even mycobacterioses should be discussed. Bronchopulmonary carcinoid tumors that trigger Cushing’217;s syndrome owing to ectopic production of ACTH are usually small (<2 mm). Thin-layer CT and somatostatin receptor imaging are helpful in detecting tumors (30, 31).
Diagnostic imaging and staging
CT using contrast medium is the best method for identifying extrabronchial proportions and mediastinal lymph node enlargement in central tumors. Because of their hypervascularization, carcinoid tumors absorb contrast medium and are often seen as well defined, obstructing tumors (Figure 1a and 1b).
Up to 20% of TC are accompanied by hilar or mediastinal lymphadenopathy, which is mostly caused by a reactive inflammatory reaction (32). Most carcinoid tumors are accessible by means of bronchoscopy with transbronchial biopsy, since 75% are centrally localized. In peripherally localized tumors, percutaneous needle biopsy can be undertaken. Half of AC have lymph node metastases. Endobronchial ultrasound guided transbronchial needle aspiration (EPBUS-TBNA) or mediastinoscopy are undertaken for the purpose of staging according to the TNM classification for lung cancer (33). The histological differentiation grade follows the classification of WHO/the International Association for the Study of Lung Cancer (IASLC) (17). TC are mostly diagnosed at stage I, AC mostly at stage II (N1, [hilar] lymph node involvement) or stage III (N2, [mediastinal] lymph node involvement). Hepatic metastases (stage IV) can be detected by using three-phase CT or, alternatively, by using ultrasonography. In analogy to GEP-NET, functional somatostatin receptor (SSTR) imaging using conventional somatostatin receptor scintigraphy (SRS) is used in the diagnostic evaluation. For example, 111Indium (In)-DTPA-octreotide or positron emission tomography (PET) or PET/CT using 68gadolinium-marked somatostatin receptor ligands, such as 68Ga-DOTATATE, are used in this setting. The immunohistochemical SSTR expression correlated in 70% of bronchopulmonary carcinoids with the SRS (34). In other studies, 100% of TC and 80% of AC were confirmed by using somatostatin receptor PET (34– 37). One big advantage of receptor imaging is whole-body imaging, which especially in AC enables the detection of extrapulmonary metastases (Figure 3) (9, 13, 32, 38). Diagnostic evaluation by PET has advantages compared with conventional scintigraphy in terms of higher spatial resolution and stronger SSTR binding affinity (39, 40). Again in analogy to the GEP-NET, diagnostic evaluation using 18fluordeoxyglucose (18F-FDG) positron emission tomography is of lesser importance in well differentiated bronchopulmonary NET, whereas the sensitivity increases with an increasingly lower histological differentiation grade (TC to AC) (36). Imaging is no substitute for tissue sampling in this setting (Figure 2).
