Abstract
Context:
Body fat-free mass (FFM), energy expenditure (EE), and respiratory quotient (RQ) are known predictors of daily food intake. Because FFM largely determines EE, it is unclear whether body composition per se or the underlying metabolism drives dietary intake.
Objective:
The objective of the study was to test whether 24-hour measures of EE and RQ and their components influence ad libitum food intake independently of FFM.
Design and Participants:
One hundred seven healthy individuals (62 males/45 females, 84 Native Americans/23 whites; age 33 ± 8 y; body mass index 33 ± 8 kg/m2; body fat 31% ± 8%) had 24-hour measures of EE in a whole-room indirect calorimeter during energy balance, followed by 3 days of ad libitum food intake using computerized vending machine systems. Body composition was estimated by dual-energy x-ray absorptiometry.
Main Outcome Measures:
FFM, 24-hour EE, RQ, spontaneous physical activity, sleeping EE (sleeping metabolic rate), awake and fed thermogenesis, and ad libitum food intake (INTAKE) were measured.
Results:
Higher 24-hour RQ (P < .001, partial R2 = 16%) and EE (P = .01, partial R2 = 7%), but not FFM (P = .65), were independent predictors of INTAKE. Mediation analysis demonstrated that 24-hour EE is responsible for 80% of the FFM effect on INTAKE (44.5 ± 16.9 kcal ingested per kilogram of FFM, P= .01), whereas the unique effect due to solely FFM was negligible (10.6 ± 23.2, P = .65). Spontaneous physical activity (r = 0.33, P = .001), but not sleeping metabolic rate (P = .71), positively predicted INTAKE, whereas higher awake and fed thermogenesis determined greater INTAKE only in subjects with a body mass index of 29 kg/m2 or less (r = 0.44, P = .01).
Conclusions:
EE and RQ, rather than FFM, independently determine INTAKE, suggesting that competitive energy-sensing mechanisms driven by the preferential macronutrient oxidation and total energy demands may regulate food intake.
The fundamental principle of energy balance states that energy intake and expenditure (EE) are the key counterbalancing factors that determine body weight change. Both components are influenced by environmental and genetic factors (1, 2); however, whereas energy intake varies largely from day to day due to psychological and social influences, daily sedentary EE is largely determined by body size and composition (3). Because EE highly depends on the metabolically active part of the human body, it has been suggested that EE may exert influence on food intake as a physiological signal for hunger modulated by body energy requirements (4, 5). Recent developments in the field of food intake regulation indicate that body fat-free mass (FFM), the main determinant of EE, but not fat mass (FM), may act as a powerful driver of energy intake (6–8). However, the link between FFM/EE and dietary intake is still not fully understood; in particular, it is unclear whether the relationship between FFM and food intake is mediated by EE or whether FFM has an independent effect on intake beyond that arising from the underlying metabolism. Furthermore, the 24-hour respiratory quotient (RQ) during energy balance was also positively associated with ad libitum food intake (9). Yet it is unclear whether both RQ and EE are independent predictors of energy intake or the metabolic effects that elicit dietary intake are specific of either preferential macronutrient oxidation or higher energy demands.
Both our research group (8) and others (6) have shown a positive association between EE and ad libitum food intake. However, it is not clearly established whether the link between EE and intake arises from sleeping EE, the cost of being awake, thermic effect of food (TEF), or spontaneous physical activity (SPA), the four components of daily EE. Therefore, a deeper and more detailed analysis of daily EE components is warranted to better understand the mechanisms by which EE may influence food intake. Sleeping EE [sleeping metabolic rate (SMR)] accounts for approximately 70% of 24-hour EE in sedentary conditions (10), suggesting that SMR might explain part of the relationship between 24-hour EE and food intake. The awake and fed thermogenesis (AFT) is defined as the nonactivity-related increase in sedentary EE over SMR that includes the cost of being awake and TEF, representing the overall thermogenic response to necessary physiological activities related to survival (11). Given the importance of meal consumption in real-life settings, it is important to evaluate the metabolic response to feeding in relation to daily energy intake. In addition, it is well known that physical activity is a determinant of daily EE (3, 12), and exercising has a significant impact on body weight regulation (4, 13, 14); nevertheless, the contribution of SPA in sedentary condition to ad libitum food intake and macronutrient preference is not fully understood.
The aim of this study is to evaluate the effects of EE and macronutrient oxidation during energy balance in relation to subsequent ad libitum food intake. Specifically, we sought to determine the following: 1) whether FFM or EE (as the FFM mediator on food intake) specifically influence dietary intake; 2) which components of 24-hour EE including sleeping EE, AFT (as an integrative measure of TEF and the cost of being awake), and SPA (as an index of nonvolitional daily activities) predict subsequent food intake; and 3) whether the RQ determines ad libitum food intake independently of 24-hour EE.
Materials and Methods
The volunteers participated in a study that investigated eating behaviors on our inpatient clinical research unit, which included measurements of food intake and EE. The present study includes 107 healthy individuals (62 males/45 females, 84 Native Americans/23 whites) who had 24-hour measures of EE in a whole-room indirect calorimeter (respiratory chamber) followed by 3 days of ad libitum food intake using computerized vending machine systems. Glucose tolerance was assessed by a 75-g oral glucose tolerance test, and all subjects were free from diabetes according to the American Diabetes Association diagnostic criteria (15). Before participation, volunteers were fully informed of the nature and purpose of the studies, and written informed consent was obtained. The experimental protocols were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Study protocol
On admission, subjects were given a standard weight-maintaining diet of 50% carbohydrates, 30% fats, and 20% proteins with a food quotient (FQ) of 0.87 (16). The weight-maintaining energy needs (WMEN) were calculated on the basis of weight and gender (9, 17) and adjusted to maintain body weight within ±1%. Body composition was estimated by dual energy X-ray absorptiometry (DPX-1; Lunar Radiation Corp) for the calculation of percentage of body fat, FM and FFM.
After at least 3 days on the weight-maintaining diet, subjects resided for 24 hours inside the respiratory chamber. Subsequently they were asked to self-select all their food on a computer-operated vending machine system for the following 3 days.
