Abstract
Directional beating of ependymal (E) cells' cilia in the walls of the ventricles in the brain is essential for proper CSF flow. E cells display two forms of planar cell polarity (PCP): rotational polarity of individual cilium and translational polarity (asymmetric positioning of cilia in the apical area). The orientation of individual E cells varies according to their location in the ventricular wall (location-specific PCP). It has been hypothesized that hydrodynamic forces on the apical surface of radial glia cells (RGCs), the embryonic precursors of E cells, could guide location-specific PCP in the ventricular epithelium. However, the detection mechanisms for these hydrodynamic forces have not been identified. Here, we show that the mechanosensory proteins polycystic kidney disease 1 (Pkd1) and Pkd2 are present in primary cilia of RGCs. Ablation of Pkd1 or Pkd2 in Nestin-Cre;Pkd1flox/flox or Nestin-Cre;Pkd2flox/flox mice, affected PCP development in RGCs and E cells. Early shear forces on the ventricular epithelium may activate Pkd1 and Pkd2 in primary cilia of RGCs to properly polarize RGCs and E cells. Consistently, Pkd1, Pkd2, or primary cilia on RGCs were required for the proper asymmetric localization of the PCP protein Vangl2 in E cells' apical area. Analyses of single- and double-heterozygous mutants for Pkd1 and/or Vangl2 suggest that these genes function in the same pathway to establish E cells' PCP. We conclude that Pkd1 and Pkd2 mechanosensory proteins contribute to the development of brain PCP and prevention of hydrocephalus.
SIGNIFICANCE STATEMENT This study identifies key molecules in the development of planar cell polarity (PCP) in the brain and prevention of hydrocephalus. Multiciliated ependymal (E) cells within the brain ventricular epithelium generate CSF flow through ciliary beating. E cells display location-specific PCP in the orientation and asymmetric positioning of their cilia. Defects in this PCP can result in hydrocephalus. Hydrodynamic forces on radial glial cells (RGCs), the embryonic progenitors of E cells, have been suggested to guide PCP. We show that the mechanosensory proteins Pkd1 and Pkd2 localize to primary cilia in RGCs, and their ablation disrupts the development of PCP in E cells. Early shear forces on RGCs may activate Pkd1 and Pkd2 in RGCs' primary cilia to properly orient E cells. This study identifies key molecules in the development of brain PCP and prevention of hydrocephalus.
Keywords: cilia, ependymal cell, epithelium, neural stem cell, planar cell polarity, polycystin
Introduction
Ependymal (E) cells are multiciliated epithelial cells that line the brain ventricles and generate CSF flow through ciliary beating (Miyan et al., 2003). E cells exhibit two types of planar cell polarity (PCP): (1) rotational polarity, the unidirectional orientation of individual basal bodies (BBs), and (2) translational polarity, the asymmetric positioning of the cluster of BBs in the apical region of E cells toward the direction of CSF flow (Mirzadeh et al., 2010b). Defects in E cells' PCP result in abnormal CSF accumulation and hydrocephalus (Boutin et al., 2014; Ohata et al., 2014; Ying et al., 2014). Understanding the mechanism underlying the establishment of E cell PCP is important for the diagnosis and prevention of hydrocephalus.
Translational polarity begins in the embryonic progenitors of E cells, called radial glial cells (RGCs), by the embryonic day (E)16 (Mirzadeh et al., 2010b; Boutin et al., 2014). The early asymmetric localization of BB in RGCs is further refined as they differentiate into E cells (Mirzadeh et al., 2010b). Rotational polarity of individual motile cilia, which in immature E cells is random, becomes unidirectional during E cells' maturation between postnatal day (P)4 and P20 (Guirao et al., 2010). Both rotational and translational polarity are well correlated with the direction of CSF flow (location-specific PCP; Mirzadeh et al., 2010b; Boutin et al., 2014), suggesting that the early passive CSF flow could influence the development of PCP in RGCs and E cells. Hydrodynamic forces can instruct rotational polarity in cultured developing E cells and multiciliated cells in the Xenopus larval skin (Mitchell et al., 2007; Guirao et al., 2010). Interestingly, RGCs' primary cilia, which protrude into the ventricles, are required for the proper polarization of E cells (Mirzadeh et al., 2010b). The primary cilium is emerging as a key sensory organelle with many functions, including mechanosensation (Guemez-Gamboa et al., 2014). Early hydrodynamic forces on the ventricular surface of RGCs has been proposed to help guide the planar polarization of E cells (Mirzadeh et al., 2010b). The molecular components involved in the establishment of PCP in RGCs and E cells remain unknown.
The mechanosensory protein polycystic kidney disease 1 (Pkd1; also known as polycystin-1 and PC-1) is an 11-pass transmembrane protein, enriched in primary cilia, and has been proposed to mediate mechanosensation of urine flow in the kidney (Nauli et al., 2003; Zhou, 2009; Kotsis et al., 2013). Activation of Pkd1 by fluid flow triggers Ca2+ intake through its associated ion channel, Pkd2 (also known as polycystin-2, PC-2, and transient receptor potential polycystic 2; Nauli et al., 2003). Genetic inactivation of Pkd1 affects convergent extension-like movement and oriented cell division in kidney epithelial cells (Luyten et al., 2010; Castelli et al., 2013), suggesting its involvement in PCP. Interestingly, ablation of Pkd1 in the developing mouse brain results in hydrocephalus (Wodarczyk et al., 2009). However, whether Pkd1 or Pkd2 play a role in the E cells' planar polarization, and how these mechanoreceptor components help organize the ventricular epithelium remains unknown.
Here, we show that Pkd1 and Pkd2 are expressed in primary cilia of RGCs. Ablation of Pkd1 or Pkd2 in early RGCs using Nestin-Cre;Pkd1flox/flox or Nestin-Cre;Pkd2flox/flox mutant mice did not affect the differentiation of E cells, but significantly disrupted PCP in both RGCs and E cells. The core PCP protein van Gogh-like 2 (Vangl2) is asymmetrically localized in RGCs' and E cells' apical compartment, and it is required for their PCP (Guirao et al., 2010; Boutin et al., 2014). Interestingly, in the Nestin-Cre;Pkd1flox/flox, Nestin-Cre;Pkd2flox/flox or ciliary mutants (Nestin-Cre;Kif3aflox/flox and Nestin-Cre;Ift88flox/flox), the asymmetric localization of Vangl2 in the apical region of E cells was severely disrupted. Double-heterozygous mutants for Pkd1 and Vangl2 resulted in synergistic phenotypes, suggesting that these genes function in the same pathway for E cells' PCP. These results identify Pkd1 and Pkd2 as key mechanosensory components in RGCs to establish PCP in the ventricular epithelium of the postnatal brain.
Materials and Methods
Animals.
All animal experiments were approved by the Institutional Animal Care and Use Committee and Laboratory Animal Resource Center at UCSF, and maintained following the NIH, American Veterinary Medical Association, and UCSF guidelines. hGFAP-Cre (Zhuo et al., 2001), Ift88flox (Haycraft et al., 2007), Kif3aflox (Marszalek et al., 2000), Nestin-Cre (Tronche et al., 1999), Pkd1flox (Wodarczyk et al., 2009), and Pkd2flox (Garcia-Gonzalez et al., 2010; The Jackson Laboratory, 017292) mice have been previously generated and were genotyped as described. Vangl2Lp mice (The Jackson Laboratory, 000220) were identified previously (Strong and Hollander, 1949; Kibar et al., 2001; Murdoch et al., 2001) and were genotyped by PCR using the following primers (Lp WT F, 5′-CAA ACA GTG GAC CTT GGT GTG-3′; Lp mutant F, 5′-CAA ACA GTG GAC CTT GGT GTA-3′; Lp WT and mutant R, 5′-TGG CAG AAA TGT GTC AGG G-3′). Both males and females were used for all experiments.
Reverse transcription-PCR.
cDNAs were generated from microdissected lateral walls of the lateral ventricles at P0 as described previously (Ohata et al., 2009, 2011). The following primers were used; Pkd1 F, 5′-GGA CTA TGA GAT GGT GGA GC-3′; Pkd1 R, 5′-GAG AGG AAG GAG GTC CAT TG-3′; Pkd2 F, 5′-TCA AGC TGG AGA TCA TGG AG-3′; Pkd2 R, 5′-CTC AGG AAC ACA CAA ACA CG-3′; ef1αF, 5′-GCT TTG AGT GAA GCT CTT CC-3′; ef1αR, 5′-CCT TCT TGT CCA CAG CTT TG-3′.
Immunohistochemistry.
Mice were deeply anesthetized with 700 mg/kg bodyweight tribromoethanol (Sigma-Aldrich) by intraperitoneal injections. For the preparation of cryosections, brains were dissected after transcardial perfusion with saline (0.9% NaCl) and 4% paraformaldehyde (PFA, Sigma-Aldrich) in 100 mm phosphate buffer (PB), pH 7.4, using a 323E pump (Watson Marlow, 3–4 ml/min), postfixed with 4% PFA at 4°C overnight, rinsed with PBS, incubated in 30% sucrose overnight, immersed in OCT compound (Sakura) at 4°C for 1 h, frozen on dry ice in OCT compound, and sectioned at 12 μm thickness with a HM 500 OM cryostat (Microm). The cryosections were washed with PB at 4°C for 15 min, and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS at room temperature (20–22°C) for 15 min. Antigen retrieval was performed in 10 mm citrate buffer, pH 6.0, at 95°C for 10 min. Next, blocking of nonspecific binding sites was performed with PBS with 10% normal donkey serum (Sigma-Aldrich) and 0.5% Triton X-100 (blocking solution). Primary antibodies were incubated in the blocking solution overnight at 4°C followed by secondary antibody and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) incubation at room temperature for 2 h.
Whole-mounts of the lateral ventricle walls were prepared as described previously (Mirzadeh et al., 2010a). The exposed walls were fixed in 4% PFA at 4°C overnight and washed with PBS/0.2% Triton X-100 three times. For Pkd2 staining, antigen retrieval was performed in 10 mm citrate buffer, pH 6.0, at 95°C for 10 min. After blocking, whole-mounts were incubated with primary antibodies in the blocking solution overnight at 4°C, washed with PBS/0.2% Triton X-100, and incubated with secondary antibodies in the blocking solution overnight at 4°C.
Confocal images were taken on a Leica SP5 using a HCX PL APO CS 100× oil-immersion objective lens (NA 1.46). To analyze BB patch angle in E cells and BB angle in RGCs, the vectors were drawn using MetaMorph software as described previously (Mirzadeh et al., 2010b). The angles of the vectors were normalized so that the average of the angle of the vectors is 180° in each picture. The percentages of vectors were plotted on a histogram in 15° bins. BB patch displacement in E cells and BB displacement in RGCs were quantified using the ImageJ software (NIH). To normalize the magnitude of the vector for the size and shape of the apical surface in each E cell, we divided it by the length of line that is drawn from the center of the apical surface to the edge of the apical surface passing through the center of BB patch (see Fig. 7a, black line). The intensity of Vangl2-immunoreactivity in the apical area of E cells was quantified using the MetaMorph software and normalized to control mice.