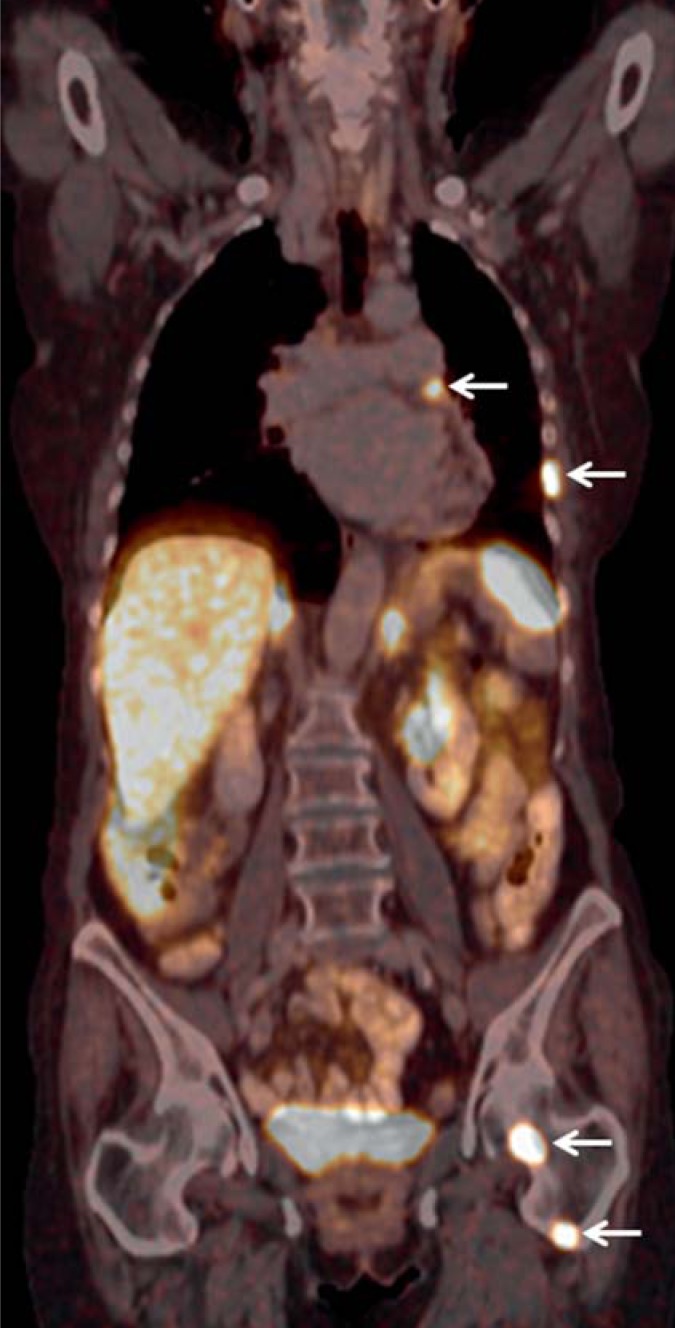
Figure 3.
Somatostatin receptor (SSTR)-PET/CT using 68gadolinium-DOTATATE in a 72-year-old woman, who had been recurrence-free 4 years after resection of an AC (stage IA) of the left upper pulmonary lobe in a non-local hospital. Owing to a follow-up CT, a metastasis in the 12th thoracic vertebra was suspected. Further investigations using SSTR/PET/CT showed multiple metastases in mediastinal lymph nodes, the thoracic wall, the liver, and the skeletal system. Palliative peptide receptor radionuclide therapy (PRRT) using 177Lutetium-DOTATATE was initiated. The patient was in partial remission after three therapeutic cycles.
Serum parameters
In GEP-NET, hormonal activity occurs in some 10% of cases, in bronchopulmonary NET in <1% of cases. The serotonin breakdown product 5-hydroxyindoleacetic acid (5-HIAA) is detectable in urine. Its concentration may be raised even in the absence of carcinoid syndrome (e1). Chromogranin A (CgA) detected in serum has a sensitivity of 85% and specificity of 96% in NET (e2). Measuring CgA is more used in the follow-up care of patients with (metastatic) disease rather than primary diagnostic evaluation (e3).
Tissue sampling
Bronchoscopy shows in central localization almost pathognomonically a strongly vascularized tumor that is mostly covered by bronchial epithelium (Figure 1b). Such tumors are mostly broad-based and grow intraluminally as well as extraluminally—the so called iceberg phenomenon. In order to confirm the diagnosis, brush cytology or biopsy should be done. TC and AC can be distinguished only by using a prepared resected tissue specimen (e4, e5). In spite of vascularization, serious problems with hemorrhage are rare during the biopsy procedure (<1%). Where concerns arise, the indication for rigid bronchoscopy should be defined generously. In peripheral tumors that are suspected to be carcinoid tumors, thoracoscopic resection is the method of choice that should be undertaken immediately (Figure 2).
Histology
The histological differentiation grade follows the WHO/IASLC classification (4, 17):
TC (<2 mitoses/2 mm2 and no necroses)
AC (2–10 mitoses/2 mm2 and/or confirmed necroses).
Both should be distinguished from large-cell and small-cell lung cancers, although neuroendocrine differentiation manifests in the latter as well as in AC and TC (e7, e8). As proof, either the expression of NCAM/CD56, CgA, or synaptophysin are immunohistochemically determined or the neuroendocrine granula are determined by electron microscopy. Typically, growth patterns in the shape of rosettes or trabeculae are clearly histologically detectable in carcinoid tumors.