Respiratory chamber
The method for measuring 24-hour EE and substrate oxidation in the respiratory chamber was previously described in detail (3). The total energy content of four meals was calculated using previously described equations (18). Carbon dioxide production, oxygen consumption, RQ, and the rate of EE were measured continuously, calculated for each 15-minute interval, averaged, and then extrapolated to the 24-hour interval. Calculation of 24-hour EE components is reported in the footnote of Table 1 and in the Supplemental Data.
Table 1.
Demographic, Anthropometric, Metabolic, and ad Libitum Food Intake Measures of the Study Group
| All Subjects (n = 107) | Males (n = 62) | Females (n = 45) | Native Americans (n = 84) | Whites (n = 23) | BMI ≤29 kg/m2 (n = 32) | BMI >29 kg/m2 (n = 75) | |
|---|---|---|---|---|---|---|---|
| Age, y | 32.9 ± 8.1 | 31.9 ± 7.2 | 34.4 ± 9.1 | 32.1 ± 7.9a | 35.9 ± 8.1 | 31.4 ± 7.9 | 33.6 ± 8.1 |
| Height, cm | 168.3 ± 8.2 | 173.5 ± 5.5b | 161 ± 5.2 | 167.2 ± 8.1a | 172.2 ± 7.6 | 169.4 ± 8.1 | 167.8 ± 8.3 |
| Body weight, kg | 94.7 ± 24.4 | 96.4 ± 25.0 | 92.3 ± 23.7 | 95.4 ± 25.4 | 92.3 ± 20.6 | 71.0 ± 9.9c | 104.8 ± 21.6 |
| BMI, kg/m2 | 33.5 ± 8.3 | 32.0 ± 7.8b | 35.6 ± 8.7 | 34.1 ± 8.6 | 31.2 ± 6.9 | 24.8 ± 3.2c | 37.2 ± 6.9 |
| Body fat, % | 31.4 ± 8.4 | 26.8 ± 6.6b | 38.4 ± 5.4 | 32.1 ± 7.8 | 29.3 ± 9.8 | 24.2 ± 7.4c | 34.8 ± 6.4 |
| FM, kg | 30.2 ± 13.0 | 26.9 ± 12.0b | 35.1 ± 13 | 30.9 ± 13.2 | 28.1 ± 12.2 | 17.4 ± 5.1c | 36.2 ± 11.1 |
| FFM, kg | 62.7 ± 14.2 | 68.9 ± 13.4b | 53.6 ± 9.9 | 62.2 ± 14.7 | 64.3 ± 12.5 | 53.6 ± 9.5c | 66.8 ± 14.1 |
| Fasting glucose concentration, mg/dL | 90.4 ± 8.9 | 88.5 ± 7.8b | 93.1 ± 9.7 | 90.7 ± 9.4 | 89.6 ± 6.7 | 86.4 ± 6.0c | 92.1 ± 9.4 |
| 2-hour glucose concentration, mg/dL | 123.4 ± 33.1 | 116.5 ± 29.2b | 133 ± 35.9 | 126.0 ± 32.4 | 113.9 ± 34.5 | 111.8 ± 27.9c | 128.3 ± 34.0 |
| Glucose tolerance status | |||||||
| NGT | 76 (71%) | 49 (79%) | 27 (60%) | 58 (69%) | 18 (78%) | 25 (78%) | 51 (68%) |
| IGT | 31 (29%) | 13 (21%) | 18 (40%) | 26 (31%) | 5 (22%) | 7 (22%) | 24 (32%) |
| Respiratory chamber | |||||||
| 24-hour energy intake, kcal | 2277 ± 351 | 2422 ± 300b | 2078 ± 318 | 2271 ± 363 | 2301 ± 307 | 2017 ± 263c | 2389 ± 325 |
| 24-hour EE, kcal/d | 2452 ± 381 | 2621 ± 329b | 2218 ± 321 | 2457 ± 381 | 2434 ± 386 | 2176 ± 310c | 2570 ± 347 |
| 24-hour energy balance, kcald | −174 ± 167 | −199 ± 172 | −141 ± 153 | −186 ± 163 | −132 ± 175 | −159 ± 159 | −181 ± 170 |
| SPA, %e | 7.5 ± 2.6 | 7.8 ± 2.6 | 7.0 ± 2.6 | 7.4 ± 2.4 | 7.7 ± 3.2 | 7.3 ± 2.3 | 7.5 ± 2.8 |
| Energy cost of SPA, kcal/d·% SPAf | 63.1 ± 22.2 | 65.5 ± 22.4 | 60.0 ± 21.7 | 64.7 ± 22.4 | 57.5 ± 21.1 | 52.9 ± 18.4c | 67.5 ± 22.3 |
| SPA EE, kcal/dg | 445 ± 172 | 487 ± 177b | 388 ± 148 | 453 ± 169 | 416 ± 182 | 366 ± 136c | 479 ± 175 |
| EE0 activity, kcal/14 hh | 1267 ± 206 | 1350 ± 188b | 1152 ± 174 | 1264 ± 200 | 1280 ± 231 | 1134 ± 178c | 1324 ± 191 |
| SMR, kcal/di | 1724 ± 280 | 1819 ± 265b | 1593 ± 248 | 1723 ± 279 | 1728 ± 291 | 1517 ± 188c | 1812 ± 267 |
| AFT, kcal/14 hj | 262 ± 99 | 289 ± 108b | 223 ± 70 | 259 ± 98 | 272 ± 104 | 249 ± 100 | 267 ± 98 |
| AFT, % of energy intake | 11.6 ± 4.1 | 12.1 ± 4.6 | 10.9 ± 3.3 | 11.5 ± 4.2 | 11.7 ± 3.9 | 12.3 ± 4.4 | 11.3 ± 4.0 |
| 24-hour RQ, ratio | 0.84 ± 0.02 | 0.84 ± 0.02 | 0.84 ± 0.03 | 0.84 ± 0.02 | 0.84 ± 0.03 | 0.84 ± 0.02 | 0.84 ± 0.02 |
| Daily (fed) RQ | 0.85 ± 0.03 | 0.85 ± 0.03 | 0.85 ± 0.03 | 0.85 ± 0.03 | 0.85 ± 0.02 | 0.85 ± 0.03 | 0.85 ± 0.03 |
| Sleeping (fasting) RQ | 0.81 ± 0.04 | 0.81 ± 0.04 | 0.82 ± 0.04 | 0.81 ± 0.04 | 0.81 ± 0.04 | 0.82 ± 0.04 | 0.81 ± 0.04 |
| 24-hour carbohydrate oxidation rate, kcal/d | 1020 ± 242 | 1112 ± 242b | 896 ± 179 | 1027 ± 249 | 995 ± 218 | 916 ± 211c | 1065 ± 241 |
| 24-hour fat oxidation rate, kcal/d | 1091 ± 298 | 1150 ± 264b | 1012 ± 325 | 1104 ± 311 | 1045 ± 250 | 948 ± 258c | 1153 ± 295 |
| Computerized vending machine systemsk | |||||||
| Total ad libitum food intake, kcal/d | 4401 ± 1202 | 4931 ± 1016b | 3670 ± 1051 | 4395 ± 1185 | 4422 ± 1286 | 4351 ± 1103 | 4422 ± 1248 |
| WMEN | 2798 ± 263 | 2907 ± 243b | 2646 ± 213 | 2795 ± 270 | 2809 ± 243 | 2606 ± 167c | 2881 ± 255 |
| Total ad libitum food intake, % of WMEN | 158 ± 40 | 170 ± 34b | 142 ± 43 | 158 ± 41 | 160 ± 37 | 167 ± 40 | 155 ± 40 |
| Carbohydrate intake, g/d | 558 ± 152 | 625 ± 123b | 466 ± 140 | 560 ± 148 | 554 ± 170 | 567 ± 154 | 555 ± 152 |
| Fat intake, g/d | 189 ± 61 | 213 ± 54b | 157 ± 56 | 190 ± 63 | 185 ± 57 | 181 ± 55 | 193 ± 64 |
| Protein intake, g/d | 145 ± 45 | 158 ± 43b | 126 ± 42 | 135 ± 39a | 180 ± 49 | 144 ± 45 | 145 ± 45 |
Abbreviations: EE0 activity, EE in the inactive awake state; IGT, impaired glucose tolerance (2 h plasma glucose concentration: 140–199 mg/dL) according to American Diabetes Association diagnostic criteria (15). Values in each cell are reported as mean ± SD.