Figure 7.
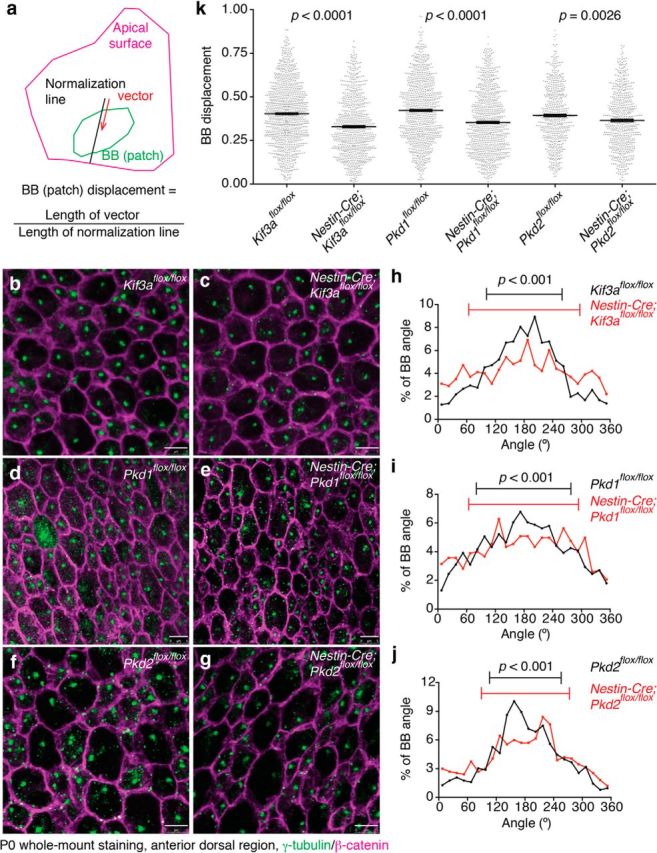
Primary cilia, Pkd1, and Pkd2 were involved in the establishment of translational polarity in RGCs. a, Schematic drawing showing BB patch angle and BB patch displacement. A vector (red arrow) was drawn from the center of apical surface to that of BB patch (in case of RGCs, BBs of primary cilia). To calculate BB patch displacement, the length of the vector was normalized with the length of the line extended to the edge of the apical surface (black line). b–g, Whole-mount preparations of the lateral walls of the lateral ventricle at P0 were stained with antibodies against γ-tubulin (green) and β-catenin (magenta) in Kif3aflox/flox (b), Nestin-Cre;Kif3aflox/flox (c), Pkd1flox/flox (d), Nestin-Cre;Pkd1flox/flox (e), Pkd2flox/flox (f), and Nestin-Cre;Pkd2flox/flox (g) mice. Scale bar, 5 μm. h–j, Histogram showing the distribution of BB angles in Kif3aflox/flox (h, black; CSD = 79°; n = 1018 RGCs, five mice), Nestin-Cre;Kif3aflox/flox (h, red; CSD = 116°; n = 997 RGCs, five mice; p < 0.001), Pkd1flox/flox (i, black; CSD = 99°; n = 1225 RGCs, eight mice) and Nestin-Cre;Pkd1flox/flox (i, red; CSD = 115°; n = 924 RGCs, five mice; p < 0.001), Pkd2flox/flox (j, black; CSD = 75°; n = 627 RGCs, four mice) and Nestin-Cre;Pkd2flox/flox (j, red; CSD = 92°; n = 667 RGCs, four mice; p < 0.001) mice at P0. Error bars on the graph show CSD. k, Quantification of BB displacement. Data shown are mean ± SEM. Each point on the graphs is BB displacement of an individual RGC or E cell. Kif3aflox/flox; n = 1125 RGCs, five mice: Nestin-Cre;Kif3aflox/flox; n = 852 RGCs, five mice; p < 0.0001. Pkd1flox/flox; n = 948 RGCs, six mice: Nestin-Cre;Pkd1flox/flox; n = 927 RGCs, five mice; p < 0.0001. Pkd2flox/flox; n = 600 RGCs, four mice: Nestin-Cre;Pkd2flox/flox; n = 657 RGCs, four mice; p = 0.0026.
Antibodies.
Primary antibodies: rabbit anti-adenylyl cyclase type III (ACIII, Santa Cruz Biotechnology, sc-588), mouse anti-β-catenin (BD Biosciences, 610153), rabbit anti-β-catenin (Sigma-Aldrich, C2206), rat anti-CD24-conjugated with phycoerythrin (PE; BD Biosciences, 553262), mouse anti-FoxJ1 (eBiosciences, 14-9965), chicken anti-GFAP (aves, GFAP), mouse anti-HA (Covance Research Products, MMS-101P-200), rabbit isotype control (Cell Signaling Technology, no. 3900), rabbit anti-Pkd1 (Bioss, bs-2157R), rabbit anti-Pkd2 (EMD Millipore, AB9088), rabbit anti-S100β (Dako, Z0311), mouse anti-acetylated tubulin (Sigma-Aldrich, T6793), rabbit anti-γ-tubulin (Sigma-Aldrich, T5192), rabbit anti-Vangl2 (Santa Cruz Biotechnology, sc-67136). Secondary antibodies: conjugated to AlexaFluor dyes (donkey or goat polyclonal, Thermo Fisher Scientific).
Scanning electron microscopy.
Whole-mount preparations of the lateral wall of lateral ventricles were fixed with 2% PFA and 2.5% glutaraldehyde (Electron Microscopy Sciences; EMS) in PB at room-temperature for 1 h, washed with PB, postfixed with 1% osmium tetroxide (EMS) in PB for 2 h at room temperature, rinsed with deionized water, and dehydrated first in ethanol then with CO2 by critical point drying method. The samples were coated with gold/palladium alloy by sputter coating. The surface of the lateral wall was studied under a Hitachi S-4800 scanning electron microscope using Quantax 400 software (Bruker) for image acquisition.
Transmission electron microscopy.
Whole-mount preparations of the lateral wall of lateral ventricles were fixed overnight with 2% PFA and 2.5% glutaraldehyde (EMS) in PB at 4°C, washed with PB, postfixed with 2% osmium tetroxide (EMS) in PB for 2 h at room temperature, rinsed with PB, stained with 2% uranyl acetate (EMS) in 70% ethanol at 4°C for 2.5 h, dehydrated with ethanol, incubated in propylene oxide (EMS), and embedded in Durcupan resin (Sigma-Aldrich). Serial semithin sections (1.5 μm) were cut with an UC-6 ultramicrotome (Leica) and stained lightly with 1% toluidine blue. Subsequently, selected levels were glued with Super Glue-3 Loctite (Henkel) to Araldite blocks and detached from the glass slide by repeated freezing in liquid nitrogen and thawing. Ultrathin sections (60–70 nm) were prepared with an ultramicrotome and stained with lead citrate (Reynolds' solution). Photomicrographs were obtained under a FEI Tecnai G2 Spirit transmission electron microscope (FEI Europe) using a Morada digital camera (Olympus Soft Image Solutions). Acquired images were analyzed using ImageJ software.
Image processing.
Adjustment of brightness and contrast of the pictures, if required, was performed using Photoshop CS5 (Adobe Systems) or ImageJ software.
High-speed live imaging of ciliary beating and ependymal flow assay.
Whole-mounts of the lateral wall of the lateral ventricle were freshly dissected as above. For high-speed imaging of ciliary beating, the whole-mount preparations were incubated with rat anti-CD24 antibody conjugated with PE in neurobasal medium (Thermo Fisher Scientific) supplemented with B-27 serum-free supplement (Thermo Fisher Scientific), glutamine and antibiotics for 20 min at room temperature, rinsed with L-15 medium (Thermo Fisher Scientific), and placed in a glass bottomed dish (BD Biosciences). Low melting point agarose (1–2%; Thermo Fisher Scientific) and Neurobasal medium with the supplements were placed on the whole-mounts. Ciliary beating was recorded with 15 ms exposure time at 61 frames per second (fps) for 200 frames at room temperature using a Leica DMI600 B microscope, HCX PL APO 100× oil-immersion objective lens (NA 1.44, Leica), Rolera EM-C2 high-speed camera (QImaging), and MetaMorph software.
For the ependymal flow assay, a glass micropipette filled with fluorescent polystyrene latex microbeads (2 μm, Polysciences) attached to an MO-10 micromanipulator (Narishige) was lowered onto the whole-mount and microbeads were deposited onto the ventricular surface. The movement of microbeads was recorded using a Leica MZFLIII fluorescent dissection microscope and Retiga 2000R high-speed digital camera (QImaging) plugged into OpenLab imaging software (Improvision) at 10 fps. The speeds of migrating fluorescent beads were quantified using the Manual Tracking plugin for ImageJ software.
Magnetic resonance imaging.
T2-weighted axial image sequences of fixed brains were acquired using a 7T MR scanner (Unity Inova system; Agilent Technologies) and a handmade proton surface coil (repetition time/echo time = 4200/36 ms, 10 × 10 mm, thickness = 0.5 mm, slice gap = 0 mm). Obtained images were analyzed using ImageJ software.
Statistics.
All results shown in the dot plot graphs are expressed as mean ± SEM. The means of experimental groups were compared with two-tailed Student's t test. The distributions of angles were compared with Watson's U2 test using Oriana software (Kovach Computing Services). Distribution of the Vangl2 asymmetric localization positive and negative cells between control and mutant mice was compared with Fisher's exact test using Prism 6 software (Graphpad). Differences were considered significant at p < 0.05.