Resection methods
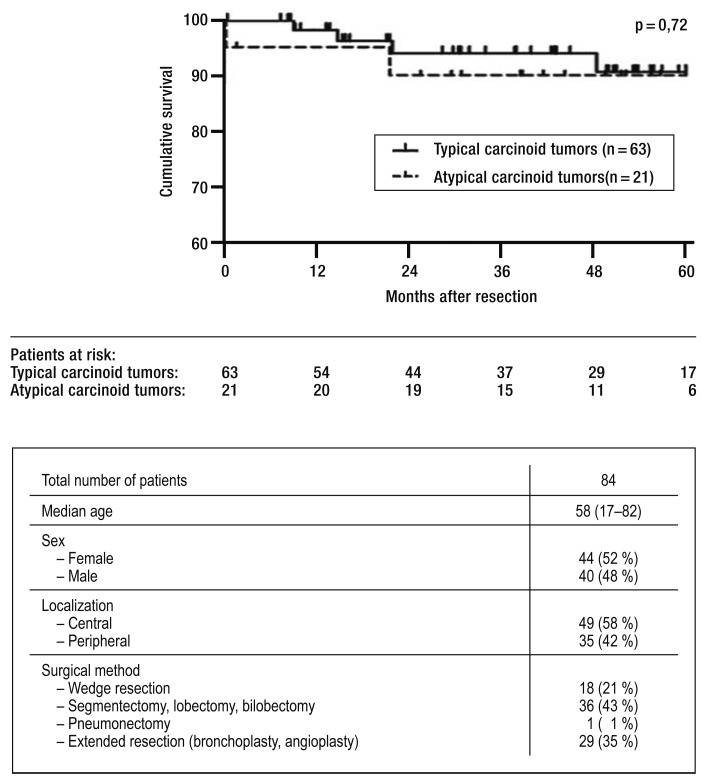
The recommendations for surgical treatment are based on retrospective case series and database analyses (13, 18, e5). Surgery is the only curative approach, and, in view of the lack of prospective studies, it is the therapeutic mode of choice in all recommendations (Figures 2 and 4) (18, e5). The most important objective is a microscopically tumor-free resection margin (R0), which is associated with a good prognosis (e9). The European Society of Thoracic Surgeons Neuroendocrine Tumours Working Group showed in 1109 patients with TC that resection is associated with a 5-year survival rate of 94% (e10). A database analysis from the United States including 441 patients with AC showed that surgical resection leads to a 3-year survival rate of 67% (13). A retrospective analysis in 84 patients who had undergone surgical resection that was conducted by the authors also showed that survival after 5 years is excellent: 91% in patients with TC (n=63) and 90% in patients with AC (n=21) (Figure 4). Radical mediastinal lymph node dissection is indicated in TC since lymph node metastases may be present (13, e5). It should be attempted to preserve healthy pulmonary parenchyma; in a peripherally localized tumor, wedge resection therefore seems sufficient (e11). If the tumor is localized in the central airways, complex resections with angioplasty/bronchoplasty are often required (e12). These can be undertaken with low morbidity and mortality, as can be seen from the high proportion (35%) of patients who were successfully treated with extended resection at the University of Freiburg Medical Center (Fgure 4). Since 50% of patients with AC develop lymph node metastases, more drastic resections will be necessary in these patients (segmentectomy/lobectomy with radical mediastinal lymphadenectomy). This helps prevent local recurrences in the residual lobe or the mediastinal lymph nodes (18, 21).
Figure 4.
Total survival at 5 years after surgical resection. Survival rates of patients whose carcinoid tumors were curatively resected at the University Medical Center Freiburg. From 2003 to 2013, 84 patients were operated on, using the described resection methods. 5-year survival in patients with TC was 91% and in patients with AC, 90%. The median observation period was 43 months; survival data were available for all patients.
Inoperable tumors requiring palliative treatment can be resected bronchoscopically, in order to alleviate symptoms such as retention pneumonia. Even in case of the rare endobronchial growth without expansion through the cartilage, bronchoscopic resection should not be undertaken, although descriptions of this form of limited resection exist (e13).
Long term survival and follow-up care
After complete resection of bronchopulmonary carcinoid tumors, survival rates of more than 80% have been observed consistently (Figure 4) (e14). The prognosis is significantly associated with the degree of differentiation and lymph node metastases. TC have the best prognosis, with a 10-year survival rate of more than 80% (11). The 5-year survival rate in AC without lymph node metastases is 80%, and for AC with lymph node metastases, 60% (Figure 4) (7, 13). A US analysis over 40 years including more than 5500 patients with bronchopulmonary carcinoid tumors showed a 5-year survival rate of 61% (e15).
