Abstract
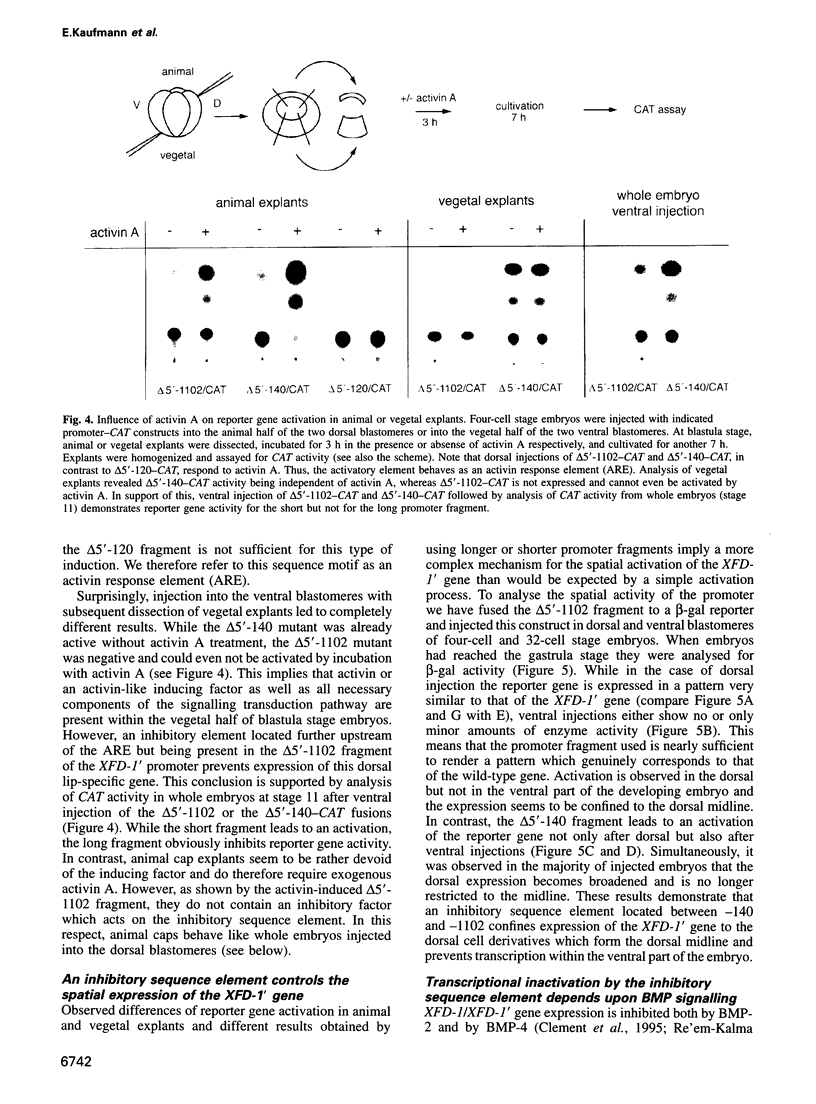
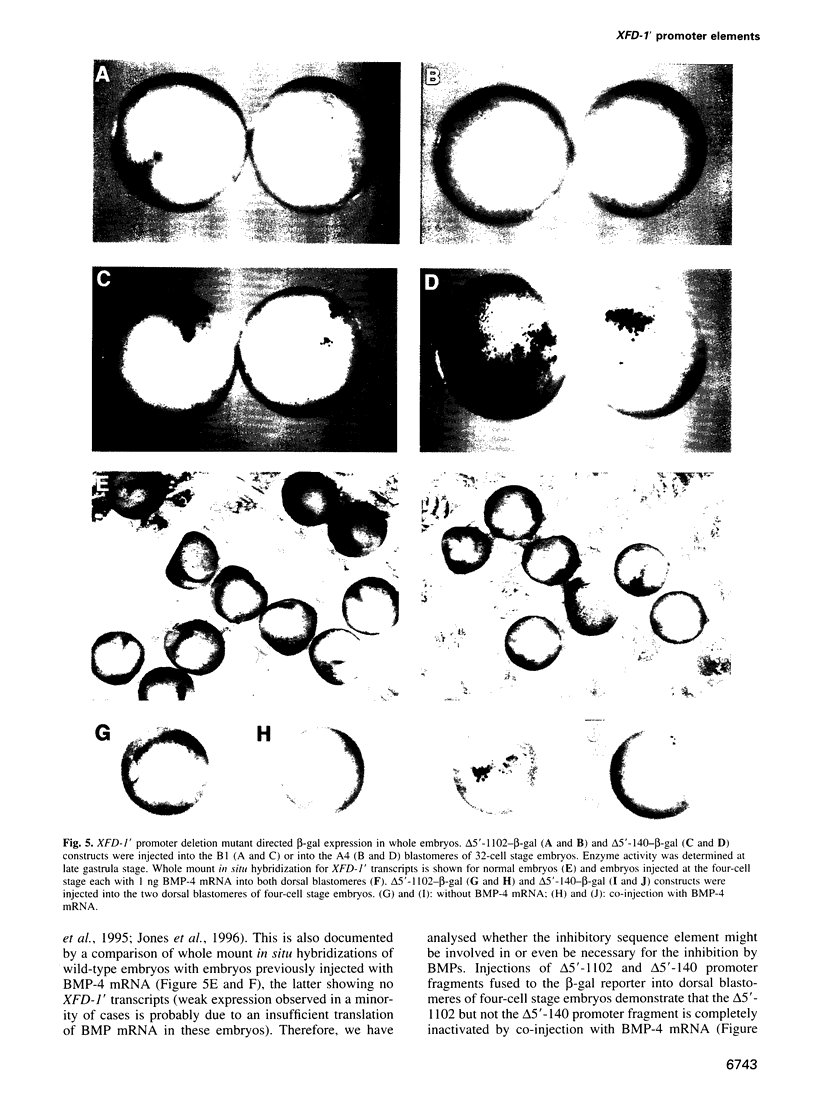
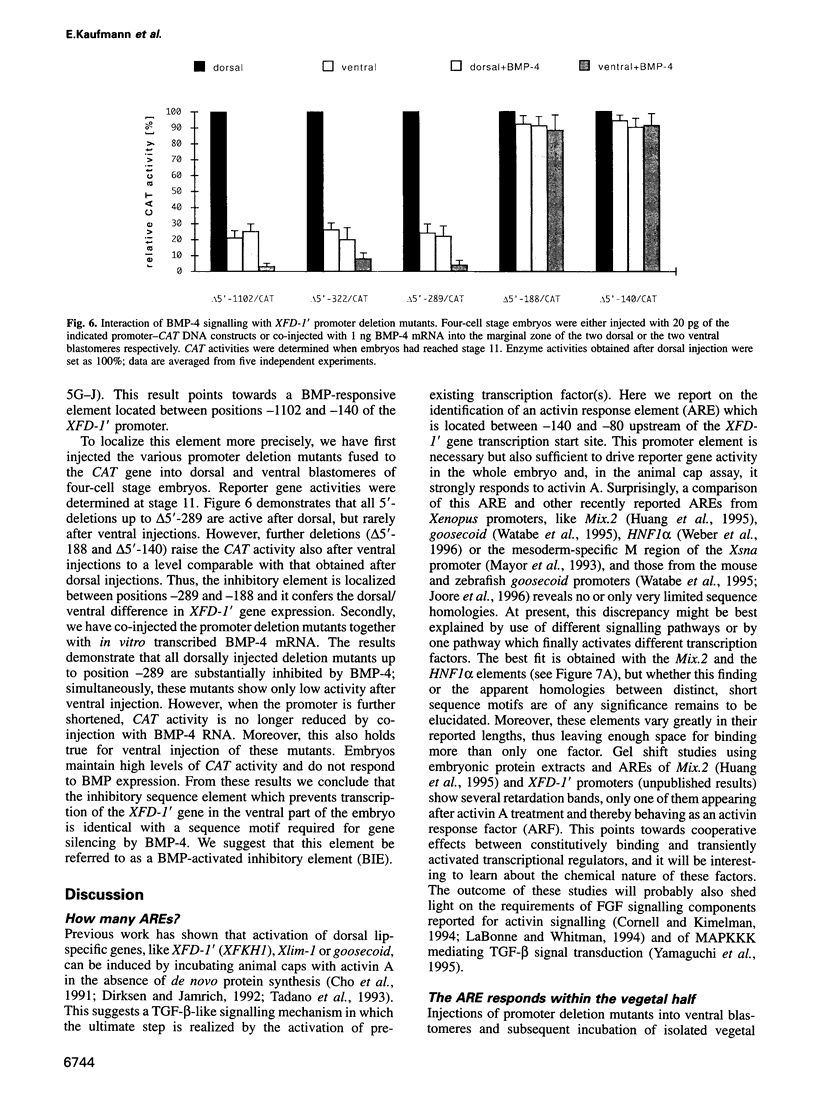
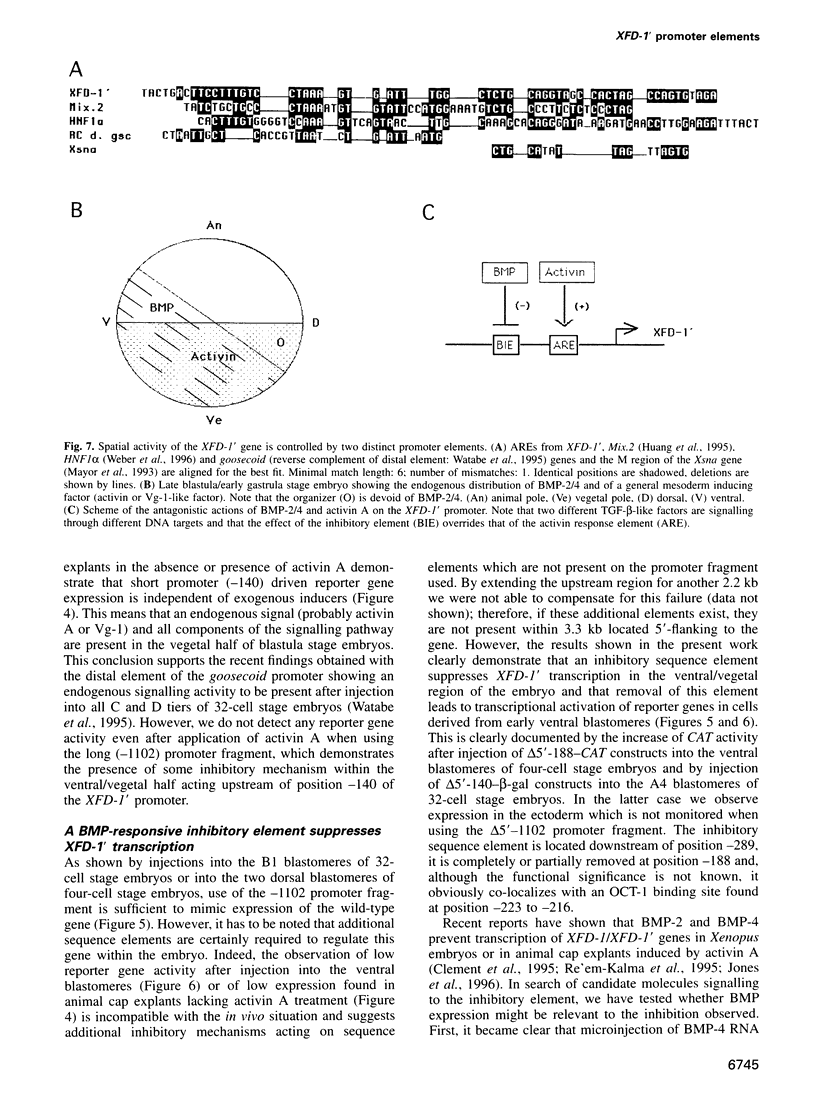
Transcription of the early response gene XFD-1' (XFKH1) in the dorsal lip (Spemann organizer) of Xenopus embryos is activated by dorsal mesoderm inducing factors. Promoter studies revealed the presence of an activin A response element (ARE) which is both necessary and sufficient for transcriptional activation of reporter genes in animal cap explants incubated with activin A. Surprisingly, this ARE is also active within vegetal explants in the absence of exogenously added inducers, but an additional inhibitory response element prevents transcription of the XFD-1' gene in the ventral/vegetal region of the embryo in vivo. This element is located upstream of the ARE, it responds to bone morphogenic proteins 2 and 4 (BMP-2/4) triggered signals and it overrides the activating properties of the ARE. Expression patterns of BMP-2 and BMP-4 in the late blastula stage embryo and, especially, their absence from the dorsal blastopore lip may thus control the spatial transcription of the XFD-1' gene. Accordingly, the temporal activation and the spatial restriction of XFD-1' gene activity to the Spemann organizer is regulated by antagonistic actions of two distinct members of the TGF-beta family (activin and BMP) which act on different promoter elements.