P < .05 vs whites.
P < .05 vs females.
P < .05 vs BMI greater than 29 kg/m2.
Twenty-four-hour energy balance inside the respiratory chamber was the difference between the total caloric intake of four meals (24 h energy intake) and 24-hour EE.
SPA was calculated as the average value of all the 15-minute periods between 11:00 am and 1:00 am (14 h) inside the respiratory chamber.
The energy cost of SPA was quantified by the slope of the regression line between EE and SPA between 11:00 am and 1:00 am.
The SPA-related EE was derived as the product of SPA times the slope of the regression line between EE and SPA between 11:00 am and 1:00 am.
The EE0 activity was calculated as the intercept of the regression line between EE and SPA between 11:00 am and 1:00 am.
SMR was defined as the average EE of all 15-minute nightly periods between 1:00 am and 5:00 am during which SPA was less than 1.5%.
The AFT was calculated as the difference between EE0 activity and SMR (11).
Ad libitum food intake measures are reported as the average over 3 days on the vending machines.
Computerized vending machine system
The measurement of ad libitum food intake by an automated food-selection system was previously described, validated, and tested for reproducibility (19, 20) (Supplemental Data). Briefly, an automated food-selection system is made up of a refrigerated vending machine (model 3007; U-Select-It) including the 40 items for breakfast, lunch, and dinner, which were made available every day on the basis of the responses to a food-preference questionnaire, as previously described (21). Each volunteer was assigned to a single vending machine, had unrestricted access to the machine for 23.5 h/d for 3 days and was asked to follow his/her typical eating pattern as closely as possible. Subjects were instructed to eat only in the vending room, whatever they wished whenever they desired and to return the unconsumed food portions to the metabolic kitchen for calculation of actual calories consumed obtained using a food database.
Statistical analysis
Multiple linear regression analysis was used to identify the independent determinants of ad libitum food intake including demographic, anthropometric, and metabolic parameters. Mediation analysis based on hierarchical multivariable regression models (22) was used to evaluate whether the effect of FFM on ad libitum food intake was exerted through the influence of FFM on 24-hour EE. More specifically, the total effect of FFM on food intake was partitioned in two components according to the causal model shown in Figure 1: the indirect effect through 24-hour EE (mediator), and the direct effect independent of 24-hour EE. The Sobel test (23) was used to test whether the indirect effect was significantly greater than zero, namely that the effects of FFM on food intake are mediated by 24-hour EE.
Figure 1.
Results of the mediation analysis for FFM, 24-hour EE, and ad libitum food intake. Path coefficient between FFM (independent variable) and 24-hour EE (dependent variable) blocks was calculated as the β-coefficient from the multivariable regression model including all covariates. Path coefficient between 24-hour EE (independent variable) and ad libitum food intake (dependent variable) blocks is the β-coefficient obtained from the multivariable regression model including all covariates plus FFM. The path coefficient between FFM (independent variable) and ad libitum food intake (dependent variable) blocks is the β-coefficient obtained from the multivariable regression model including all covariates. The direct effect of FFM (independent variable) on ad libitum food intake (dependent variable) was calculated as the β-coefficient from the multivariable regression model including all covariates plus 24-hour EE. The indirect effect of FFM on ad libitum food intake via 24-hour EE (mediator) is obtained as the product of the two path coefficients between FFM→24-hour EE and 24-hour EE→ad libitum food intake, and it is tested for significance by the Sobel test.
Multivariable regression models were used to calculate the residuals of EE parameters (24 h EE, RQ, SMR, and AFT) after accounting for known covariates, as previously described (11, 24, 25). The Pearson's correlation coefficient (r) was used to quantify the associations between EE residuals and food-intake measures. Based on the previously reported nonlinear relationship between AFT and BMI, showing an inflection point at a BMI of 29 kg/m2 (11), the relationships between residuals of AFT and food intake measures were calculated separately in subjects with a BMI below and above 29 kg/m2.
A value of P < .05 was considered significant. Data are presented as mean ± SD, except for path coefficients from a mediation analysis that are reported as mean ± SE. Statistical analyses were performed using SPSS (version 21; IBM Corp).
