Abstract
Context:
Successful long-term weight loss is challenging. Brain endogenous opioid systems regulate associated processes; however, their role in the maintenance of weight loss has not been adequately explored in humans.
Objective:
In a preliminary study, the objective was to assess central μ-opioid receptor (MOR) system involvement in eating behaviors and their relationship to long-term maintenance of weight loss.
Design:
This was a case-control study with follow-up of the treatment group at 1 year after intervention.
Setting:
The study was conducted at a tertiary care university medical center.
Participants:
Lean healthy (n = 7) and chronically obese (n = 7) men matched for age and ethnicity participated in the study.
Interventions:
MOR availability measures were acquired with positron emission tomography and [11C]carfentanil. Lean healthy men were scanned twice under both fasted and fed conditions. Obese men were placed on a very low-calorie diet to achieve 15% weight loss from baseline weight and underwent two positron emission tomography scans before and two after weight loss, incorporating both fasted and fed states.
Main Outcome Measures:
Brain MOR availability and activation were measured by reductions in MOR availability (nondisplaceable binding potential) from the fed compared with the fasted-state scans.
Results:
Baseline MOR nondisplaceable binding potential was reduced in obese compared with the lean and partially recovered obese after weight loss in regions that regulate homeostatic, hedonic, and emotional responses to feeding. Reductions in negative affect and feeding-induced MOR system activation in the right temporal pole were highly correlated in leans but not in obese men. A trend for an association between MOR activation in the right temporal pole before weight loss and weight regain 1 year was found.
Conclusions:
Although these preliminary studies have a small sample size, these results suggest that obesity and diet-induced weight loss impact central MOR binding and endogenous opioid system function. MOR system activation in response to an acute meal may be related to the risk of weight regain.
Obesity has reached epidemic levels despite the knowledge of the negative health impacts of excessive weight. Although development of systems primed to sustain positive energy balance have provided evolutionary advantage, it is very likely that the biological processes regulating energy balance have not had sufficient time to adapt to the rapid development of our current stress-prone and obesogenic environment (1). Food reduces negative affect and is used as a coping strategy in response to diffuse and uncontrollable stress (2, 3), and individual differences exist in how stress impacts food selection (4, 5). In our current environment, the capacity for easily acquired palatable food to acutely modulate affect and reduce stress may contribute to an individual's inability to self-regulate body weight despite knowledge of the adverse consequences of excessive weight on health. A greater understanding of the underlying biological processes involved at the interface of eating and emotion will be crucial in understanding triggers that may promote behaviors that undermine successful maintenance of healthy body weight.
Central regulation of food intake and energy management is complex. The endogenous opioid system and μ-opioid receptors have long been known to regulate food and energy balance, particularly by modulating consummatory behavior beyond satiety (6). Additionally, the opioid system is involved in the regulation of affective and stress responses and is therefore positioned as a common mediator that underlies the interface of food intake, hedonic response, and emotional regulation (7–9). What is not known is how central opioid systems that regulate emotion and food intake respond to acute bouts of feeding in humans, how they are impacted by chronic obesity, and whether they play a role in mediating the long-term maintenance of weight loss.
Given the role for μ-opioid receptor (MOR)-mediated neurotransmission in regulating emotional and feeding responses, we sought to determine the affective and central MOR system response [using [11C]carfentanil positron emission tomography (PET)] to a standardized meal and how obesity and weight loss may impact the response of this system to feeding. Lean individuals were assessed once. Obese individuals were assessed before and after a targeted 15% weight loss using a very low-calorie diet (VLCD). For each assessment individuals were scanned after an overnight fast, and then after the consumption of a standard meal. Under those conditions, endogenous opioid release after feeding is reflected by reductions in the in vivo availability of MOR [nondisplaceable binding potential (BPND)] from fasted to fed conditions, which were then related to changes in affect and weight regain at 1 year after the completion of the VLCD intervention.
Materials and Methods
Subjects
Seven right-handed obese men were recruited from the Investigational Weight Management Clinic at the University of Michigan, and seven right-handed lean men were recruited by advertisement and provided informed consent before participating in the study. The study was approved by the University of Michigan Institutional Review Board and the Radioactive Drug Research Committee (University of Michigan Institutional Review Board: HUM00040929). Subjects were matched for age and ethnicity and excluded if they screened positive for any of the following: history of psychiatric disease, substance abuse, claustrophobia, illicit drug use, alcohol intake within 48 hours prior to scanning, disorders of lipid metabolism, cancer within the last 5 years, solid organ transplants, gastrointestinal disease, renal or hepatic disease, prior bariatric surgery, autoimmune disorders, unstable angina or New York Heart Association class II failure or greater, and absolute girth greater than 53 cm around the widest part of the body while lying down.