Results
Localization of Pkd1 and Pkd2 in primary cilia of RGCs
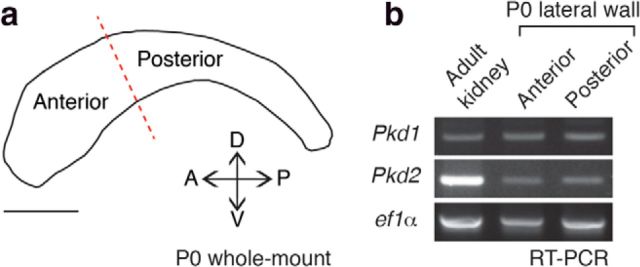
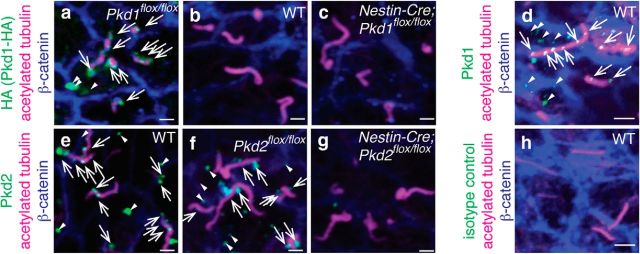
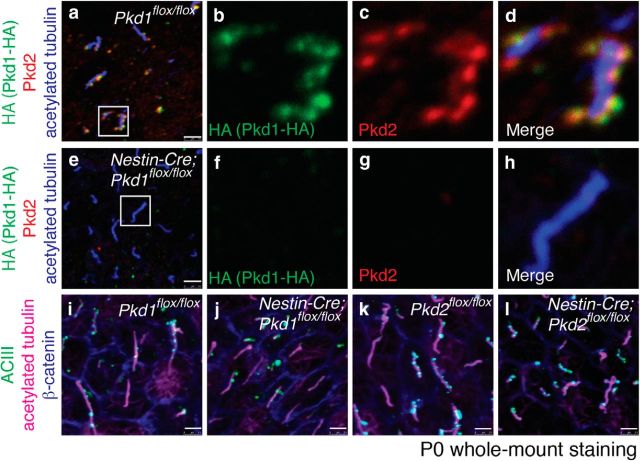
We first investigated, using reverse transcription-PCR (RT-PCR), whether Pkd1 and Pkd2 were expressed in lateral walls of the lateral ventricle at P0 (Fig. 1a). Both mRNAs were present in microdissected ventricular wall (Fig. 1b). To determine the subcellular localization of Pkd1 in RGCs, we used a knock-in mouse line that expresses influenza hemagglutinin (HA)-tagged Pkd1 (hereafter referred to as Pkd1flox/flox, also known as Pkd1HA/HA; Wodarczyk et al., 2009). Immunolabeling for Pkd1-HA revealed staining associated with the shaft of P0 RGCs' primary cilia (Fig. 2a, arrows), with some punctate staining outside the cilia in the apical domain (Fig. 2a, arrowheads). This staining was not observed in wild-type (WT) controls (Fig. 2b). To confirm the specificity of the HA-immunostaining, we crossed the Pkd1flox/flox mice with Nestin-Cre transgenic mice (hereafter referred to as Nestin-Cre;Pkd1flox/flox) to eliminate Pkd1 C terminus domain, including the HA-tag, in the developing brain beginning at E9.5 (Tronche et al., 1999). In these mice, no Pkd1-HA staining was observed in cilia (Fig. 2c), demonstrating the specificity of Pkd1-HA immunoreactivity. Immunostaining using an antibody against Pkd1 also showed punctate staining associated with primary cilia and in the apical region of RGCs (Fig. 2d, arrows and arrowheads, respectively). Pkd2 was also detected in primary cilia of P0 RGCs in WT and Pkd2flox/flox mice, but not in Nestin-Cre;Pkd2flox/flox mice (Fig. 2e–g). Immunoreactivity was not detected in RGCs stained with isotype control antibody (Fig. 2h), further suggesting the specificity of the antibody against Pkd1 (Fig. 2d) or Pkd2 (Fig. 2e–g). In the primary cilia of P0 Pkd1flox/flox RGCs, Pkd1-HA and Pkd2 colocalized (Fig. 3a–d). Interestingly, Pkd2 immunolabeling was significantly reduced in primary cilia of RGCs in Nestin-Cre;Pkd1flox/flox mice (Fig. 3e–h), suggesting that Pkd2 requires Pkd1 to be present in primary cilia of RGCs. These results are consistent with previous reports showing that Pkd1 and Pkd2 physically associate to each other (Qian et al., 1997; Tsiokas et al., 1997; Hanaoka et al., 2000) and that the ciliary localization of Pkd2 depends on the presence of Pkd1 (Nauli et al., 2003; Zhou, 2009; Kim et al., 2014). Adenylyl cyclase type III (ACIII) localizes to the primary cilia of RGCs (Higginbotham et al., 2013; Tong et al., 2014). Ciliary localization of ACIII was not affected in P0 RGCs in Nestin-Cre;Pkd1flox/flox or Nestin-Cre;Pkd2flox/flox mice (Fig. 3i–l), demonstrating the specificity of ciliary localization defects in the Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox RGCs. Altogether, our data indicate that Pkd1 and Pkd2 are present in the developing brain ventricular epithelium and are localized to the primary cilia of RGCs.
Figure 1.
Pkd1 and Pkd2 were expressed in microdissected lateral walls of the lateral ventricle. a, Schematic drawing of the lateral wall of the lateral ventricle at P0. The red dotted line indicates the border between the anterior and posterior regions used for RT-PCR analysis. A, Anterior; D, dorsal; P, posterior; V, ventral. Scale bar, 1 mm. b, RT-PCR showing that Pkd1 and Pkd2 are expressed both in the anterior and posterior regions of lateral walls of the lateral ventricle at P0. ef1α was used as a loading control.
Figure 2.
Pkd1 and Pkd2 localized to RGCs' primary cilia. Whole-mount preparations of the lateral walls of lateral ventricle from Pkd1flox/flox (a), WT (b, d, e, h), Nestin-Cre;Pkd1flox/flox (c), Pkd2flox/flox (f), and Nestin-Cre;Pkd2flox/flox (g) at P0 were stained with antibodies against HA (a–c, green, marking Pkd1-HA), Pkd1 (green in d), Pkd2 (e–g, green), acetylated tubulin (a–h, magenta, marking the primary cilia of RGCs), β-catenin (a–h, blue, marking the intercellular junctions between RGCs), or isotype control antibody (h, green). White arrows and arrowheads indicate the Pkd1-HA (a), Pkd1 (d), and Pkd2 (e, f) immunoreactivities inside and outside of cilia, respectively. Scale bars: a–c, e–g, 1.0 μm; d, h, 2.5 μm.
Figure 3.
Pkd1 and Pkd2 colocalized in RGCs' primary cilia. Whole-mount preparations of the lateral walls of lateral ventricle from Pkd1flox/flox (a–d, i), Nestin-Cre;Pkd1flox/flox (e–h, j), Pkd2flox/flox (k), and Nestin-Cre;Pkd2flox/flox (l) at P0 were stained with antibodies against HA (a, b, d–f, h, green), Pkd2 (a, c–e, g, h, red), acetylated tubulin (a, d, e, h, blue; i–l, magenta), ACIII (i–l, green), and β-catenin (i–l, blue). The insets in a and e are magnified in b–d and f–h, respectively, and shown in separate channels (Pkd1-HA in b, f, green; Pkd2 in c, g, red) and merged images (d, h). Yellow signals in d indicate colocalization of Pkd1-HA and Pkd2. Scale bar, 2.5 μm.
Normal differentiation of E cells in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice
To address the roles of Pkd1 and Pkd2 in E cells, we used the Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice. E cells are derived from Nestin-Cre-expressing RGCs (Spassky et al., 2005), allowing us to test the role of these genes in the development of E cells. Nestin-Cre;Pkd1flox/flox or Nestin-Cre;Pkd2flox/flox mice did not display major developmental defect, but by P21 their body size was smaller than that of control littermates (Pkd1flox/flox and Pkd2flox/flox with no Cre, respectively; Pkd1flox/flox, 11.1 ± 0.3 g; mean ± SEM; n = 22; Nestin-Cre;Pkd1flox/flox, 8.3 ± 0.3 g, n = 18, p = 0.0001; Pkd2flox/flox, 9.8 ± 0.5 g, n = 9; Nestin-Cre;Pkd2flox/flox, 7.0 ± 0.6 g, n = 9, p = 0.0012), possibly due to polycystic kidney disease (Shillingford et al., 2010). Nestin-Cre;Pkd1flox/flox mice also develop hydrocephalus (Wodarczyk et al., 2009), suggesting defects in the developing ventricular system. It remains unknown whether the development or function of E cells is disrupted in Nestin-Cre;Pkd1flox/flox mice. The walls of the lateral ventricle contain, in addition to E cells, the small apical surfaces of B1 cells (the ventricular-subventricular zone adult neural stem cells; Fuentealba et al., 2012). E and B1 cells form rosette-like structures known as pinwheels (Mirzadeh et al., 2008). The pinwheel organization, as revealed in whole-mount staining for glial fibrillary acidic protein (GFAP; localized in B1 cells' apical endfoot) and β-catenin (localized to intercellular junctions), was normal in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21 (Fig. 4a–d). Observation of P21 whole-mounts using scanning electron microscopy revealed that the morphology, number, and length of motile cilia in E cells were similar in Nestin-Cre;Pkd1flox/flox, Nestin-Cre;Pkd2flox/flox, and control mice (Fig. 4e–j). Whole-mount staining for γ-tubulin, a protein which localizes to BBs, showed that the number of BBs in the apical region of E cells at P21 was unaffected in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox compared with control mice (Fig. 4k–o). These results suggest that the generation and the apical docking of the BBs (Guirao et al., 2010; Al Jord et al., 2014) was unaffected in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice. The forkhead transcription factor FoxJ1 and calcium binding protein S100β, which label E cells (Ohata et al., 2014), were normally expressed in E cells in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21 (Fig. 4p–s, arrows). Beating of E cell motile cilia can be visualized using PE-labeled CD24 antibodies (Ohata et al., 2014). We quantified ciliary beating frequency (CBF; strokes/s) in E cells from Nestin-Cre;Pkd1flox/flox, Nestin-Cre;Pkd2flox/flox and control mice at P21. CBF was not affected by deletion of Pkd1 or Pkd2 in these mutant mice (Fig. 4t; Movies 1–4). These results indicate that E cells differentiate, develop motile cilia, and become organized into pinwheels in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice.
Figure 4.