Full text
PDF
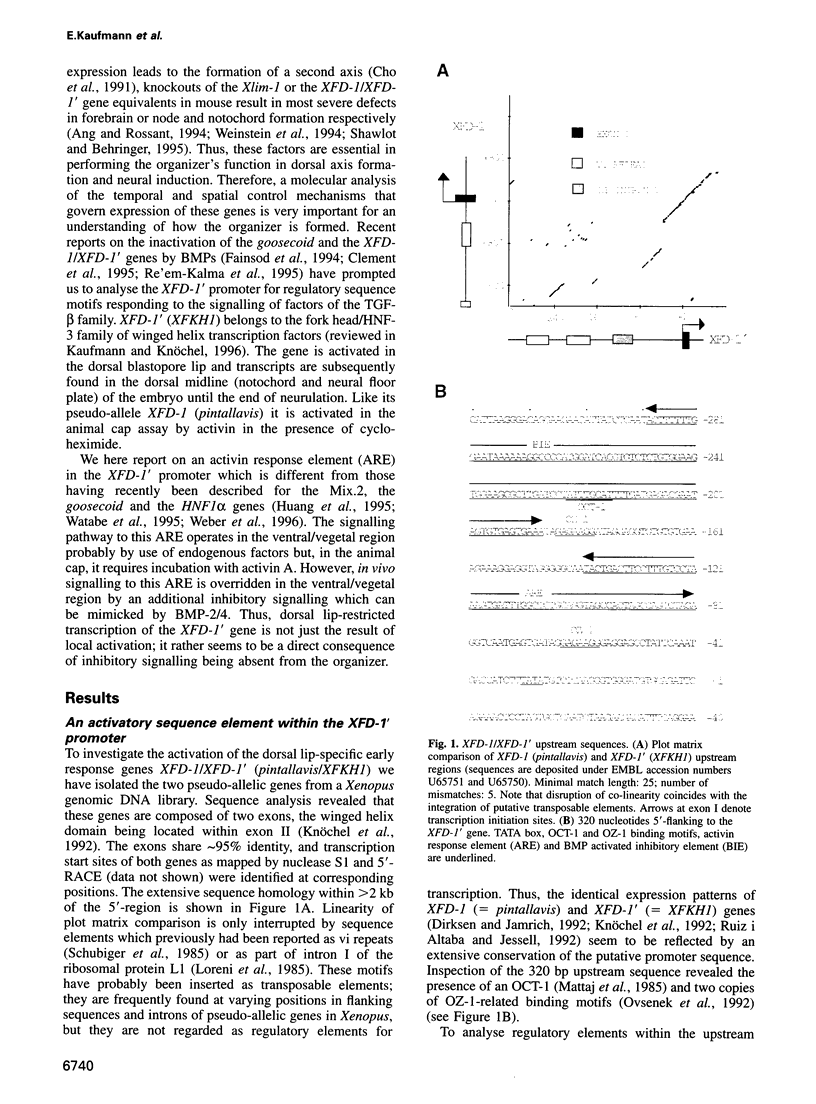
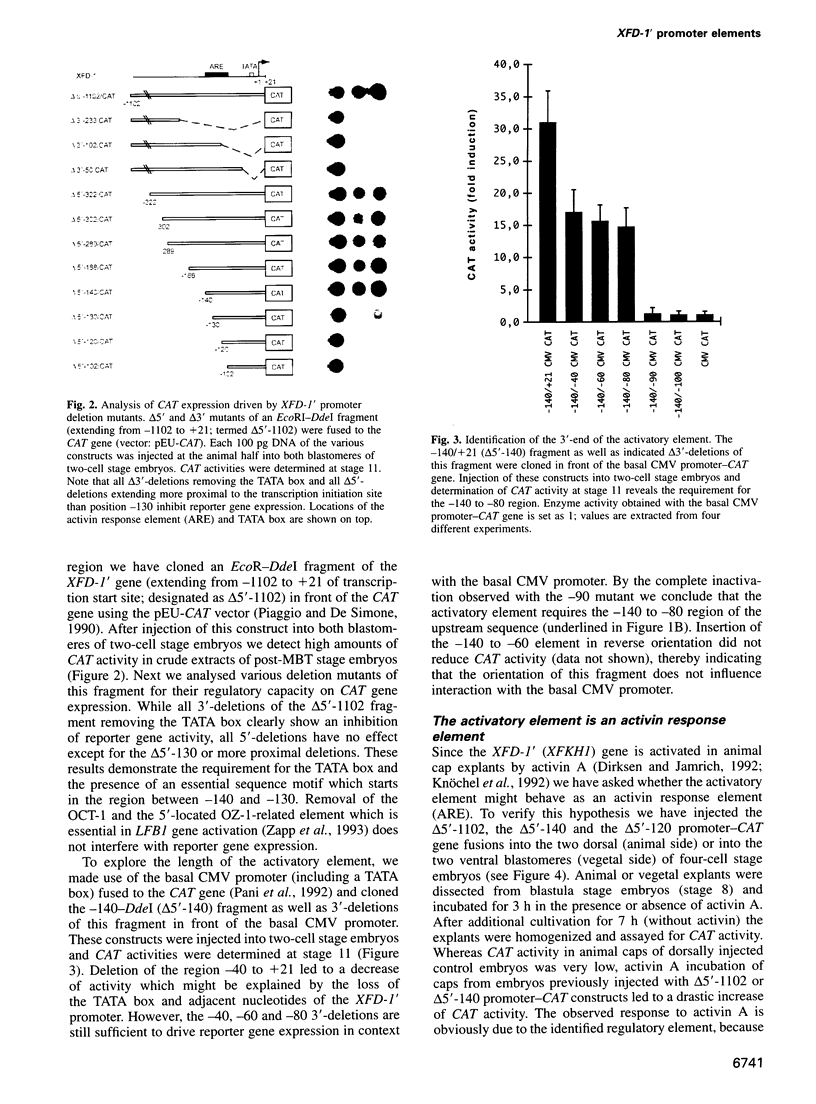




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang S. L., Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994 Aug 26;78(4):561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J. H., Fettes P., Knöchel S., Lef J., Knöchel W. Bone morphogenetic protein 2 in the early development of Xenopus laevis. Mech Dev. 1995 Aug;52(2-3):357–370. doi: 10.1016/0925-4773(95)00413-u. [DOI] [PubMed] [Google Scholar]
- Cornell R. A., Kimelman D. Activin-mediated mesoderm induction requires FGF. Development. 1994 Feb;120(2):453–462. doi: 10.1242/dev.120.2.453. [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987 Jun;100(2):279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Dawid I. B. Intercellular signaling and gene regulation during early embryogenesis of Xenopus laevis. J Biol Chem. 1994 Mar 4;269(9):6259–6262. [PubMed] [Google Scholar]
- Detrick R. J., Dickey D., Kintner C. R. The effects of N-cadherin misexpression on morphogenesis in Xenopus embryos. Neuron. 1990 Apr;4(4):493–506. doi: 10.1016/0896-6273(90)90108-r. [DOI] [PubMed] [Google Scholar]
- Dirksen M. L., Jamrich M. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992 Apr;6(4):599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Steinbeisser H., De Robertis E. M. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994 Nov 1;13(21):5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Bansal A., Melton D. A. Xenopus Mad proteins transduce distinct subsets of signals for the TGF beta superfamily. Cell. 1996 May 17;85(4):479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hawley S. H., Wünnenberg-Stapleton K., Hashimoto C., Laurent M. N., Watabe T., Blumberg B. W., Cho K. W. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995 Dec 1;9(23):2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Kelly O. G., Melton D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994 Apr 22;77(2):283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hoodless P. A., Haerry T., Abdollah S., Stapleton M., O'Connor M. B., Attisano L., Wrana J. L. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996 May 17;85(4):489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Murtaugh L. C., Vize P. D., Whitman M. Identification of a potential regulator of early transcriptional responses to mesoderm inducers in the frog embryo. EMBO J. 1995 Dec 1;14(23):5965–5973. doi: 10.1002/j.1460-2075.1995.tb00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Dale L., Hogan B. L., Wright C. V., Smith J. C. Bone morphogenetic protein-4 (BMP-4) acts during gastrula stages to cause ventralization of Xenopus embryos. Development. 