Scanning time points
To assess central MOR system response to hunger and feeding in healthy lean (n = 7) and chronically obese men before (n = 7) and after (n = 6) diet-induced weight loss (Table 1), we used [11C]carfentanil positron emission tomography (PET) to measure MOR binding (availability in vivo, BPND). Obese subjects were scanned twice: in the fasted and fed (after a shake consumption) states, prior to weight loss achieved through a VLCD intervention, and again after the intervention. A detailed description of the clinic and VLCD intervention have been described elsewhere (10). Briefly, all patients conformed to an intensive caloric restriction (VLCD) (800 kcal/d) in the form of total meal replacement (HMR). For this group of subjects, an average of 111 ± 20 days (range 89–136) of a VLCD was required to reach the targeted 15% reduction in body weight. Patients were asked to keep daily diaries of consumption behavior including number of shakes/soup consumed, deviation with other foodstuffs, hunger/satiety scales, and physical activity. These diaries were reviewed weekly with the dietitian.
Table 1.
Participant Demographics
| Subjects | Age, y | Weight, kg | BMI |
|---|---|---|---|
| Lean | |||
| Baseline (n = 7) | 52.43 ± 8.98 | 76.10 ± 6.29 | 24.00 ± 1.69 |
| Obese | |||
| Baseline (n = 7) | 51.43 ± 11.18 | 117.20 ± 10.00 | 37.96 ± 3.39 |
| PWL (n = 6) | 54.50 ± 8.41 | 98.22 ± 4.53 | 31.78 ± 1.83 |
| One year (n = 5) | 52.6 ± 7.83 | 98.77 ± 4.83 | 33.00 ± 3.81 |
Four scans were collected for obese individuals: two before weight loss [baseline (BL)-fasted and BL-fed] and two after weight loss before the termination of the VLCD [post weight loss (PWL) fasted and PWL-fed]. Two scans for lean individuals were performed in the fasted and postshake states (BL-fasted and BL-fed, respectively) on the same day. Both PET scans were performed on the same day, given the lack of carryover between scans within the testing day (11) and to reduce travel time and experimental burden for the subjects.
All subjects were instructed not to eat (with the exception of water) after 10:00 pm the night before their scans. On the day of the scan, subjects arrived at the University of Michigan Medical Center at 7:00 am for check-in, drug screen, and measurement of vital signs prior to the PET scans.
Measurements of hunger and affect
Subjects filled out a visual analog scale (VAS) of appetite (12) in the fasted state (before the fasted PET scan) and immediately after their fed scan, which was approximately 100 minutes after the consumption of the standard meal (500 kcal chocolate flavored Ensure Muscle Health; Abbot Laboratories). The Positive and Negative Affect Schedule (13) was administered prior to the fasted scan and after the fed scan.
Neuroimaging
PET scans were acquired with an HR+ scanner (CTI-Siemens) in the three-dimensional mode with septa retracted and with an intrinsic full width at half-maximum resolution of approximately 6 mm in-plane and 5 mm in the z-axis. Participants were positioned in the PET scanner gantry, and two antecubital iv catheters were placed. A light forehead restraint was used to eliminate intrascan movement. [11C]carfentanil was synthesized at high specific activity by the reaction of [11C]methyl iodide and a nonmethyl precursor, as previously described (14, 15). 15 ± 1 mCi (555 ± 55 MBq) was administered to each subject. The total mass of carfentanil injected was mean ± SD 0.028 ± 0.004 μg/kg of body weight per scan to ensure that the compound was administered in tracer quantities (ie, subpharmacological doses). Receptor occupancy at peak carfentanil concentrations was calculated at 0.2%–0.5% for brain regions with low (cerebellum), intermediate (prefrontal cortex), and high (thalamus) μ-opioid receptor concentrations. Fifty percent of the [11C]carfentanil dose was administered as a bolus and the remainder as a continuous infusion by using a computer-controlled pump to more rapidly achieve steady-state tracer levels. For each study, 19 sets of dynamic scans were acquired with an increasing duration (four 30 sec frames, three 1 min frames, two 2.5 min frames, eight 5 min frames, and two 10 min frames) over 70 minutes.