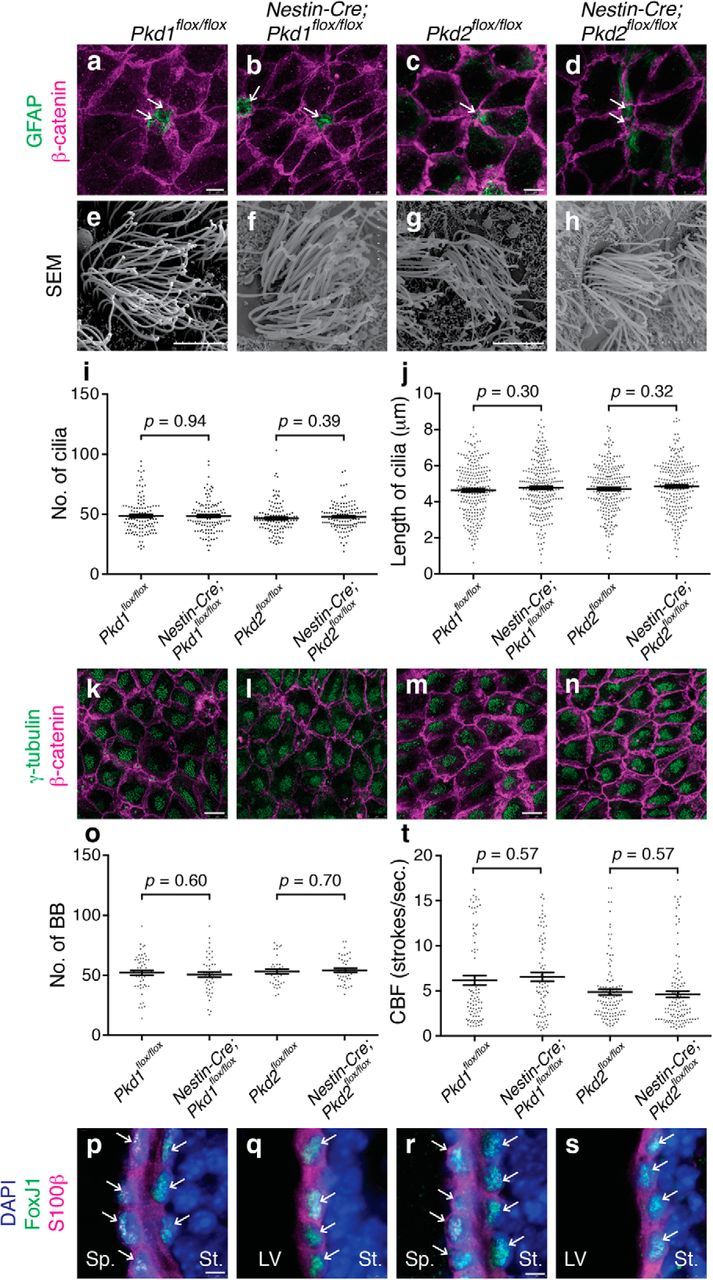
Differentiation of E cells was largely normal in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice. a–d, The pinwheel organization of E cells surrounding B1 cells' apical endings was preserved in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21. Whole-mount preparations of the lateral walls of lateral ventricle were stained with antibodies against GFAP (green; apical endings of B1 cells) and β-catenin (magenta, cell borders) in Pkd1flox/flox (a), Nestin-Cre;Pkd1flox/flox (b), Pkd2flox/flox (c), and Nestin-Cre;Pkd2flox/flox (d) mice at P21. White arrows indicate B1 cells at the center of pinwheel structures. Scale bar, 7.5 μm. e–j, Multiciliation of E cells was normal in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21. e–h, Whole-mount preparations of the lateral walls of the lateral ventricle were observed by scanning electron microscopy in Pkd1flox/flox (e), Nestin-Cre;Pkd1flox/flox (f), Pkd2flox/flox (g), and Nestin-Cre;Pkd2flox/flox (h) mice. Scale bar, 5 μm. i, j, The number (i) and length (j) of E cells' motile cilia were quantified and plotted. Data shown are mean ± SEM. Each point on the graph is the number of an individual E cell (i) and the length of an individual cilium (j). i, 118 cells from four Pkd1flox/flox mice, 121 cells from four Nestin-Cre;Pkd1flox/flox mice, p = 0.94; 121 cells from four Pkd2flox/flox mice, 122 cells from four Nestin-Cre;Pkd2flox/flox mice, p = 0.39. j, 243 cilia from four Pkd1flox/flox mice, 248 cilia from four Nestin-Cre;Pkd1flox/flox mice, p = 0.30; 244 cilia from four Pkd2flox/flox mice, 247 cilia from four Nestin-Cre;Pkd2flox/flox mice; p = 0.32. k–n, Whole-mount preparations of the lateral walls of the lateral ventricle at P21 were stained with antibodies against γ-tubulin (green) and β-catenin (magenta) in Pkd1flox/flox (k), Nestin-Cre;Pkd1flox/flox (l), Pkd2flox/flox (m), and Nestin-Cre;Pkd2flox/flox (n) mice. Scale bars, 10 μm. o, The number of BBs was similar in the Pkd1flox/flox (n = 56 cells from three mice), Nestin-Cre;Pkd1flox/flox (n = 48 cells from three mice; p = 0.60), Pkd2flox/flox (n = 35 cells from three mice), and Nestin-Cre;Pkd2flox/flox (n = 42 cells from three mice, p = 0.70) E cells. Data shown are mean ± SEM. Each point on the graph is the number of BBs in an individual E cell. p–s, Coronal cryosections were stained with anti-FoxJ1 (green) and anti-S100β (magenta) antibodies and DAPI (blue) in Pkd1flox/flox (p), Nestin-Cre;Pkd1flox/flox (q), Pkd2flox/flox (r), and Nestin-Cre;Pkd2flox/flox (s) mice. Scale bar, 5 μm. White arrows indicate FoxJ1 and S100β double-positive cells. LV, Lateral ventricle; Sp, septum; St, striatum. Note that two layers of ependyma from the lateral (striatum side) and medial (septum side) walls of the lateral ventricles are shown in controls (p, r), whereas only one layer from the lateral wall is shown in Nestin-Cre;Pkd1flox/flox (q) and Nestin-Cre;Pkd2flox/flox (s) due to the expansion of the LV in these mutants. t, CBF was unaffected in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21. Data shown are mean ± SEM. Each point on the graph is CBF of an individual E cell. 86 cells from three Pkd1flox/flox mice, 83 cells from three Nestin-Cre;Pkd1flox/flox mice, p = 0.57; 112 cells from four Pkd2flox/flox mice, 120 cells from four Nestin-Cre;Pkd2flox/flox mice, p = 0.57 (Movies 1–4).
Live imaging movie showing ciliary beating of E cells in Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Pkd2flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd2flox/flox mouse.
Requirement of primary cilia, Pkd1, and Pkd2 in the asymmetric localization of Vangl2 in the apical region of E cells
The core PCP protein Vangl2 is a four-pass transmembrane protein that is asymmetrically localized to the apical region of epithelial cells (Montcouquiol et al., 2006; Tissir et al., 2010; Peng and Axelrod, 2012). This protein is required for establishing PCP in various cell types, including E cells (Guirao et al., 2010; Boutin et al., 2014). Primary cilia in RGCs are required for proper establishment of PCP in E cells (Mirzadeh et al., 2010b). To determine whether primary cilia are required for the asymmetric distribution of Vangl2, we used 3 mouse lines: Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Ift88flox/flox and hGFAP-Cre;Kif3aflox/flox. Kif3a and Ift88 are essential for ciliogenesis (Marszalek et al., 2000; Haycraft et al., 2007). In Nestin-Cre;Kif3aflox/flox mice, primary cilia in RGCs are eliminated by E14.5 (Tong et al., 2014) and consequently, E cells never develop motile cilia (Mirzadeh et al., 2010b). In contrast, in hGFAP-Cre;Kif3aflox/flox mice Cre-expression is delayed, preserving RGCs primary cilia until around P2, but eliminating E cells' motile cilia (Mirzadeh et al., 2010b). Interestingly, severe PCP defects are observed in the Nestin-Cre;Kif3aflox/flox E cells but not in the hGFAP-Cre;Kif3aflox/flox E cells (Mirzadeh et al., 2010b), suggesting that the primary cilia in RGCs play a key role in the planar polarization of E cells. The asymmetric localization of Vangl2 on the apical surface of E cells at P21 was conserved in hGFAP-Cre;Kif3aflox/flox mice (Fig. 5a,b; Kif3aflox/flox, E cells with asymmetric localization of Vangl2/total E cells examined = 76/83, 89%, 4 mice; hGFAP-Cre;Kif3aflox/flox, 76/92, 84%, 4 mice, p = 0.21) as previously demonstrated (Guirao et al., 2010), but it was randomized in Nestin-Cre;Kif3aflox/flox and Nestin-Cre;Ift88flox/flox mice (Fig. 5c–f; Kif3aflox/flox, 69/119, 58%, 3 mice; Nestin-Cre;Kif3aflox/flox, 10/83, 12%, 3 mice, p < 0.0001; Ift88flox/flox, 162/243, 67%, 4 mice; Nestin-Cre;Ift88flox/flox, 10/103, 10%, 4 mice, p < 0.0001). This defect in the asymmetric localization of Vangl2 is not due to the long-term expression of Cre in RGCs and E cells, because Vangl2 localization was similar in E cells from Kif3aflox/+ mice and Nestin-Cre;Kif3aflox/+ mice (Fig. 5g,h; Kif3aflox/+, 114/146, 78%, 4 mice; Nestin-Cre;Kif3aflox/+, 147/181, 81%, 4 mice; p = 0.49). These results indicate that primary cilia in RGCs are required for the asymmetric distribution of Vangl2 in E cells.
Figure 5.