Results
The characteristics of the study group including metabolic and food-intake measures are reported in Table 1. The average daily total ad libitum energy intake (INTAKE) on the vending machines was 4401 ± 1202 kcal/d, equal to 159% ± 40% of WMEN, indicating that overall subjects overate approximately 60% as compared with their weight-maintaining energy needs (P < .001). Half of the ingested calories derived from carbohydrates (50% ± 6% of INTAKE), followed by fats (37% ± 5%) and proteins (13% ± 3%), with a FQ for the ad libitum diet equal to 0.867.
Predictors of ad libitum food intake
In bivariate analyses, FFM (r = 0.42, P < .001), unadjusted 24-hour EE (r = 0.46, P < .001), and RQ (r = 0.32, P= .001) each were positively associated with INTAKE. Additionally, 24-hour energy balance inversely predicted subsequent INTAKE (r = −0.30, P = .002). However, in a multivariable model, 24-hour RQ (partial R2 = 16%, P < .001), FM (partial R2 = 12%, P = .001), 24-hour EE (partial R2 = 7%, P = .01), but not FFM (P = .65) or 24-hour energy balance (P = .43) were independent predictors of INTAKE, explaining approximately 50% of its variance (Table 2). Similar results were obtained for INTAKE expressed as percentage of WMEN and macronutrient intake (Table 2). The independent effect of 24-hour EE on total intake was reflected in fat (partial R2 = 9%, P = .004) and protein (partial R2 = 13%, P < .001) but not in carbohydrate (P = .11) intake.
Table 2.
Multivariable Models for the Determinants of ad Libitum Food Intake
| Predictors | Total Food Intake, kcal/d | Total Food Intake, % WMEN | Carbohydrate Intake, g/d | Fat Intake, g/d | Protein Intake, g/d |
|---|---|---|---|---|---|
| Age, y | −21.0 ± 11.6 (P = .073) |
−0.6 ± 0.5 (P = .195) |
−1.7 ± 1.6 (P = .292) |
−1.5 ± 0.6 (P = .015)a |
−1.0 ± 0.4 (P = .031)a |
| Gender (female) | 949.1 ± 505.2 (P = .064) |
44.6 ± 19 (P = .021)a |
69.4 ± 68.8 (P = .316) |
61.9 ± 26.4 (P = .021)a |
55.0 ± 19.0 (P = .005)a |
| Ethnicity (Native American) | 27.0 ± 212.6 (P = .899) |
2.6 ± 8.2 (P = .752) |
8.7 ± 29.0 (P = .765) |
8.6 ± 11.1 (P = .439) |
−42.9 ± 8.0 (P < .001)a |
| FM, kg | −71.0 ± 20.1 (P = .001)a |
−2.9 ± 0.8 (P < .001)a |
−8.2 ± 2.7 (P = .004)a |
−3.5 ± 1.1 (P = .001)a |
−2.6 ± 0.8 (P = .001)a |
| FFM, kg | 10.6 ± 23.2 (P = .649) |
−0.1 ± 0.9 (P = .926) |
2.4 ± 3.2 (P = .454) |
0.1 ± 1.2 (P = .924) |
−0.8 ± 0.9 (P = .379) |
| 24-h energy balance, kcal | 1.01 ± 1.27 (P = .426) |
0.03 ± 0.05 (P = .470) |
−0.02 ± 0.17 (P = .894) |
0.10 ± 0.07 (P = .133) |
0.11 ± 0.05 (P = .023)a |
| SPA, % | 30.7 ± 35.4 (P = .389) |
1.0 ± 1.3 (P = .460) |
−0.7 ± 4.8 (P = .892) |
3.3 ± 1.9 (P = .076) |
0.4 ± 1.3 (P = .783) |
| 24-h EE, 100 kcal/d |
316.7 ± 123.5 (P = .012)a |
10.5 ± 4.6 (P = .026)a |
27.2 ± 16.8 (P = .110) |
18.9 ± 6.5 (P = .004)a |
17.0 ± 4.6 (P < .001)a |
| 24-h RQ, % |
183.7 ± 44.3 (P < 0.001)a |
6.7 ± 1.7 (P < 0.001)a |
19.1 ± 6.1 (P = 0.002)a |
8.9 ± 2.3 (P < 0.001)a |
7.4 ± 1.7 (P < 0.001)a |
| Intercept | −16 076 ± 3989 | −525 ± 150 | −1513 ± 543 | −838 ± 209 | −693 ± 150 |
| Total R2 | 54%a | 42%a | 48%a | 52%a | 54%a |
β-Coefficients in each cell are reported as mean ± SE. β-Coefficients for 24-hour EE and 24-hour RQ are expressed per 100-kcal increase and per 0.01-unit increase, respectively. Bold indicates P < .05.
P < .05.
To further explore the relationships linking FFM and 24-hour EE (r = 0.90, P < .001) with INTAKE, we performed a mediation analysis to quantify the indirect effect of FFM on INTAKE achieved through 24-hour EE (Figure 1). All the conditions for running a mediation analysis using 24-hour EE as a mediator variable were satisfied, given the strong effect of FFM on 24-hour EE (14.0 ± 1.3 kcal EE per kilogram of FFM, P < .001) and the significant association of 24-hour EE on INTAKE after adjustment for FFM (3.2 ± 1.2 kcal intake per kilocalorie EE, adjusted P = .01). The indirect effect of FFM on INTAKE exerted through 24-hour EE (44.5 ± 16.9 kcal intake per kilogram of FFM, P = .01) accounted for 80% of the total effect of FFM on INTAKE (55.1 ± 15.9 kcal intake per kilogram of FFM, P < .001) and was 4-fold higher than the direct effect uniquely due to FFM, which in turn was negligible (10.6 ± 23.2, kcal intake per kilogram of FFM, P = .65).