Images were reconstructed using iterative algorithms (brain mode; Fourier rebinning algorithm with ordered subset expectation maximization, four iterations, and 16 subsets; no smoothing) into a 128- × 128-pixel matrix in a 28.8-cm-diameter field of view. Attenuation correction was performed through a 6-minute transmission scan (Ge68 source) obtained before the PET study and with iterative reconstruction of the blank/transmission data, followed by segmentation of the attenuation image. Small head motions during PET were corrected by an automated computer algorithm for each subject before analysis, and the images were coregistered with the same software (16). Time points were then decay corrected during reconstruction of the PET data. Image data were then transformed on a voxel-by-voxel basis, into two sets of parametric maps: 1) a tracer transport measure (K1 ratio) and 2) two receptor-related measures. For all individuals both sets of parametric maps were produced for each scanning condition (ie, fasted or fed) at each time point (ie, baseline and PWL). To avoid the need for arterial blood sampling, these measures were calculated with a modified Logan graphical analysis (17) using the occipital cortex (an area devoid of μ-opioid receptors) as the reference region.
Using the bolus-continuous infusion protocol described above, the slope of the Logan plot becomes linear 5–7 minutes after the tracer administration and is proportional to the receptor concentration divided by its affinity for the radiotracer [BPND + 1, or (f2Bmax/Kd) +1]. Bmax is the receptor concentration and Kd, the receptor-ligand dissociation constant. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value. Reductions in the in vivo availability of receptors, the BPND measure, after an acute challenge (ie, food administration) are thought to reflect processes, such as competition between radiotracer and endogenous ligand and changes in the concentration of membrane bound receptors and their affinity related to endogenous ligand-receptor interactions associated with neurotransmitter release (18, 19). A 1- × 1- × 1-mm three-dimensional T1-weighted MPRAGE structural magnetic resonance imaging was collected for coregistration on each subject using a Philips Ingenia 3T magnetic resonance scanner in the Department of Radiology at the University of Michigan. The K1 and BPND images for each experimental period and spoiled gradient-recalled-echo magnetic resonance images [echo time, 5.5 msec; repetition time, 14 msec; inversion time, 300 msec; flip angle, 20°; number of signals acquired, one; thickness, 1.5 mm] were coregistered to each other and to the International Consortium for Brain Mapping stereotactic atlas orientation (20) after 6- × 6- × 6-mm smoothing.
Statistics
Whole-brain voxel-by-voxel analysis was conducted in SPM8 (Welcome Trust Centre for Neuroimaging, University College London, London, UK). Contrasts for receptor BPND in the fasted state were constructed as an unpaired two-tailed t test for lean-BL greater than obese-BL, and lean-BL greater than obese-PWL. A paired t test was used to assess the changes in receptor BPND for obese-BL greater than obese-PWL, and in the assessment of feeding-induced activation of MOR systems for each group (fasted greater than fed for lean-BL, obese-BL, and obese-PWL). The significance of the whole-brain voxel-by-voxel analyses was set at P = .005, with a minimum cluster size of 10 voxels for this pilot study (Table 2).
Table 2.