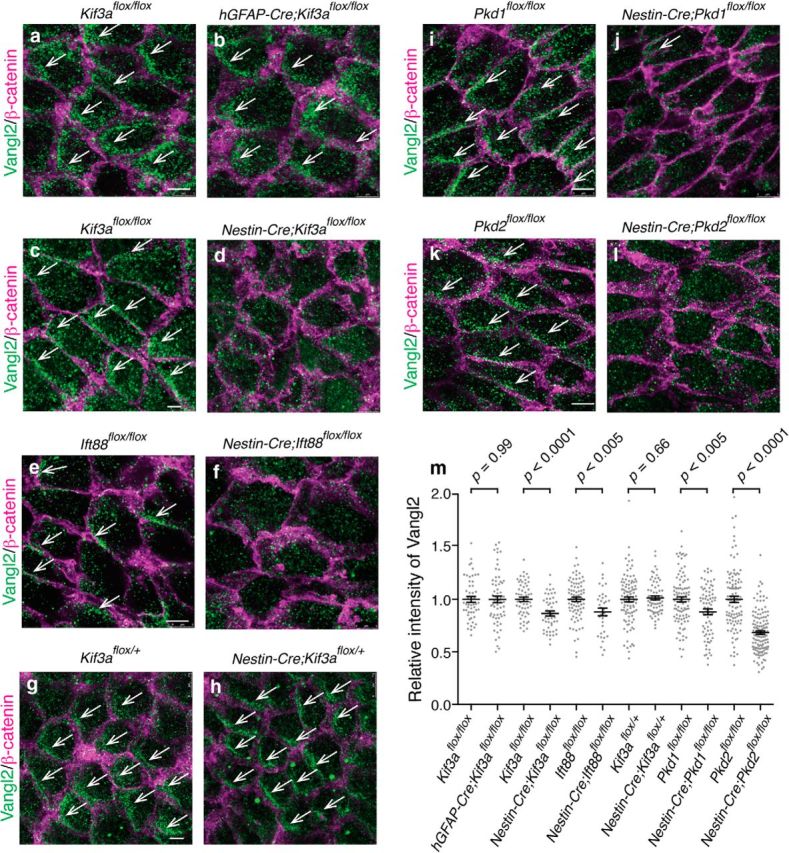
Primary cilia of RGCs, Pkd1, and Pkd2 were required for the asymmetric localization of Vangl2. a–l, Whole-mount preparations of the lateral walls of the lateral ventricle were stained with antibodies against Vangl2 (green) and β-catenin (magenta) in Kif3aflox/flox (a, sibling control for hGFAP-Cre;Kif3aflox/flox in b), hGFAP-Cre;Kif3aflox/flox (b), Kif3aflox/flox (c, sibling control for Nestin-Cre;Kif3aflox/flox in d), Nestin-Cre;Kif3aflox/flox (d), Ift88flox/flox (e), Nestin-Cre;Ift88flox/flox (f), Kif3aflox/+ (g), Nestin-Cre;Kif3aflox/+ (h), Pkd1flox/flox (i), Nestin-Cre;Pkd1flox/flox (j), Pkd2flox/flox (k), and Nestin-Cre;Pkd2flox/flox (l) mice at P21. White arrows indicate asymmetric localization of Vangl2. Scale bar, 5 μm. m, Ablation of primary cilia in RGCs, Pkd1, or Pkd2 resulted in a reduction in the apical levels of Vangl2 staining in E cells at P21. Data shown are mean ± SEM. Each point on the graph is relative intensity of Vangl2 immunosignal from an individual E cell. Kif3aflox/flox (sibling control for hGFAP-Cre;Kif3aflox/flox), 51 cells, four mice; hGFAP-Cre;Kif3aflox/flox, 63 cells, four mice, p = 0.99. Kif3aflox/flox (sibling control for Nestin-Cre;Kif3aflox/flox), 57 cells, three mice; Nestin-Cre;Kif3aflox/flox, 48 cells, three mice, p < 0.0001. Ift88flox/flox, 84 cells, four mice; Nestin-Cre;Ift88flox/flox, 35 cells, four mice, p = 0.0035. Kif3aflox/+, 98 cells, four mice; Nestin-Cre;Kif3aflox/+, 71 cells, four mice; p = 0.66. Pkd1flox/flox, 98 cells, five mice; Nestin-Cre;Pkd1flox/flox, 75 cells, six mice; p = 0.0013. Pkd2flox/flox, 106 cells, six mice; Nestin-Cre;Pkd2flox/flox, 136 cells, six mice; p < 0.0001.
As shown in Figures 2 and 3, Pkd1 and Pkd2 were expressed in the primary cilia of RGCs. So we asked whether Pkd1 and Pkd2 were required for Vangl2 asymmetrical localization in the apical area of E cells at P21 (Fig. 5i–l). In Pkd1flox/flox and Pkd2flox/flox control mice, Vangl2 staining was observed in a crescent-shape close to the intercellular junctions on one side of E cells apical membrane (Fig. 5i,k; Pkd1flox/flox, 68/95 cells, 5 mice, 72%; Pkd2flox/flox, 74/128, 4 mice, 58%). In contrast, in the Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox E cells, this asymmetric accumulation of Vangl2 was not observed (Fig. 5j,l; Nestin-Cre;Pkd1flox/flox,18/93, 6 mice, 19%, p < 0.0001; Nestin-Cre;Pkd2flox/flox,25/121, 3 mice, 21%, p < 0.0001). These results suggest that Pkd1 and Pkd2 are required for Vangl2 asymmetric localization in E cells. Interestingly, the relative intensity of Vangl2-immunosignal in the apical area of E cells was also significantly reduced in Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Ift88flox/flox, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice but not in Nestin-Cre;Kif3aflox/+ or hGFAP-Cre;Kif3aflox/flox mice (Fig. 5m). These results suggest that primary cilia of RGCs, Pkd1, or Pkd2 also affect the apical levels of Vangl2 in E cells directly or indirectly.
Aberrant rotational polarity in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice
To examine the role of Pkd1 and Pkd2 in rotational polarity of individual E cells' cilia, we used sequential tangential ultrathin sections and transmission electron microscopy (TEM) to determine the location of the basal foot next to E cells' BBs (Guirao et al., 2010; Hirota et al., 2010; Mirzadeh et al., 2010b). In control animals, the majority of basal feet in E cells were oriented in the same direction, while this rotational polarity was significantly disrupted in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox (Fig. 6a–p): the total number of cilia oriented in the same direction and the circular SD (CSD) of basal foot orientation (Fig. 6m–o, error bars) were significantly disrupted. These results suggest that both Pkd1 and Pkd2 are required for the proper alignment of BBs within E cells.
Figure 6.
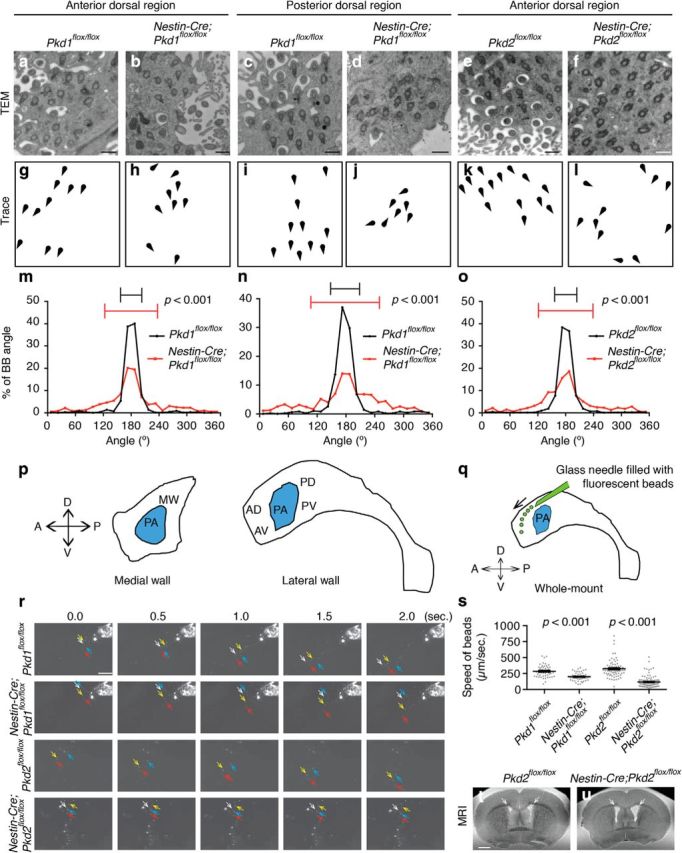
Rotational polarity was disrupted in the Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox E cells. a–f, TEM analysis of rotational polarity in E cells in the anterior-dorsal region in Pkd1flox/flox (a), Nestin-Cre;Pkd1flox/flox (b), Pkd2flox/flox (e), and Nestin-Cre;Pkd2flox/flox (f) mice, and in the posterior dorsal region in Pkd1flox/flox (c) and Nestin-Cre;Pkd1flox/flox (d) mice at P21. See Figure 6p for the annotation of anterior dorsal and posterior dorsal regions. Scale bar, 500 nm. g–l, Schematic drawings of BBs shown in a-f illustrate the misalignment of BB rotational orientation in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice. m–o, Histogram showing the distribution of the BB angle in the Pkd1flox/flox (m, black; CSD = 23°; n = 674 BBs from 74 cells, three mice), Nestin-Cre;Pkd1flox/flox (m, red; CSD = 57°; n = 1116 BBs from 101 cells, three mice; p < 0.001), Pkd2flox/flox (o, black; CSD = 24°; n = 1575 BBs from 141 cells, five mice), and Nestin-Cre;Pkd2flox/flox (o, red; CSD = 59°; n = 607 BBs from 76 cells, four mice; p < 0.001) E cells in the anterior dorsal region, and in the Pkd1flox/flox (n, black; CSD = 30°; n = 497 BBs from 68 E cells, three mice) and Nestin-Cre;Pkd1flox/flox (n, red; CSD = 55°; n = 543 BBs, 55 E cells, three mice; p < 0.001) E cells in the posterior dorsal region. Error bars on the graphs show CSD. p, Schematic drawing of the regions of the lateral and medial walls of the lateral ventricle studied in the present study. A, Anterior; D, dorsal; P, posterior; V, ventral. AD, anterior dorsal; AV, anterior ventral; MW, medial wall; PA, point of adhesion; PD, posterior dorsal; PV, posterior ventral. q–s, The speed of ependymal flow was significantly slower in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice at P21. q, Schematic of the ependymal flow assay. Fluorescent beads (green) were filled in a glass needle and placed onto whole-mount preparations of the lateral walls of lateral ventricle. A, Anterior; D, dorsal; P, posterior; PA, point of adhesion; V, ventral. r, Migration patterns of the fluorescent beads placed on the whole-mount preparations of the lateral walls of lateral ventricle in Pkd1flox/flox (top row), Nestin-Cre;Pkd1flox/flox mutant (second top row), Pkd2flox/flox (second bottom row), and Nestin-Cre;Pkd2flox/flox mutant (bottom row) mice. The arrows indicate the position of arbitrarily chosen fluorescent beads. Scale bar, 50 μm. s, The speeds of fluorescent beads on the whole-mount preparation are expressed as mean ± SEM. Each point on the graph represents the speed of individual fluorescent beads. Pkd1flox/flox; n = 102 beads, four mice. Nestin-Cre;Pkd1flox/flox; n = 120 beads, five mice. p < 0.0001. Pkd2flox/flox; n = 70 beads, five mice. Nestin-Cre;Pkd2flox/flox; n = 115 beads, four mice. p < 0.0001 (Movies 5–8). t, u, T2-weighted coronal MRI scans showing enlargement of the lateral ventricles in Nestin-Cre;Pkd2flox/flox mice (u) compared with Pkd2flox/flox mice (t) at P31. White arrows point the lateral ventricles. Scale bar, 1 mm.