Since recurrences and distant metastases in TC can develop years after resection of the primary tumor, follow-up care for a minimum of 10 years is indicated (e16). Surgical treatment at the metastatic stage can be considered if the metastases are resectable. Retrospective case series have shown that after resection of neuroendocrine hepatic metastases, the 5-year survival rate is 78% (e17, e18). Furthermore, liver transplantation is an option in NET with diffuse hepatic metastases. An analysis of data from 150 patients showed excellent 5-year survival of 49% after transplantation (e19). But prospectively randomized studies are lacking here too with regard to the selection criteria for transplantation of metastasectomy (number of metastases, length of disease-free intervals/tumor-free period). The authors take the view that interdisciplinary decisions in a center of excellence are required.
Chemotherapy and radiotherapy
Because of the increased risk of recurrence in carcinoid patients with lymph node metastases, adjuvant chemotherapy is desirable. Different drugs have been used with disappointing results (e20). A prospective, randomized study in patients with advanced carcinoid tumors, in whom—among others—5-fluorouracil with streptozotocin was administered, showed a slightly prolonged median survival period, from 16 months to 24 months (e19, e21). Somatostatin receptor analogues (SSA)—for example, octreotide or lanreotide—are primarily indicated for the control of symptoms in carcinoid syndromes. However, a prospective, randomized, placebo-controlled study showed that lanreotide in GEP-NET prolonged progression-free survival (placebo group: median not achieved; lanreotide group: median 18 months) (e21, e22). Compared with a combination of octreotide and placebo, progression-free survival improved in metastatic carcinoid tumors from 11.3 months to 16.4 months if octreotide was given in combination with the mTOR inhibitor everolimus (RADIANT-2 Study) (e23). In addition, the results of a prospective, double blind, randomized multicenter phase III study are expected, which investigated the treatment of metastatic NET with everolimus (versus placebo) (RADIANT-4). A further therapeutic approach is peptide receptor radionuclide therapy (PRRT) using radioactively marked 90Yttrium (Y) or 177Lutetium (Lu)-SSA (e1). In a phase II study, more than 74% of patients with somatostatin-refractory GEP-NET responded to the treatment or stabilized (e20). Pilot studies of the somatostatin receptor ligand 177Lu-DOTATATE showed tumor regression of more than 50% in 28% of patients with bronchopulmonary NET (e24). Furthermore, local ablative palliation should be considered in patients with unresectable hepatic metastases—for example, transarterial chemoembolization (TACE) (Figure 2) (e25).
The role of percutaneous radiotherapy in carcinoid is controversial because the tumors are mostly resistant to irradiation (23). However, since resected AC have a significantly increased risk for local recurrence, adjuvant mediastinal radiotherapy can be considered if lymph nodes are involved. Furthermore, local radiotherapy can be undertaken if the tumor is inoperable, in order to alleviate symptoms (Figure 2) (23, e26).
Key Messages.
Bronchopulmonary carcinoid tumors are rare and should be treated in an interdisciplinary center of excellence, including thoracic surgeons, oncologists, pulmonologists, nuclear medicine specialists, radiotherapists, radiologists, and pathologists.
Most neuroendocrine tumors of the lung are typical carcinoids (TC), which occur primarily in younger patients and are slow growing. Atypical carcinoids (AC) are associated with a higher rate of distant metastases, lymph node metastases, and a poorer prognosis.
Thoracic computed tomography scanning is the imaging method of choice. In tumors that are centrally localized, the diagnosis is made by means of bronchoscopy with transbronchial biopsy; in peripheral lesions, transthoracic needle biopsy or direct resection may be considered.
Carcinoid tumors have an excellent prognosis after surgical resection. 5-year survival after resection of AC is 80%; for TC, 10-year survival is at 80%.