Relationships between components of 24-hour EE and ad libitum food intake
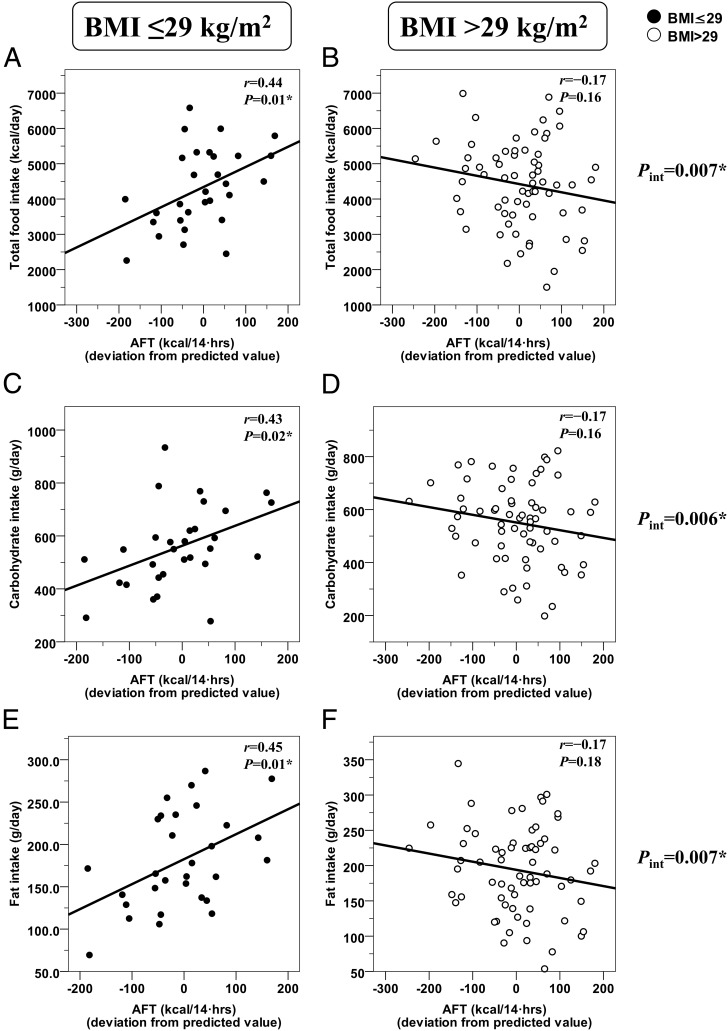
Residuals of AFT were directly related to INTAKE in subjects with a BMI of 29 kg/m2 or less (r = 0.44, P = .01) but not in those with a BMI greater than 29 kg/m2 (r = −0.17, P=0.16, interaction term P = .007) (Figure 2, A and B). The relationship between AFT and INTAKE was also different in the two subgroups when subjects were categorized as obese (BMI ≥ 30 kg/m2) and nonobese (interaction term P = .04), after excluding subjects with impaired glucose tolerance (P = .01) and expressing INTAKE as a percentage of WMEN (P = .009). In only subjects with a BMI of 29 kg/m2 or less, AFT was directly related with carbohydrate (Figure 2C) and fat (Figure 2E) intakes, whereas the same relationships tended to be inverse in the subgroup with a BMI greater than 29 kg/m2 (Figure 2, D–F). No significant relationships were found between AFT and protein intake in the whole group and in the two subgroups (all P > .20, interaction term P = .33). Results for AFT vs INTAKE in the subgroup of subjects with a BMI less than 29 kg/m2 were still significant after adjustment for fed RQ (partial r = 0.47, P = .01).
Figure 2.
Different relationships between AFT and ad libitum food intake in the two subgroups of subjects identified by a BMI cutoff of 29 kg/m2. Different relationships between the AFT (adjusted for age, gender, ethnicity, percentage of body fat, FFM, and fasting glucose concentration) and ad libitum food intake measures (total intake, panels A and B; carbohydrate intake, panels C and D; fat intake, panels E and F) in the two subgroups of subjects identified by a BMI cutoff value of 29 kg/m2. Food intake measures are reported as the average over 3 days on the vending machines. In each panel, the Pearson's correlation coefficient (r) is reported along with its significance (P). The best-fit regression line is displayed in each graph. The slopes of the regression lines in the two subgroups were compared by analysis of covariance, and the significance of the interaction term between AFT and BMI subgroups is reported on the right of each pair of graphs. In subjects with a BMI less than 29 kg/m2, a 10-kcal increase in AFT was associated with an average 58-kcal increase in INTAKE per day (equivalent to 2% of WMEN), of which 29 kcal derived from carbohydrates, 25 kcal from fats, and 4 kcal from proteins.
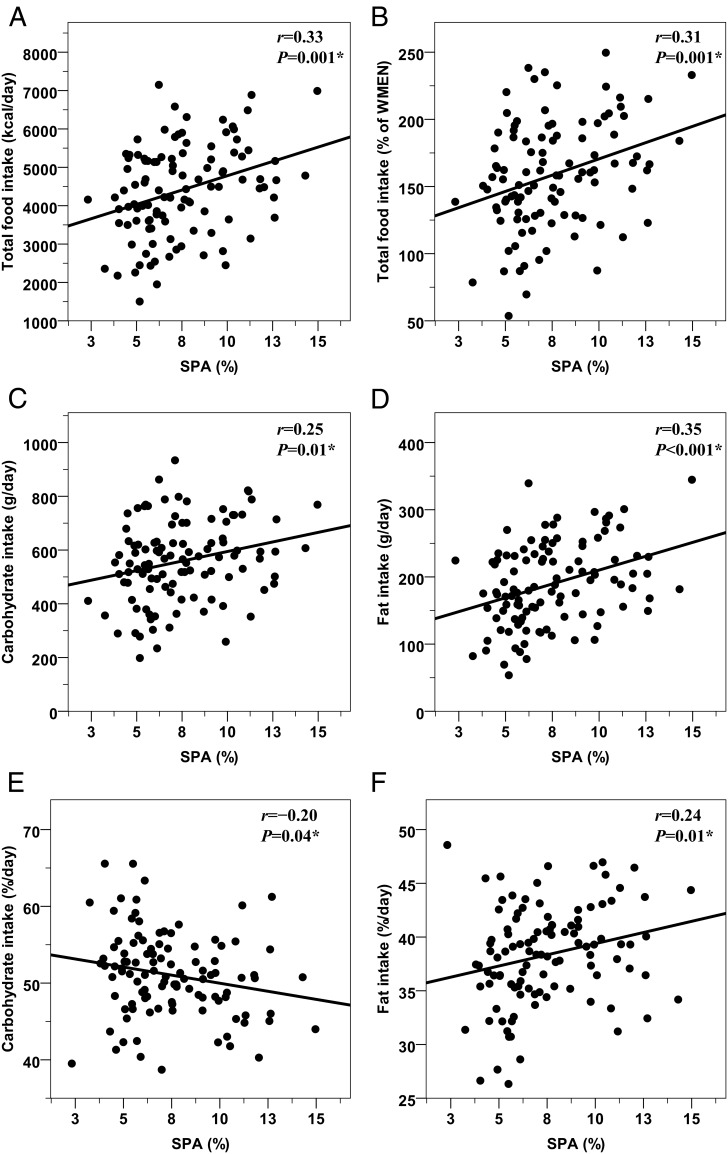
The average SPA positively predicted subsequent INTAKE (Figure 3A), INTAKE expressed as a percentage of WMEN (Figure 3B), carbohydrate (Figure 3C), fat (Figure 3D), and protein (r = 0.24, P = .01) intake. Similar results were obtained for the SPA-related EE (all P ≤ .01) but not for the energy cost of SPA (all P > .05). When macronutrient intakes were expressed as a percentage of INTAKE, SPA was inversely associated with percent carbohydrate intake (Figure 3E) and directly related with percentage of fat intake (Figure 3F) but not a percentage of protein intake (r = −0.04, P = .70). Significant results for SPA were also obtained after adjustment of 24-hour energy balance; however, SPA was not significant (P = .39) in the full model for food intake including 24-hour EE and RQ (Table 2).