Defects in rotational polarity frequently result in reduced ependymal flow speed and expansion of the ventricles (Guirao et al., 2010; Hirota et al., 2010; Ohata et al., 2014; Ying et al., 2014). The continuous beating of cilia on the apical surface of E cells generates this unidirectional fluid flow (Miyan et al., 2003; Del Bigio, 2010; Lee, 2013), which can be visualized and quantified by placing polystyrene latex fluorescent microbeads on live preparations of the lateral wall of the lateral ventricle (Fig. 6q; Sawamoto et al., 2006; Mirzadeh et al., 2010a,b; Ohata et al., 2014). We next investigated the ependymal flow in whole-mount preparations from control, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice. When a small number of microbeads were placed in the anterior dorsal region of the lateral ventricles in control mice, a strong posterior-dorsal to anterior-ventral current was observed (Fig. 6r; Movies 5, 7). In Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice, the overall directionality of this flow was similar to that in controls (Fig. 6r; Movies 6, 8), but the speed of the fluorescent beads was significantly slower compared with controls (Fig. 6s). Nestin-Cre;Pkd1flox/flox mice have been shown to develop hydrocephalus (Wodarczyk et al., 2009). Consistently, the volume of the lateral ventricles was significantly larger in Nestin-Cre;Pkd2flox/flox mice compared with Pkd2flox/flox mice at P31 (Figure 6t,u; Pkd2flox/flox, 0.63 ± 0.067 mm3; mean ± SEM; n = 11; Nestin-Cre;Pkd2flox/flox, 1.6 ± 0.031 mm3, n = 9, p = 0.00060). These results indicate that in mice lacking Pkd1 or Pkd2, the rotational polarity of basal body, ependymal flow, and the size of the lateral ventricles are abnormal.
Live imaging movie showing the migration of fluorescent beads on the wholemount preparation of lateral wall of lateral ventricle in Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd1flox/flox mouse.
Live imaging movie showing the migration of fluorescent beads on the wholemount preparation of lateral wall of lateral ventricle in Pkd2flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd2flox/flox mouse.
Abnormal translational polarity in Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice
Translational polarity is established in RGCs ∼E16 (Mirzadeh et al., 2010b; Boutin et al., 2014), and a subpopulation of these RGCs differentiate into E cells by P5 (Spassky et al., 2005). To assess whether primary cilia, Pkd1, and Pkd2 are involved in the establishment of translational polarity in RGCs at P0 (a time-point before most RGCs differentiate into E cells), we drew vectors from the center of the apical surface to the BB of RGCs in control, Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice (Fig. 7a–g). We measured the angles of these vectors (BB angle) and the displacement of the BBs from the center of the apical surface (BB displacement; Fig. 7a). BB angle in RGCs at P0 distributed significantly more widely in Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice compared with that in control mice (Fig. 7b–j). The BB displacement was significantly smaller in Nestin-Cre;Kif3aflox/flox, Nestin-Cre;Pkd1flox/flox, and Nestin-Cre;Pkd2flox/flox mice compared with that in control mice (Fig. 7k). These data indicate that primary cilia, Pkd1, and Pkd2 contribute to the establishment of translational polarity in RGCs before they differentiate into E cells.
We next investigated whether translational polarity in differentiated E cells at P21 was affected following ablation of Pkd1 or Pkd2 using Nestin-Cre. The BB patch angle in E cells had a significantly wider distribution in both Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox compared with controls (Figs. 4k–n, 8a–f). However, E cells' BB patch displacement was not significantly different between control and Nestin-Cre;Pkd1flox/flox, or Nestin-Cre;Pkd2flox/flox mice (Fig. 8g). These results suggest that Pkd1 and Pkd2 help coordinate BB patch angle in E cells.
Figure 8.
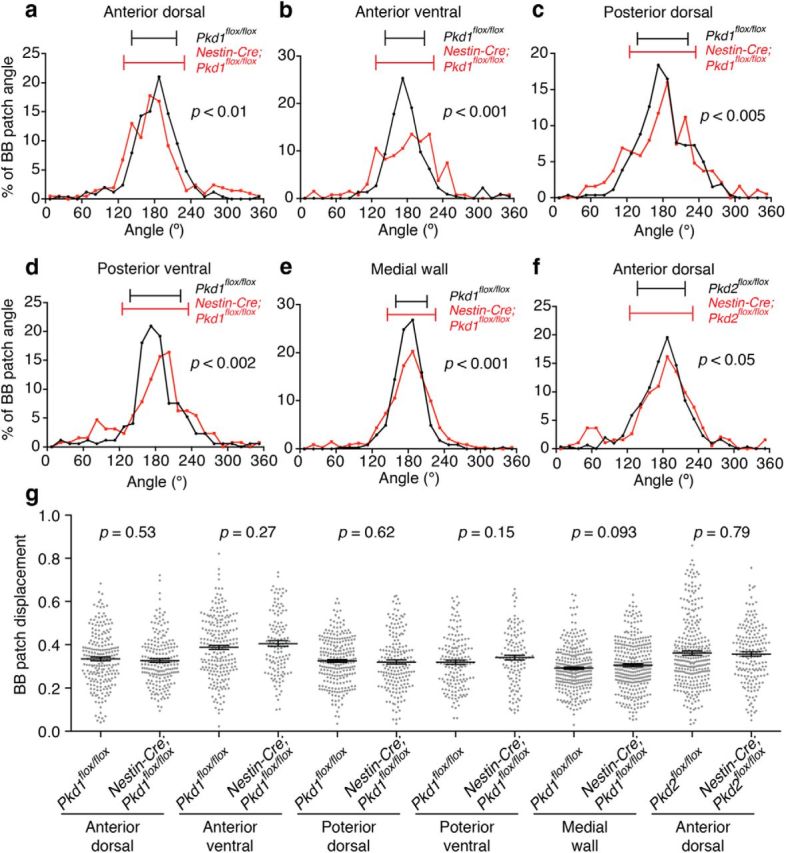
Pkd1 and Pkd2 contributed to translational polarity in E cells. a–f, Histograms showing the distribution of the BB patch angles in the Pkd1flox/flox (a–e, black), Nestin-Cre;Pkd1flox/flox (a–e, red), Pkd2flox/flox (f, black), and Nestin-Cre;Pkd2flox/flox (f, red) E cells in the anterior dorsal (a, f), anterior ventral (b), posterior dorsal (c), and posterior ventral (d) regions of the lateral wall and medial wall (e) of the lateral ventricle. Error bars on the graph show CSD. a, Pkd1flox/flox; CSD = 37°, n = 252 E cells, three mice. Nestin-Cre;Pkd1flox/flox; CSD = 50°, n = 208 E cells, four mice; p < 0.01. b, Pkd1flox/flox; CSD = 34°, n = 225 E cells, three mice. Nestin-Cre;Pkd1flox/flox; CSD = 49°, n = 133 E cells, three mice; p < 0.001. c, Pkd1flox/flox, CSD = 42°, n = 261 E cells, three mice; Nestin-Cre;Pkd1flox/flox, CSD = 55°, n = 188 E cells, three mice; p < 0.005. d, Pkd1flox/flox, CSD = 42°, n = 172 E cells, three mice; Nestin-Cre;Pkd1flox/flox, CSD = 55°, n = 128 E cells, three mice; p < 0.002. e, Pkd1flox/flox, CSD = 26°, n = 347 E cells, three mice; Nestin-Cre;Pkd1flox/flox, CSD = 40°, n = 341 E cells, three mice; p < 0.001. f, Pkd2flox/flox; CSD = 40°; n = 307 E cells, three mice: Nestin-Cre;Pkd2flox/flox; CSD = 53°; n = 192 E cells, three mice; p < 0.05. See Figure 6p for the annotation of regions examined in the present study. g, Quantification of BB patch displacement. Data shown are mean ± SEM. Each point on the graphs is BB patch displacement of an individual E cell. Anterior dorsal region: Pkd1flox/flox; n = 222 E cells, three mice: Nestin-Cre;Pkd1flox/flox; n = 197 E cells, four mice: p = 0.53. Anterior ventral region: Pkd1flox/flox; n = 219 E cells, three mice: Nestin-Cre;Pkd1flox/flox; n = 126 E cells, three mice: p = 0.27. Posterior dorsal region: Pkd1flox/flox; n = 250 E cells, three mice: Nestin-Cre;Pkd1flox/flox; n = 186 E cells, three mice: p = 0.62. Posterior ventral region: Pkd1flox/flox; n = 162 E cells, three mice: Nestin-Cre;Pkd1flox/flox; n = 124 E cells, three mice: p = 0.15. Medial wall: Pkd1flox/flox, n = 309 E cells, three mice: Nestin-Cre;Pkd1flox/flox, n = 320 E cells, three mice: p = 0.093. Anterior dorsal region: Pkd2flox/flox; n = 307 E cells, three mice: Nestin-Cre;Pkd2flox/flox; n = 184 E cells, three mice: p = 0.79. See Figure 6p for the annotation of regions examined in the present study.
Synergistic enhancement of PCP defects in theVangl2 heterozygous mutant E cells by Pkd1 heterozygous mutation
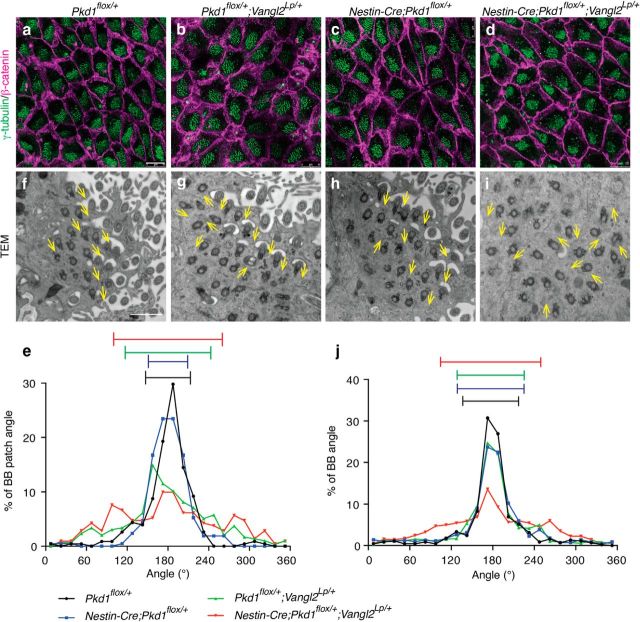
In the developing kidney epithelia, ablation of Pkd1 affects multiple cellular processes related to PCP, including oriented cell division and convergent-extension-like movements (Luyten et al., 2010; Castelli et al., 2013). However, whether and how Pkd1 regulates the PCP pathway remains unknown. The PCP gene Vangl2 is required for both rotational and translational polarity in E cells (Guirao et al., 2010; Boutin et al., 2014). The Loop-tail mutation (Vangl2Lp, S464N) affects the stability and membrane localization of Vangl2 (Montcouquiol et al., 2006; Guirao et al., 2010; Merte et al., 2010; Iliescu et al., 2011). To address whether Pkd1 and Vangl2 function cooperatively or independently in the establishment of translational and rotational polarity, we analyzed single and double-heterozygous mutant mice for these genes. Heterozygous Vangl2Lp/+ mutation was sufficient to significantly affect the BB patch angle in E cells at P21 (Fig. 9a,b,e; Table 1). In contrast, the BB patch angle was unaffected in Nestin-Cre;Pkd1flox/+ mice compared with Pkd1flox/+ mice (Fig. 9a,c,e; Table 1). If Pkd1 and Vangl2 were to function independently, double-heterozygous mutations of Pkd1 and Vangl2 in Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ mice should result in an additive effect on the randomization of BB patch angle (i.e., similar effects to those observed in Vangl2Lp/+ mutants). However, we observed a synergistic effect on the disorientation of BB patch angle by double-heterozygous mutations for Pkd1 and Vangl2 in Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ mice compared with Pkd1 single heterozygous mice (Nestin-Cre;Pkd1flox/+) or Vangl2 single heterozygous mice (Pkd1flox/+;Vangl2Lp/+) individually (Fig. 9b–e; Table 1). These results suggest that Pkd1 and Vangl2 function in the same pathway to coordinate BB patch angle.