By using somatostatin receptor imaging, metastases can be confirmed.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Pelosi G, Papotti M, Rindi G, Scarpa A. Unraveling tumor grading and genomic landscape in lung neuroendocrine tumors. Endocr Pathol. 2014;25:151–164. doi: 10.1007/s12022-014-9320-0. [DOI] [PubMed] [Google Scholar]
- 2.Oberndorfer S. Karzinoide Tumoren des Dünndarmes. Frankfurter Zeitschrift für Pathologie. 1907;1:426–429. [Google Scholar]
- 3.Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg. 1972;64:413–421. [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Press. 4th ed. Geneva, Switzerland: World Health Organization Classification of Tumors; 2015. WHO classification of tumours of the lung, pleura, thymus and heart; pp. 9–97. [DOI] [PubMed] [Google Scholar]
- 5.Noel-Savina E, Descourt R. Focus on treatment of lung carcinoid tumor. Onco Targets Ther. 2013;6:1533–1537. doi: 10.2147/OTT.S32464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong WK, Schaapveld M, Blaauwgeers JL, Groen HJ, editors. Pulmonary tumours in the Netherlands: focus on temporal trends in histology and stage and on rare tumours. Thorax. 2008;63:1096–1102. doi: 10.1136/thx.2007.095067. [DOI] [PubMed] [Google Scholar]
- 7.Skuladottir H, Hirsch FR, Hansen HH, Olsen JH. Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer. 2002;37:127–135. doi: 10.1016/s0169-5002(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 8.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 9.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 11.Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001;119:1647–1651. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 12.Duh QY, Hybarger CP, Geist R, et al. Carcinoids associated with multiple endocrine neoplasia syndromes. Am J Surg. 1987;154:142–148. doi: 10.1016/0002-9610(87)90305-9. [DOI] [PubMed] [Google Scholar]
- 13.Steuer CE, Behera M, Kim S, et al. Atypical carcinoid tumor of the lung: a surveillance, epidemiology, and end results database analysis. J Thorac Oncol. 2015;10:479–485. doi: 10.1097/JTO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 14.Hurt R, Bates M. Carcinoid tumours of the bronchus: a 33 year experience. Thorax. 1984;39:617–623. doi: 10.1136/thx.39.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harpole DH, Jr, Feldman JM, Buchanan S, Young WG, Wolfe WG. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. Ann Thorac Surg. 1992;54:50–54. doi: 10.1016/0003-4975(92)91139-z. [DOI] [PubMed] [Google Scholar]
- 16.Deutsches Register Neuroendokrine Tumore (NET-Register) www.net-register.org. (last accessed on 9 June 2015)
- 17.Lim E, Goldstraw P, Nicholson AG, et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J Thorac Oncol. 2008;3:1194–1201. doi: 10.1097/JTO.0b013e3181861d7b. [DOI] [PubMed] [Google Scholar]
- 18.Horsch D, Schmid KW, Anlauf M, et al. Neuroendocrine tumors of the bronchopulmonary system (typical and atypical carcinoid tumors): current strategies in diagnosis and treatment. Conclusions of an expert meeting February 2011 in Weimar, Germany. Oncol Res Treat. 2014;37:266–276. doi: 10.1159/000362430. [DOI] [PubMed] [Google Scholar]
- 19.Jeung MY, Gasser B, Gangi A, et al. Bronchial carcinoid tumors of the thorax: spectrum of radiologic findings. Radiographics. 2002;22:351–365. doi: 10.1148/radiographics.22.2.g02mr01351. [DOI] [PubMed] [Google Scholar]
- 20.Nessi R, Basso Ricci P, Basso Ricci S, Bosco M, Blanc M, Uslenghi C. Bronchial carcinoid tumors: radiologic observations in 49 cases. J Thorac Imaging. 1991;6:47–53. [PubMed] [Google Scholar]
- 21.Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89:998–1005. doi: 10.1016/j.athoracsur.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 22.Tomassetti P. Clinical aspects of carcinoid tumours. Ital J Gastroenterol Hepatol. 1999;31(Suppl 2):143–146. [PubMed] [Google Scholar]
- 23.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 24.Shrager JB, Wright CD, Wain JC, Torchiana DF, Grillo HC, Mathisen DJ. Bronchopulmonary carcinoid tumors associated with Cushing’217;s syndrome: a more aggressive variant of typical carcinoid. J Thorac Cardiovasc Surg. 1997;114:367–375. doi: 10.1016/S0022-5223(97)70182-X. [DOI] [PubMed] [Google Scholar]