Figure 3.
Relationships between SPA inside the respiratory chamber and subsequent ad libitum food intake. Positive relationships between the SPA (expressed as a percentage of time from 11:00 am to 1:00 am during which the subject was moving inside the chamber as detected by motion sensors) and total caloric intake (panels A and B), carbohydrate (panel C), and fat (panel D) intakes. Panels E and F show the direct and inverse relationships between SPA and carbohydrate and fat intakes expressed as a percentage of total caloric intake. Food intake measures are reported as the average over 3 days on the vending machines. In each panel, the Pearson's correlation coefficient (r) is reported along with its significance (P). The best-fit lines are also displayed. On average, a 1% increase in SPA (equivalent to approximately 8 min and 63 kcal expended over 14 h) predicted a 131-kcal increase in INTAKE (equivalent to 5% of WMEN), of which 74 kcal were derived from fats and 57 from carbohydrates.
Residuals of SMR were not associated with any food-intake measure (all P > .50, Supplemental Figure 1).
Independent effects of 24-hour EE and RQ on ad libitum food intake
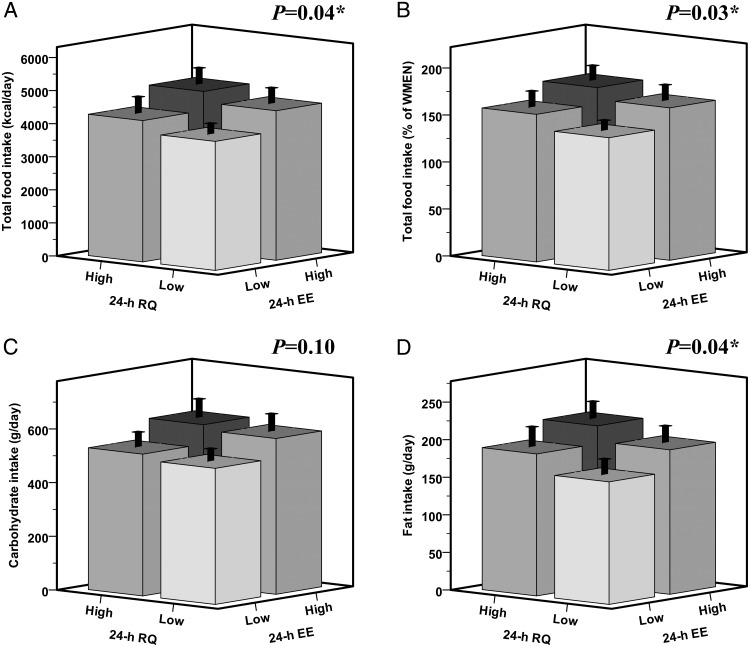
In multivariable models including residuals of 24-hour EE and RQ, both were independent predictors of INTAKE and fat intake. Results were stronger in a sensitivity analysis including only the 76 individuals with normal glucose tolerance (NGT) (data not shown). On average, a surplus of 100 kcal/d in 24-hour EE (independent of 24 h RQ) was associated with a 175-kcal increase in INTAKE per day (equivalent to 5% of WMEN), of which 87 kcal derived from carbohydrates, 70 kcal from fats, and 18 kcal from proteins. An increase of 0.01 unit in 24-hour RQ (independent of 24 h EE) was associated with a 204-kcal increase in INTAKE per day (equivalent to 8% of WMEN), of which 94 kcal derived from fats, 79 kcal from carbohydrates, and 31 kcal from proteins. Results for 24-hour RQ were similar to those for fed RQ (data not shown), whereas sleeping/fasting RQ did not show any significant association with any food intake measure (all P > .05). When subjects were categorized in four subgroups based on the sign of the residuals of 24-hour EE and RQ (Figure 4), individuals with both relatively higher 24-hour EE and RQ (black bar) consumed on average 947 kcal/d (P = .006) more than the individuals with both relatively lower EE and RQ (white bar), despite similar body weight (P = .71) and FFM (P = .75). This difference in INTAKE between the two extreme EE-RQ groups was equivalent to a 34% of WMEN (P = .004) and reflected a higher consumption of 448 kcal/d from fats (P = .004), 373 kcal/d from carbohydrates (P = .03), and 126 kcal/d from proteins (P = .01).
Figure 4.
Phenotypes of ad libitum food intake based on the cumulative effects of 24-hour EE and 24-hour RQ. Subjects were categorized in two groups (High and Low) based on the sign of the residuals of 24-hour EE and 24-hour RQ after adjustment for age, gender, ethnicity, FM, FFM (24 h EE), age, gender, ethnicity, deviation from energy balance, and percentage of body fat (24 h RQ) by multiple regression analysis. The High groups include individuals with a positive residual (ie, the measured value of 24 h EE or RQ was higher than the predicted value calculated based on the multivariable regression model including all the determinants), whereas the Low groups include those having a negative residual. Total caloric intake is calculated as the average daily kilocalories consumed over 3 days (panel A) and expressed as a percentage of the WMEN (panel B). Carbohydrate (panel C) and fat (panel D) intakes are calculated as the average weight in grams of macronutrient consumed over the 3 days of ad libitum food intake. P values are obtained by ANOVA. Individuals with both relatively higher 24-hour EE and RQ (black bar, n = 23) consumed on average 947 kcal/d (P = .006) more than the individuals with both relatively lower EE and RQ (white bar, n = 23). There were no differences among the four groups based on subjects' age (P = .38), gender (P = .66), ethnicity (P = .60), body weight (P = .71), body fat (P = .54), FM (P = .71), and FFM (P = .75).