Figure 9.
Pkd1 and Vangl2 functioned in the same pathway to coordinate BB patch angle and rotational alignment of BBs in E cells. a–d, Whole-mount preparations of the lateral walls of the lateral ventricle at P21 were stained with antibodies against γ-tubulin (green) and β-catenin (magenta) in Pkd1flox/+ (a), Pkd1flox/+;Vangl2Lp/+ (b), Nestin-Cre;Pkd1flox/+ (c), and Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ (d) mice. E cells in the anterior dorsal region were analyzed. Scale bars, 10 μm. e, Histograms showing the distribution of the BB patch angles in Pkd1flox/+ (black; CSD = 33°; n = 228 E cells, three mice), Nestin-Cre;Pkd1flox/+ (blue; CSD = 29°; n = 209 E cells, three mice), Pkd1flox/+;Vangl2Lp/+ (green; CSD = 63°; n = 295 E cells, three mice), and Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ (red; CSD = 77°; n = 211 E cells, three mice) mice. Error bars on the graph show CSD. f–i, TEM analysis of rotational polarity in Pkd1flox/+ (f), Pkd1flox/+;Vangl2Lp/+ (g), Nestin-Cre;Pkd1flox/+ (h), and Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ (i) mice. E cells in the anterior dorsal region were analyzed. Scale bar, 1 μm. Yellow arrows indicate the directions of individual BBs. j, Histogram showing the distribution of the rotational angle of BBs in the Pkd1flox/+ (black; CSD = 40°; n = 446 BBs from 55 cells, three mice), Nestin-Cre;Pkd1flox/+ (blue; CSD = 48°; n = 704 BBs from 68 E cells, three mice), Pkd1flox/+;Vangl2Lp/+ (green; CSD = 49°; n = 835 BBs from 81 E cells, three mice), and Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ (red; CSD = 72°; n = 1104 BBs from 102 E cells, three mice) E cells. Error bars on the graph show CSD.
Table 1.
p values for the comparison of BB patch angles by Watson's U2 test
| Pkd1flox/+ | Nestin-Cre; Pkd1flox/+ | Pkd1flox/+; Vangl2Lp/+ | Nestin-Cre; Pkd1flox/+; Vangl2Lp/+ | |
|---|---|---|---|---|
| Pkd1flox/+ | — | 0.05 < p < 0.1 | p < 0.001 | p < 0.001 |
| Nestin-Cre;Pkd1flox/+ | 0.05 < p < 0.1 | — | p < 0.001 | p < 0.001 |
| Pkd1flox/+;Vangl2Lp/+ | p < 0.001 | p < 0.001 | — | p < 0.002 |
| Nestin-Cre;Pkd1flox/+; Vangl2Lp/+ | p < 0.001 | p < 0.001 | p < 0.002 | — |
p values comparing the distribution of BB patch angle shown in Fig. 9e.
Next, we examined rotational polarity in these mutant mice. Both Pkd1 and Vangl2 heterozygous mutations affected rotational polarity (Fig. 9f–h,j; Table 2). The difference in CSD between Nestin-Cre;Pkd1flox/+ mice and Pkd1flox/+ mice was 8° (48° and 40°, respectively) and that between Pkd1flox/+;Vangl2Lp/+ mice and Pkd1flox/+ mice was 9° (49° and 40°, respectively). Interestingly, double-heterozygous mutant mice for these genes (Nestin-Cre;Pkd1flox/+;Vangl2Lp/+) displayed more severe defect in rotational polarity than that of Nestin-Cre;Pkd1flox/+ or Pkd1flox/+;Vangl2Lp/+ mice (Fig. 9g–j; Table 2). The difference in CSD between Nestin-Cre;Pkd1flox/+;Vangl2Lp/+ mice and Pkd1flox/+ mice was 32° (72° and 40°, respectively), almost twice the sum of the differences in CSD for Nestin-Cre;Pkd1flox/+ and Pkd1flox/+;Vangl2Lp/+ mice (8° and 9°, respectively). As with translational polarity, these results for rotational polarity suggest that Pkd1 and Vangl2 do not function independently, but are part of the same pathway to establish PCP in E cells.
Table 2.
p values for the comparison of BB angles by Watson's U2 test
| Pkd1flox/+ | Nestin-Cre; Pkd1flox/+ | Pkd1flox/+; Vangl2Lp/+ | Nestin-Cre; Pkd1flox/+; Vangl2Lp/+ | |
|---|---|---|---|---|
| Pkd1flox/+ | — | p < 0.001 | p < 0.001 | p < 0.001 |
| Nestin-Cre;Pkd1flox/+ | p < 0.001 | — | 0.2 < p < 0.5 | p < 0.001 |
| Pkd1flox/+;Vangl2Lp/+ | p < 0.001 | 0.2 < p < 0.5 | — | p < 0.001 |
| Nestin-Cre;Pkd1flox/+; Vangl2Lp/+ | p < 0.001 | p < 0.001 | p < 0.001 | — |
p values comparing the distribution of BB angle shown in Fig. 9j.
Discussion
The present study demonstrates that the planar organization of E cells, essential for the proper CSF circulation, requires RGCs' primary cilia and mechanosensory genes, Pkd1 and Pkd2. Together with our previous report (Mirzadeh et al., 2010b), we propose that Pkd1 and Pkd2 in the primary cilia of RGCs could help sense small perturbations in early hydrodynamic forces on the ventricular epithelium (e.g., passive CSF flow) to establish an initial translational polarity in RGCs. Given that E cells' PCP in Pkd1 mutant mice was affected in all ventricular wall subdomains, this planar polarization mechanism may be widely used along the ventricular epithelium. How this early polarization of RGCs is then translated into polarized E cells remains unknown. During differentiation of RGCs to E cells, newly generated BBs for motile cilia migrate apically into the vicinity of the primary cilia's BBs (Al Jord et al., 2014). The asymmetric localizations of the primary cilia and its BBs in RGCs may be key for the subsequent asymmetric positioning of motile cilia and BBs in E cells.
Ciliary localization of Pkd1 and Pkd2 in RGCs
Primary cilium compartmentalizes membrane-bound and cytoplasmic signaling components and functions as a signaling center for chemical and mechanical signals (Nauli et al., 2003; Su et al., 2013; Guemez-Gamboa et al., 2014; Lee et al., 2015). Here we show that mechanoreceptor proteins Pkd1 and Pkd2 are present in RGCs' primary cilia, which extend into the developing ventricular system and contact the CSF directly (Mirzadeh et al., 2010b; Tong et al., 2014). The elongated shape of the primary cilium and the compartmentalization of Pkd1 and Pkd2 within its small volume could enable the primary cilium to function as a highly sensitive mechanosensory organelle.
Pkd1 and Pkd2 were also present as small puncta in the apical compartment of RGCs. A possible role for these mechanosensors in the apical region remains unknown. In vascular smooth muscle, these proteins localize on the apical surface and are involved in sensing pressure generated by the stretching of the plasma membrane (Sharif-Naeini et al., 2009). Apical Pkd1 and Pkd2, outside the cilia, could have a similar function in RGCs. It is also possible that the punctate staining we detect in the apical domain of RGCs corresponds to Pkd1 or Pkd2 being transported or recycled.
Regulation of PCP pathway by Pkd1, Pkd2, and primary cilia
In the present study, we show that primary cilia, Pkd1, and Pkd2 are required for the asymmetric localization of Vangl2, a core PCP protein essential for planar polarization of E cells (Guirao et al., 2010; Boutin et al., 2014). The double-heterozygous mutant experiments suggest that Pkd1 and Vangl2 function in the same pathway for both rotational and translational polarity in E cells. Pkd1 and Pkd2 in RGCs' primary cilia may control E cell PCP through the regulation of the asymmetric localization of Vangl2. Our results suggest a molecular link between the hydrodynamic forces created by CSF movements on the ventricular surface of RGCs and the planar polarization within this wall. An alternative, but not mutually exclusive, possibility is that Pkd1 and Pkd2 participate in the sensing of gradients of unknown soluble factors in the CSF. For example, Pkd2 family protein Pkd2-like1 (Pkd2l1) can enhance Sonic hedgehog signaling (Delling et al., 2013). Gradients of secreted factors could help modulate PCP in RGCs.
It remains unclear how primary cilia, Pkd1, and Pkd2 are involved in the establishment of the asymmetric positioning of PCP proteins. In the present study, we show that primary cilia, Pkd1, and Pkd2 contribute to the translational polarity in RGCs. Interestingly, translational polarity in RGCs is observed as early as E16.5, preceding the asymmetric accumulation of PCP proteins that occurs after P0 (Mirzadeh et al., 2010b; Boutin et al., 2014). Positionally biased signaling mediated by primary cilia in RGCs may be important for directional sorting of PCP proteins along microtubules. In the developing fly wing, GFP-tagged Frizzled (Fzd), another core PCP protein, is preferentially transported toward the distal side along microtubule arrays to establish its asymmetric accumulation (Shimada et al., 2006). The BBs of RGCs' primary cilia function as microtubule organizing center (Boutin et al., 2014). Pharmacological disruption of microtubule organization significantly affects the asymmetric localization of core PCP proteins in RGCs and cultured tracheal epithelial cells (Vladar et al., 2012; Boutin et al., 2014). Pkd1 is involved in the stabilization of microtubules in cultured cells (Castelli et al., 2015). Mechanosensory signals may contribute to the initial rearrangement of the apical microtubule networks in RGCs. Proper asymmetric accumulation of PCP proteins in the apical area of RGCs would be important to refine PCP and ultimately instructive for E cells. We also found that ablation of cilia, Pkd1, or Pkd2 resulted in a reduction in apical Vangl2 levels in E cells. Primary cilia of RGCs, Pkd1, or Pkd2, may directly or indirectly affect synthesis, stability, and/or apical migration of Vangl2.