1996 May;122(5):1545–1554. doi: 10.1242/dev.122.5.1545. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Joore J., Fasciana C., Speksnijder J. E., Kruijer W., Destrée O. H., van den Eijnden-van Raaij A. J., de Laat S. W., Zivkovic D. Regulation of the zebrafish goosecoid promoter by mesoderm inducing factors and Xwnt1. Mech Dev. 1996 Mar;55(1):3–18. doi: 10.1016/0925-4773(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann E., Knöchel W. Five years on the wings of fork head. Mech Dev. 1996 Jun;57(1):3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Melton D. A. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994 Oct 28;266(5185):596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Knöchel S., Lef J., Clement J., Klocke B., Hille S., Köster M., Knöchel W. Activin A induced expression of a fork head related gene in posterior chordamesoderm (notochord) of Xenopus laevis embryos. Mech Dev. 1992 Aug;38(2):157–165. doi: 10.1016/0925-4773(92)90007-7. [DOI] [PubMed] [Google Scholar]
- Kothary R., Clapoff S., Darling S., Perry M. D., Moran L. A., Rossant J. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development. 1989 Apr;105(4):707–714. doi: 10.1242/dev.105.4.707. [DOI] [PubMed] [Google Scholar]
- Köster M., Plessow S., Clement J. H., Lorenz A., Tiedemann H., Knöchel W. Bone morphogenetic protein 4 (BMP-4), a member of the TGF-beta family, in early embryos of Xenopus laevis: analysis of mesoderm inducing activity. Mech Dev. 1991 Mar;33(3):191–199. doi: 10.1016/0925-4773(91)90027-4. [DOI] [PubMed] [Google Scholar]
- LaBonne C., Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994 Feb;120(2):463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D., Harland R. M. Neural induction by the secreted polypeptide noggin. Science. 1993 Oct 29;262(5134):713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Liu F., Hata A., Baker J. C., Doody J., Cárcamo J., Harland R. M., Massagué J. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996 Jun 13;381(6583):620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- Loreni F., Ruberti I., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Nucleotide sequence of the L1 ribosomal protein gene of Xenopus laevis: remarkable sequence homology among introns. EMBO J. 1985 Dec 16;4(13A):3483–3488. doi: 10.1002/j.1460-2075.1985.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R., Essex L. J., Bennett M. F., Sargent M. G. Distinct elements of the xsna promoter are required for mesodermal and ectodermal expression. Development. 1993 Nov;119(3):661–671. doi: 10.1242/dev.119.3.661. [DOI] [PubMed] [Google Scholar]