Discussion
In the present study, we show that relatively higher daily EE and RQ during weight maintenance and relative energy balance, both independently predicted ad libitum food intake over 3 days. The results for EE and RQ were independent of FFM, whose effect on food intake was in turn negligible and largely accounted for via EE. SPA, but neither SMR nor sleeping RQ, as well as higher AFT in only subjects with a BMI less than 29 kg/m2, determined a greater dietary intake when subjects self-selected their food on the computerized vending machine systems.
The physiological mechanisms underlying the positive relationship between EE and food intake are not fully understood, but recent evidence suggests that energy requirements may act as a physiological signal for hunger (5, 6). Individually, FFM and 24-hour EE were positively associated with ad libitum food intake in a previous study (8), indicating that energy requirements are tightly linked to intake by the metabolically active part of the body that largely determines daily EE. Yet the effects of each component of daily EE (SMR, AFT, and SPA), resulting daily energy balance or concomitant substrate oxidation on food intake were not evaluated in that study. Higher resting EE (6) and FFM (7) were both associated with energy intake, appetite, and self-determined meal size in recent studies from another research group. The effects of FFM and, consequently, 24-EE on food intake appear to act at the level of the brain through central pathways that modulate appetite in regions implicated in energy homeostasis (26). Yet because of the tight link between FFM and EE, it is not clear whether their single effects on food intake represent the same signal that elicits hunger or whether both FFM and EE may contribute independently in driving dietary intake. In the present study, we therefore explored the link between FFM and food intake by dissecting solely the effect of total body lean mass from that exerted exclusively via 24-hour EE. The results of our mediation analysis clearly indicate that the largest part of the FFM effect on food intake is accounted by 24-hour EE, indicating that body energy metabolism, rather than the amount of body active tissues, is the main driver of dietary intake. Furthermore, after accounting for 24-hour EE, the unique effect of FFM on food intake was greatly reduced by 80% and ultimately became not significant, suggesting that EE is the main mechanism by which FFM exerts its influence on food intake.
On average, a 100-kcal surplus in daily EE and a positive 1% shift in 24-hour RQ were associated with independent increases in ad libitum food intake of approximately 175 and 204 kcal/d, respectively, indicating that both the underlying metabolism and substrate oxidation are competing drives for food intake. These novel results provide important implications for understanding the mechanisms underlying food intake regulation, especially in the current obesogenic environment characterized by wide availability of calorically dense food. The 24-hour RQ was the strongest determinant of ad libitum food intake, accounting for 16% of its variance. As a balance of carbohydrate to lipid oxidation, possible mechanisms by which higher RQ may drive to overeating may be due to the depletion of glycogen stores (27, 28) or the regulation of peripheral (muscle) fatty acid oxidation, which may occur via central mechanisms also implicated in satiety (29). We also observed that not only a reduced 24-hour EE but also a negative 24-hour energy balance predicted subsequent food intake. Nevertheless, in a multivariable model, only 24-hour EE (but not 24 h energy balance nor FFM) and RQ were the only parameters predicting ad libitum food intake. Yet because energy balance is calculated as the difference between the total energy intake of four meals provided during the 24 hours in the chamber and 24-hour EE and energy intake was fixed and calculated based on subject's body size and gender (18), more detailed studies are warranted to elucidate whether the energy deficit per se or, specifically, the increase in EE (even with concomitant energy balance) drives appetite and dietary intake.
The level of SPA, but not the energy cost of SPA, was found to be a potent driver of subsequent food intake and higher SPA induced a shift from carbohydrate to fat intake when both macronutrient intakes were normalized to the total caloric intake. SPA, as measured in the confined environment of the respiratory chamber by radar sensor, represents the percentage of time during which subjects perform unconscious activities such as fidgeting and strolling (3) but does not include the unrestricted voluntary physical activity as during free-living conditions. SPA was previously found to be a familial trait, a positive determinant of daily EE, and lower SPA predicted weight gain in male subjects (12). However, this latter finding appears to be contradictory to our results showing that higher SPA induced greater ad libitum food intake, possibly determining short-term weight gain. Possible explanations for this might be that subjects with increased SPA both ate more but also had a higher 24-hour EE through increased movement, thereby achieving energy balance, or higher SPA created an energy deficit that in turn promoted food intake during the subsequent ad libitum period. Yet the SPA effect on subsequent food intake was still observed after adjusting for 24-hour energy balance. Furthermore, it might be also speculated that subjects with higher propensity to be active may be prone to overeat in an environment characterized by a wide availability of calorically dense food, such as in our vending machine paradigm. Future research should be carried out to investigate whether SPA levels or activity-induced energy deficit are the factors driving food intake.
Daily nonexercise EE is composed of the minimum energy requirements as estimated while sleeping (SMR) and the thermogenic cost of being awake and fed (AFT) (11). We have recently shown in a large cohort of individuals with a broad range of adiposity that the relationship between AFT and body size was nonlinear; specifically, AFT was negatively related to BMI only when BMI exceeded 29 kg/m2 (11). Accordingly, in the present study, we tested the associations between AFT and food intake measures separately in the two groups identified by a BMI cutoff of 29 kg/m2. Although we did not observe any association for SMR (or sleeping RQ) despite SMR represented 70% of 24-hour EE, we found that higher AFT (even after adjustment for concomitant fed RQ) predicted overeating solely in nonobese individuals with a BMI less than 29 kg/m2. This finding suggests that the positive relationship between EE and food intake is more evident in those individuals in whom the thermogenic response to feeding is not blunted by obesity, namely those subjects who are more metabolically responsive when fed. Conversely, the lack of association for AFT in subjects with a BMI greater than 29 kg/m2 might be due to a decreased metabolic response to eating caused by increased abdominal adiposity (11), which may attenuate (or even suppress) the effects of AFT on ad libitum food intake. These findings are in accord with a previous study that showed an inverse relationship between body adiposity/waist circumference and changes in ad libitum food intake after isocaloric manipulation of macronutrients (17), suggesting that the individual efficiency of responding to dietary changes and, possibly, the metabolic efficiency may be dependent on body habitus and be reduced in obese subjects.