The requirement of cilia, Pkd1, and Pkd2 in the asymmetric localization of core PCP proteins may be tissue-dependent. The asymmetric accumulation of Vangl2-GFP fusion protein or Fzd3 was not affected in the cochlear hair cells in Foxg1-Cre;Ift88flox/flox mice (Jones et al., 2008). In the Foxg1-Cre;Kif3aflox/flox cochlea, the asymmetric localizations of Fzd3 and Dishvelled2 are slightly disorganized but largely normal (Sipe and Lu, 2011). Ablation of Pkd1 in the inner ear disrupts the ultrastructure of actin-based stereocilia but does not affect its PCP (Steigelman et al., 2011).
Primary cilia apparently have different functions at different times in development (Guemez-Gamboa et al., 2014). The early removal of primary cilia, Pkd1, or Pkd2 from RGCs affects the establishment of PCP in RGCs and E cells, whereas late removal of motile cilia from developing E cells partially disrupt E cells' PCP (Mirzadeh et al., 2010b). The early ablation of Arl13b (a small GTPase essential for ciliary function), before neuroepithelial cells convert into RGCs, dramatically reverses apical-basal polarity, whereas its late ablation does not (Higginbotham et al., 2013). Primary cilia may become essential for PCP as the size of the apical area expands in late RGCs.
Partial polarization in Pkd1 or Pkd2 mutant mice
Although PCP in RGCs and E cells were affected in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice, partial translational and rotational polarity were preserved. Members of the Pkd1 and Pkd2 gene families (Pkd1-like 1,2,3 and Pkd2-like1,2; Pkd1l1,2,3 and Pkd2l1,2, respectively; Zhou, 2009) could contribute to this remaining PCP. It has been suggested, in the mouse node and in fish Kupffer's vesicle, that Pkd1l1 interacts with Pkd2 and functions as a nodal flow sensor to establish left-right asymmetry (Field et al., 2011; Kamura et al., 2011). In future studies, it will be interesting to determine the precise localization of the different Pkd1 and Pkd2 protein family members in RGCs and E cells. Compound mutant animals could also help determine the contribution of each Pkd1 and Pkd2 family members in the development of PCP in the brain's ventricular epithelium.
We observed a defect in BB displacement in RGCs in Nestin-Cre;Pkd1flox/flox or Nestin-Cre;Pkd2flox/flox mice at P0, but in these mutant mice, BB patch displacement in E cells was unaffected by P21. As translational polarity is refined during the differentiation and maturation of RGCs into E cells (Mirzadeh et al., 2010b; Boutin et al., 2014; Ohata et al., 2014), the products of Pkd1's and Pkd2's family genes may function in this refinement. It is also possible that the refinement of BB patch displacement during the maturation of E cells is regulated by a different mechanism that does not require Pkd1 or Pkd2.
In Pkd1 heterozygous mutant mice (i.e., Nestin-Cre;Pkd1flox/+), partial defects in rotational polarity were observed. Heterozygous mutations in human, which are implicated in autosomal dominant polycystic kidney disease (Zhou, 2009), could be a risk factor for the development of hydrocephalus.
Conclusion
PCP in E cells plays key roles in the proper circulation of CSF and its defects can cause hydrocephalus (Boutin et al., 2014; Ohata et al., 2014; Ying et al., 2014). The present study shows that Pkd1 and Pkd2 are present in the primary cilia of RGCs and are involved in the development of PCP in RGCs and E cells. Hydrodynamic forces have been suggested to guide the development of PCP in E cells and multiciliated cells in the frog larval skin (Mitchell et al., 2007; Guirao et al., 2010; Mirzadeh et al., 2010b). Pkd1 and Pkd2 are involved in mechanosensation in cultured kidney epithelial cells and the embryonic node (Nauli et al., 2003; Yoshiba et al., 2012). Similarly, Pkd1 and Pkd2 could help sense hydrodynamic force in RGCs or immature E cells for the establishment and refinement of proper PCP. The disruption in E cells' PCP in Nestin-Cre;Pkd1flox/flox and Nestin-Cre;Pkd2flox/flox mice result in abnormal CSF circulation, as shown by slower ependymal flow, and expansion of the lateral ventricles. A full understanding of the mechanism leading to the intricate planar polarization of E cells, which varies by location in the ventricular wall, could help develop genetic tools for the diagnosis and treatment of hydrocephalus. This study identifies molecular components that could mediate mechanosensation in RGCs and E cells.
Footnotes
This work was sponsored by grants from the National Institutes of Health (NIH, HD032116 and NS028478) and a generous gift from the J. G. Bowes Foundation; S.O. was supported by the Cell Science Research Foundation, the Kanae Foundation for the Promotion of Medical Science, TOYOBO Biotechnology Foundation, and the Japanese Society for Promotion of Science; A.Á.-B. holds the Heather and Melanie Muss Endowed Chair. We thank Drs. L. S. Goldstein, A. Messing, G. Schütz, T. Watnick, and B. K. Yoder for providing mice; T. Inubushi, A. Kriegstein, P. McQuillen, and M. Ramalho-Santos for sharing equipment; A. Cebrián-Silla, Y.-G. Han, Z. Mirzadeh, M. F. Paredes, I. Rowe, L. Subramanian, and C. K. Tong for technical advice; L. Bogdanova, M. Castelli, J. L. Elsbernd, S. González-Granero, C. Guinto, J. R. Rodriguez, R. Romero, and A. Rudik for technical help; J. L. Elsbernd and M. F. Paredes for proofreading; and the A.Á.-B. laboratory for useful discussion.
The authors declare no competing financial interests.
References
- Al Jord A, Lemaître AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature. 2014;516:104–107. doi: 10.1038/nature13770. [DOI] [PubMed] [Google Scholar]
- Boutin C, Labedan P, Dimidschstein J, Richard F, Cremer H, André P, Yang Y, Montcouquiol M, Goffinet AM, Tissir F. A dual role for planar cell polarity genes in ciliated cells. Proc Natl Acad Sci U S A. 2014;111:E3129–E3138. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli M, Boca M, Chiaravalli M, Ramalingam H, Rowe I, Distefano G, Carroll T, Boletta A. Polycystin-1 binds Par3/aPKC and controls convergent extension during renal tubular morphogenesis. Nat Commun. 2013;4:2658. doi: 10.1038/ncomms3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli M, De Pascalis C, Distefano G, Ducano N, Oldani A, Lanzetti L, Boletta A. Regulation of the microtubular cytoskeleton by polycystin-1 favors focal adhesions turnover to modulate cell adhesion and migration. BMC Cell Biol. 2015;16:15. doi: 10.1186/s12860-015-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–1142. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez MA, Outeda P, Zhou Q, Zhou F, Menezes LF, Qian F, Huso DL, Germino GG, Piontek KB, Watnick T. Pkd1 and Pkd2 are required for normal placental development. PLoS One. 2010;5:e12821. doi: 10.1371/journal.pone.0012821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Coufal NG, Gleeson JG. Primary cilia in the developing and mature brain. Neuron. 2014;82:511–521. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, Anton ES. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16:1000–1007. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS, Inoue M, Ito T, Kato H, Sakaguchi M, Sunabori T, Nakaya MA, Nonaka S, Ogura T, Higuchi H, Okano H, Spassky N, Sawamoto K. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–3046. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- Iliescu A, Gravel M, Horth C, Kibar Z, Gros P. Loss of membrane targeting of Vangl proteins causes neural tube defects. Biochemistry. 2011;50:795–804. doi: 10.1021/bi101286d. [DOI] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development. 2011;138:1121–1129. doi: 10.1242/dev.058271. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K, Germino GG, Weinman EJ, Watnick T, Qian F. Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun. 2014;5:5482. doi: 10.1038/ncomms6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsis F, Boehlke C, Kuehn EW. The ciliary flow sensor and polycystic kidney disease. Nephrol Dial Transplant. 2013;28:518–526. doi: 10.1093/ndt/gfs524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res. 2013;91:1117–1132. doi: 10.1002/jnr.23238. [DOI] [PubMed] [Google Scholar]
- Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/S0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41–46. 1–8. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. 2010a;39:1938. doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Han YG, Soriano-Navarro M, García-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010b;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- Miyan JA, Nabiyouni M, Zendah M. Development of the brain: a vital role for cerebrospinal fluid. Can J Physiol Pharmacol. 2003;81:317–328. doi: 10.1139/y03-027. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, Tsuboi T, Watabe S, Okamoto H. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009;136:1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]
- Ohata S, Aoki R, Kinoshita S, Yamaguchi M, Tsuruoka-Kinoshita S, Tanaka H, Wada H, Watabe S, Tsuboi T, Masai I, Okamoto H. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 2011;69:215–230. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Ohata S, Nakatani J, Herranz-Pérez V, Cheng J, Belinson H, Inubushi T, Snider WD, García-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83:558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Axelrod JD. Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr Top Dev Biol. 2012;101:33–53. doi: 10.1016/B978-0-12-394592-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honoré E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Sipe CW, Lu X. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development. 2011;138:3441–3449. doi: 10.1242/dev.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigelman KA, Lelli A, Wu X, Gao J, Lin S, Piontek K, Wodarczyk C, Boletta A, Kim H, Qian F, Germino G, Géléoc GS, Holt JR, Zuo J. Polycystin-1 is required for stereocilia structure but not for mechanotransduction in inner ear hair cells. J Neurosci. 2011;31:12241–12250. doi: 10.1523/JNEUROSCI.6531-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong LC, Hollander WF. Hereditary loop-tail in the house mouse: accompanied by imperforate vagina and with lethal craniorachischisis when homozygous. J Hered. 1949;40:329–334. [Google Scholar]
- Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods. 2013;10:1105–1107. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, Poumay Y, Uemura T, Goffinet AM. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A. 2014;111:12438–12443. doi: 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarczyk C, Rowe I, Chiaravalli M, Pema M, Qian F, Boletta A. A novel mouse model reveals that polycystin-1 deficiency in ependyma and choroid plexus results in dysfunctional cilia and hydrocephalus. PLoS One. 2009;4:e7137. doi: 10.1371/journal.pone.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying G, Avasthi P, Irwin M, Gerstner CD, Frederick JM, Lucero MT, Baehr W. Centrin 2 is required for mouse olfactory ciliary trafficking and development of ependymal cilia planar polarity. J Neurosci. 2014;34:6377–6388. doi: 10.1523/JNEUROSCI.0067-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Live imaging movie showing ciliary beating of E cells in Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Pkd2flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd2flox/flox mouse.
Live imaging movie showing the migration of fluorescent beads on the wholemount preparation of lateral wall of lateral ventricle in Pkd1flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd1flox/flox mouse.
Live imaging movie showing the migration of fluorescent beads on the wholemount preparation of lateral wall of lateral ventricle in Pkd2flox/flox mouse.
Live imaging movie showing ciliary beating of E cells in Nestin-Cre;Pkd2flox/flox mouse.