- Maéno M., Ong R. C., Suzuki A., Ueno N., Kung H. F. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Wang S., Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995 Dec;121(12):4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S., Suzuki A., Shoda A., Murakami K., Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Northrop J., Woods A., Seger R., Suzuki A., Ueno N., Krebs E., Kimelman D. BMP-4 regulates the dorsal-ventral differences in FGF/MAPKK-mediated mesoderm induction in Xenopus. Dev Biol. 1995 Nov;172(1):242–252. doi: 10.1006/dbio.1995.0019. [DOI] [PubMed] [Google Scholar]
- Ovsenek N., Zorn A. M., Krieg P. A. A maternal factor, OZ-1, activates embryonic transcription of the Xenopus laevis GS17 gene. Development. 1992 Jun;115(2):649–655. doi: 10.1242/dev.115.2.649. [DOI] [PubMed] [Google Scholar]
- Pani L., Overdier D. G., Porcella A., Qian X., Lai E., Costa R. H. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Mol Cell Biol. 1992 Sep;12(9):3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re'em-Kalma Y., Lamb T., Frank D. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12141–12145. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. M. Pintallavis, a gene expressed in the organizer and midline cells of frog embryos: involvement in the development of the neural axis. Development. 1992 Sep;116(1):81–93. doi: 10.1242/dev.116.Supplement.81. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995 Jul 27;376(6538):333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger J. L., Germond J. E., ten Heggeler B., Wahli W. The Vi element. A transposon-like repeated DNA sequence interspersed in the vitellogenin locus of Xenopus laevis. J Mol Biol. 1985 Dec 5;186(3):491–503. doi: 10.1016/0022-2836(85)90124-x. [DOI] [PubMed] [Google Scholar]
- Shawlot W., Behringer R. R. Requirement for Lim1 in head-organizer function. Nature. 1995 Mar 30;374(6521):425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Shoda A., Murakami K., Ueno N. Presence of high molecular weight forms of BMP-2 in early Xenopus embryos. Growth Factors. 1993;8(3):165–172. doi: 10.3109/08977199309011019. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Inducing factors in Xenopus early embryos. Curr Biol. 1994 Feb 1;4(2):116–126. doi: 10.1016/s0960-9822(94)00027-8. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Slack J. M. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol. 1983 Dec;78:299–317. [PubMed] [Google Scholar]