- 25.Athanassiadi K, Exarchos D, Tsagarakis S, Bellenis I. Acromegaly caused by ectopic growth hormone-releasing hormone secretion by a carcinoid bronchial tumor: a rare entity. J Thorac Cardiovasc Surg. 2004;128:631–632. doi: 10.1016/j.jtcvs.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 26.de Laat JM, Pieterman CR, van den Broek MF, et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab. 2014;99:3325–3333. doi: 10.1210/jc.2014-1560. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira AM, Tazelaar HD, Wentzlaff KA, et al. Familial pulmonary carcinoid tumors. Cancer. 2001;91:2104–2109. doi: 10.1002/1097-0142(20010601)91:11<2104::aid-cncr1238>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Aguayo SM, Miller YE, Waldron JA, Jr., et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992;327:1285–1288. doi: 10.1056/NEJM199210293271806. [DOI] [PubMed] [Google Scholar]
- 29.Gorshtein A, Gross DJ, Barak D, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and the associated lung neuroendocrine tumors: clinical experience with a rare entity. Cancer. 2012;118:612–619. doi: 10.1002/cncr.26200. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JM, Trainer PJ, Reznek RH, et al. The radiological investigation of occult ectopic ACTH-dependent Cushing’217;s syndrome. Clin Radiol. 1993;48:11–17. doi: 10.1016/s0009-9260(05)80100-x. [DOI] [PubMed] [Google Scholar]
- 31.Tsagarakis S, Christoforaki M, Giannopoulou H, et al. A reappraisal of the utility of somatostatin receptor scintigraphy in patients with ectopic adrenocorticotropin Cushing’217;s syndrome. J Clin Endocrinol Metab. 2003;88:4754–4758. doi: 10.1210/jc.2003-030525. [DOI] [PubMed] [Google Scholar]
- 32.Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumours. Clin Endocrinol (Oxf) 2003;59:793–799. doi: 10.1046/j.1365-2265.2003.01931.x. [DOI] [PubMed] [Google Scholar]
- 33.JCC Cancer Staging Manual. 7th Edition. New York City: Springer; 2011. pp. 251–253. [Google Scholar]
- 34.Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ’217;clinically aggressive’217; cases. Ann Oncol. 2010;21:548–555. doi: 10.1093/annonc/mdp334. [DOI] [PubMed] [Google Scholar]
- 35.Lococo F, Cesario A, Paci M, et al. PET/CT assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumour Biol. 2014;35:8369–8377. doi: 10.1007/s13277-014-2102-y. [DOI] [PubMed] [Google Scholar]
- 36.Venkitaraman B, Karunanithi S, Kumar A, Khilnani GC, Kumar R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging. 2014;41:856–864. doi: 10.1007/s00259-013-2659-5. [DOI] [PubMed] [Google Scholar]
- 37.Yellin A, Zwas ST, Rozenman J, Simansky DA, Goshen E. Experience with somatostatin receptor scintigraphy in the management of pulmonary carcinoid tumors. Isr Med Assoc J. 2005;7:712–716. [PubMed] [Google Scholar]
- 38.Frilling A, Malago M, Martin H, Broelsch CE. Use of somatostatin receptor scintigraphy to image extrahepatic metastases of neuroendocrine tumors. Surgery. 1998;124:1000–1004. doi: 10.1067/msy.1998.93919. [DOI] [PubMed] [Google Scholar]
- 39.Sundin A, Rockall A. Therapeutic monitoring of gastroenteropancreatic neuroendocrine tumors: the challenges ahead. Neuroendocrinology. 2012;96:261–271. doi: 10.1159/000342270. [DOI] [PubMed] [Google Scholar]
- 40.Kaira K, Murakami H, Endo M, et al. Biological correlation of (1)(8)F-FDG uptake on PET in pulmonary neuroendocrine tumors. Anticancer Res. 2013;33:4219–4228. [PubMed] [Google Scholar]
- e1.Oberg K, Hellman P, Ferolla P, Papotti M. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii120–vii103. doi: 10.1093/annonc/mds267. [DOI] [PubMed] [Google Scholar]
- e2.Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967–1973. doi: 10.1200/JCO.2006.10.1535. [DOI] [PubMed] [Google Scholar]
- e3.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- e4.Nguyen GK. Cytopathology of pulmonary carcinoid tumors in sputum and bronchial brushings. Acta Cytol. 1995;39:1152–1160. [PubMed] [Google Scholar]