The main limitations of this study are the relative small sample size, the relative overeating during the vending period, a slightly negative energy balance inside the calorimeter with a 24-hour RQ less than FQ, and the limited physical activity on the metabolic ward, which may not reflect that of free-living conditions and might limit the generalization of these findings. A potential caveat in interpreting the different results between obese and nonobese subjects may be that obese subjects may have refrained from overeating due to an observational effect. However, in the present study, FM was a negative predictor of food intake, even after concomitant adjustment for FFM, 24-hour EE, and RQ, providing evidence that higher body fat is associated with relatively reduced food intake. The inhibitory effect of FM on food intake may be explained by hormonal mediators such as leptin or by lower circulating levels of ghrelin as previously observed in obese subjects (30). Finally, food intake estimated by the vending machine paradigm might not reflect that of a real-life setting. Nevertheless, this method of measuring dietary intake represents one of the most reproducible, consistent, and objective methods to measure ad libitum food intake in a clinical setting with an intraclass correlation of 0.90 (20).
In conclusion, we have shown that higher 24-hour EE rather than FFM, along with a preference for carbohydrates over fats as substrate for oxidation, both independently induced greater dietary intake when food was provided ad libitum. Furthermore, higher SPA predicted overeating, whereas an increased rate of thermogenesis drove food intake in nonobese individuals. Because we did not find a relationship between sleeping EE or RQ and intake, it raises the possibility that only the EE components related to daily activities of survival influence dietary intake. Taken together, these results imply that competitive energy-sensing mechanisms based on substrate preference for oxidation along with the total amount of kilocalories expended may regulate food intake and that higher energy demands (and perhaps consequent energy deficit) may trigger appetite and can ultimately lead to overconsumption in a food-rich environment. Given the wide availability of calorically dense and highly palatable food in modern society and increased likelihood of overeating, a better understanding of the mechanisms by which EE influences food intake may represent a novel target for the development of obesity therapies to tackle this disease.
Acknowledgments
We thank the nursing, clinical, and dietary staffs and laboratory technicians of the clinical research center for their valuable assistance and care of the volunteers. The technical support of Enrique R. Diaz, RN, for data collection is gratefully acknowledged.
The study had the identifier of NCT00342732 (clinicaltrials.gov).
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. P.P. was supported by a visiting fellowship award of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AFT
- awake and fed thermogenesis
- EE
- energy expenditure
- FFM
- fat-free mass
- FM
- fat mass
- FQ
- food quotient of the diet
- INTAKE
- total ad libitum food intake
- NGT
- normal glucose tolerance
- RQ
- respiratory quotient
- SMR
- sleeping metabolic rate
- SPA
- spontaneous physical activity
- TEF
- thermic effect of food
- WMEN
- weight-maintaining energy needs.
References
- 1. de Castro JM. Genetic influences on daily intake and meal patterns of humans. Physiol Behav. 1993;53:777–782. [DOI] [PubMed] [Google Scholar]
- 2. Bogardus C, Lillioja S, Ravussin E, et al. Familial dependence of the resting metabolic rate. N Engl J Med. 1986;315:96–100. [DOI] [PubMed] [Google Scholar]
- 3. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16(suppl 1):67–76. [DOI] [PubMed] [Google Scholar]
- 5. Blundell JE, Caudwell P, Gibbons C, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Models Mech. 2012;5:608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caudwell P, Finlayson G, Gibbons C, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97:7–14. [DOI] [PubMed] [Google Scholar]
- 7. Blundell JE, Caudwell P, Gibbons C, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–449. [DOI] [PubMed] [Google Scholar]
- 8. Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes. 2014;38(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord. 1999;23:715–722. [DOI] [PubMed] [Google Scholar]
- 11. Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol. 1992;263:E296–E300. [DOI] [PubMed] [Google Scholar]
- 13. King AC, Tribble DL. The role of exercise in weight regulation in nonathletes. Sports Med. 1991;11:331–349. [DOI] [PubMed] [Google Scholar]
- 14. Caudwell P, Gibbons C, Hopkins M, et al. The influence of physical activity on appetite control: an experimental system to understand the relationship between exercise-induced energy expenditure and energy intake. Proc Nutr Soc. 2011;70:171–180. [DOI] [PubMed] [Google Scholar]
- 15. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. 2003. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 26(Suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 16. Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. [DOI] [PubMed] [Google Scholar]
- 17. Penesova A, Venti CA, Bunt JC, Bonfiglio SM, Votruba SB, Krakoff J. Short-term isocaloric manipulation of carbohydrate intake: effect on subsequent ad libitum energy intake. Eur J Nutr. 2011;50:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbott WG, Howard BV, Christin L, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255:E332–E337. [DOI] [PubMed] [Google Scholar]
- 19. Rising R, Alger S, Boyce V, et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr. 1992;55:343–349. [DOI] [PubMed] [Google Scholar]
- 20. Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salbe AD, Tschop MH, DelParigi A, Venti CA, Tataranni PA. Negative relationship between fasting plasma ghrelin concentrations and ad libitum food intake. J Clin Endocrinol Metab. 2004;89:2951–2956. [DOI] [PubMed] [Google Scholar]
- 22. MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Routledge; 2008. [Google Scholar]
- 23. Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 24. Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–E707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piaggi P, Thearle MS, Bogardus C, Krakoff J. Fasting hyperglycemia predicts lower rates of weight gain by increased energy expenditure and fat oxidation rate. J Clin Endocrinol Metab. 2015;100:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. NeuroImage. 2013;64:712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flatt JP. Use and storage of carbohydrate and fat. Am J Clin Nutr. 1995;61:952S–959S. [DOI] [PubMed] [Google Scholar]
- 28. Flatt JP. Carbohydrate balance and body-weight regulation. Proc Nutr Soc. 1996;55:449–465. [DOI] [PubMed] [Google Scholar]
- 29. Jumpertz R, Guijarro A, Pratley RE, Mason CC, Piomelli D, Krakoff J. Associations of fatty acids in cerebrospinal fluid with peripheral glucose concentrations and energy metabolism. PloS One. 2012;7:e41503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. [DOI] [PubMed] [Google Scholar]