- Smith W. C., Knecht A. K., Wu M., Harland R. M. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993 Feb 11;361(6412):547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Smith W. C., McKendry R., Ribisi S., Jr, Harland R. M. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995 Jul 14;82(1):37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadano T., Otani H., Taira M., Dawid I. B. Differential induction of regulatory genes during mesoderm formation in Xenopus laevis embryos. Dev Genet. 1993;14(3):204–211. doi: 10.1002/dvg.1020140307. [DOI] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Thomsen G. H., Melton D. A. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993 Aug 13;74(3):433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Ueno N., Shoda A., Takebayashi K., Suzuki A., Nishimatsu S., Kikuchi T., Wakimasu M., Fujino M., Murakami K. Identification of bone morphogenetic protein-2 in early Xenopus laevis embryos. Growth Factors. 1992;7(3):233–240. doi: 10.3109/08977199209046927. [DOI] [PubMed] [Google Scholar]
- Watabe T., Kim S., Candia A., Rothbächer U., Hashimoto C., Inoue K., Cho K. W. Molecular mechanisms of Spemann's organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995 Dec 15;9(24):3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- Weber H., Holewa B., Jones E. A., Ryffel G. U. Mesoderm and endoderm differentiation in animal cap explants: identification of the HNF4-binding site as an activin A responsive element in the Xenopus HNF1alpha promoter. Development. 1996 Jun;122(6):1975–1984. doi: 10.1242/dev.122.6.1975. [DOI] [PubMed] [Google Scholar]
- Weinstein D. C., Ruiz i Altaba A., Chen W. S., Hoodless P., Prezioso V. R., Jessell T. M., Darnell J. E., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994 Aug 26;78(4):575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wilson P. A., Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995 Jul 27;376(6538):331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Xu R. H., Dong Z., Maeno M., Kim J., Suzuki A., Ueno N., Sredni D., Colburn N. H., Kung H. F. Involvement of Ras/Raf/AP-1 in BMP-4 signaling during Xenopus embryonic development. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):834–838. doi: 10.1073/pnas.93.2.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R. H., Kim J., Taira M., Zhan S., Sredni D., Kung H. F. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995 Jul 6;212(1):212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995 Dec 22;270(5244):2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Zapp D., Bartkowski S., Holewa B., Zoidl C., Klein-Hitpass L., Ryffel G. U. Elements and factors involved in tissue-specific and embryonic expression of the liver transcription factor LFB1 in Xenopus laevis. Mol Cell Biol. 1993 Oct;13(10):6416–6426. doi: 10.1128/mcb.13.10.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]