- e5.Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society Expert Consensus and Recommendations for best practice for typical and atypical pulmonary carcinoid. Ann Oncol. 2015 doi: 10.1093/annonc/mdv041. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e6.Divisi D, Crisci R. Carcinoid tumors of the lung and multimodal therapy. Thorac Cardiovasc Surg. 2005;53:168–172. doi: 10.1055/s-2005-837539. [DOI] [PubMed] [Google Scholar]
- e7.Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Ullmann R, Petzmann S, Klemen H, Fraire AE, Hasleton P, Popper HH. The position of pulmonary carcinoids within the spectrum of neuroendocrine tumors of the lung and other tissues. Genes Chromosomes Cancer. 2002;34:78–85. doi: 10.1002/gcc.10049. [DOI] [PubMed] [Google Scholar]
- e9.Ducrocq X, Thomas P, Massard G, et al. Operative risk and prognostic factors of typical bronchial carcinoid tumors. Ann Thorac Surg. 1998;65:1410–1414. doi: 10.1016/s0003-4975(98)00083-6. [DOI] [PubMed] [Google Scholar]
- e10.Filosso PL, Guerrera F, Evangelista A, et al. Prognostic model of survival for typical bronchial carcinoid tumours: analysis of 1109 patients on behalf of the European Society of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Groupdagger. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezu495. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e11.Afoke J, Tan C, Hunt I, Zakkar M. Is sublobar resection equivalent to lobectomy for surgical management of peripheral carcinoid? Interact Cardiovasc Thorac Surg. 2013;16:858–863. doi: 10.1093/icvts/ivt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Bolukbas S, Schirren J. Parenchyma-sparing bronchial sleeve resections in trauma, benign and malign diseases. Thorac Cardiovasc Surg. 2010;58:32–37. doi: 10.1055/s-0029-1186241. [DOI] [PubMed] [Google Scholar]
- e13.Brokx HA, Risse EK, Paul MA, et al. Initial bronchoscopic treatment for patients with intraluminal bronchial carcinoids. J Thorac Cardiovasc Surg. 2007;133:973–978. doi: 10.1016/j.jtcvs.2006.12.013. [DOI] [PubMed] [Google Scholar]
- e14.Machuca TN, Cardoso PF, Camargo SM, et al. Surgical treatment of bronchial carcinoid tumors: a single-center experience. Lung Cancer. 2010;70:158–162. doi: 10.1016/j.lungcan.2010.01.015. [DOI] [PubMed] [Google Scholar]
- e15.NCI. The US National Cancer Institute. Surveillance Epidemiology and End Results (SEER) data base, 1973-2004. www.seer.cancer.gov. (last accessed on 9 June 2015)
- e16.Warren WH, Gould VE. Long-term follow-up of classical bronchial carcinoid tumors. Clinicopathologic observations. Scand J Thorac Cardiovasc Surg. 1990;24:125–130. doi: 10.3109/14017439009098055. [DOI] [PubMed] [Google Scholar]
- e17.Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Watzka FM, Fottner C, Miederer M, et al. Surgical therapy of neuroendocrine neoplasm with hepatic metastasis: patient selection and prognosis. Langenbecks Arch Surg. 2015;400:349–358. doi: 10.1007/s00423-015-1277-z. [DOI] [PubMed] [Google Scholar]
- e19.Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg. 2011;146:953–958. doi: 10.1001/archsurg.2011.186. [DOI] [PubMed] [Google Scholar]
- e20.Bushnell DL, Jr., O’217;Dorisio TM, O’217;Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Narayanan S, Kunz PL. Role of somatostatin analogues in the treatment of neuroendocrine tumors. J Natl Compr Canc Netw. 2015;13:109–117. doi: 10.6004/jnccn.2015.0012. [DOI] [PubMed] [Google Scholar]
- e22.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- e23.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- e24.Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46:723–734. doi: 10.1080/02841860701441848. [DOI] [PubMed] [Google Scholar]
- e25.Diaco DS, Hajarizadeh H, Mueller CR, Fletcher WS, Pommier RF, Woltering EA. Treatment of metastatic carcinoid tumors using multimodality therapy of octreotide acetate, intra-arterial chemotherapy, and hepatic arterial chemoembolization. Am J Surg. 1995;169:523–528. doi: 10.1016/S0002-9610(99)80210-4. [DOI] [PubMed] [Google Scholar]
- e26.Mackley HB, Videtic GM. Primary carcinoid tumors of the lung: a role for radiotherapy. Oncology (Williston Park) 2006;20:1537–1543. [PubMed] [Google Scholar]