Whole-Brain Voxel-by-Voxel Comparisons for Fasted MOR BPND and Feeding-Induced MOR System Activation [%Change: 100% × (Fasted BPND-Fed BPND/Fasted BPND)] in Response to Consumption of a Standard Mildly Palatable Meal
| Region | X, Y, Z, mm | Cluster Size, mm3 | Peak t | Cluster Uncorrected (P Value) | False Discovery Rate (P Value) |
|---|---|---|---|---|---|
| MOR availability in the fasted state | |||||
| Lean-BL > obese-BL | |||||
| Right temporal pole | 28, 14, −38 | 927 | 4.58 | .172 | .982 |
| Left temporal pole | −24, 11, −36 | 279 | 4.08 | .453 | .982 |
| Lean-BL > obese-PWL | |||||
| Right temporal pole | 29, 14, −40 | 747 | 4.27 | .194 | .912 |
| Left frontal pole | −13, 62, 14 | 173 | 4.22 | .539 | .912 |
| Obese-PWL > Obese-BL | |||||
| Left ventral striatum | −8, 7, 0 | 1396 | 5.84 | .012 | .118 |
| Right thalamus | 13, −22, 14 | 458 | 5.25 | .121 | .404 |
| Left temporal pole | −27, 11, −34 | 309 | 5.07 | .197 | .493 |
| Frontal orbital cortex | 8, 24, −22 | 481 | 4.87 | .113 | .404 |
| MOR activation after standard meal (fasted > fed) | |||||
| Lean-BL | |||||
| Right temporal pole/amygdala | 25, 6, −38 | 7687 | 7.63 | .000 | .002 |
| Right frontal pole/oribital frontal cortex | 11, 56, −15 | 8654 | 5.26 | .000 | .001 |
| Right accumbens/ventral striatum | 10, 6, −7 | 2382 | 5.47 | .015 | .148 |
| Left thalamus | −1, −16, 2 | 856 | 5.66 | .119 | .712 |
| Left accumbens/ventral striatum | −15, 5, −7 | 1685 | 6.12 | .035 | .265 |
| Obese-BL | |||||
| Right accumbens/ventral striatum | 17, 19, −4 | 330 | 5.46 | .128 | .769 |
| Left frontal pole | −24, 50, −17 | 637 | 4.42 | .041 | .496 |
| Obese-PWL | |||||
| Right temporal pole/amygdala | 25, −5, −21 | 362 | 5.32 | .152 | .821 |
| Left ventral striatum | −9, 13, 4 | 692 | 4.82 | .055 | .662 |
| Right ventral striatum | 19, 11, −7 | 205 | 4.65 | .276 | .821 |
| Left thalamus | −3, −17, 5 | 100 | 4.56 | .475 | 1.00 |
Threshold of significance for all comparisons was set at P = .005, uncorrected. Bold text indicates statistically significant difference among groups by false discovery rate correction. All voxel-by-voxel comparisons set at P = .005 uncorrected.
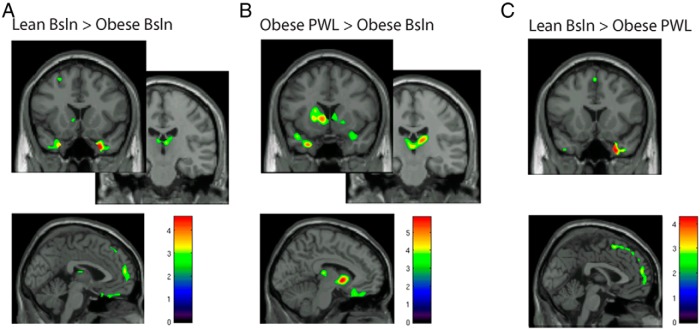
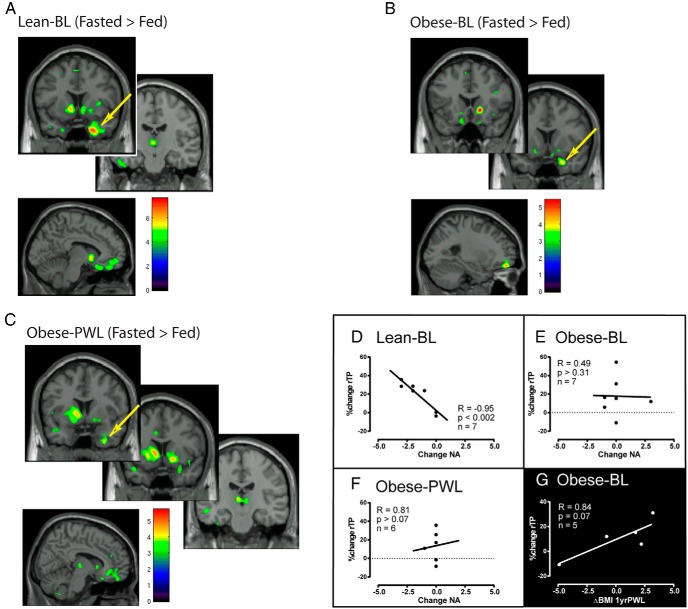
Extracted BPND values were used for the calculation of the following: 1) receptor BPND in the fasted state (Figure 1) and 2) percentage change (%change) in BPND [%change: 100% × (fastedBPND-fedBPND/fastedBPND)] between the fed and fasted state at each time point for each group (Figure 2).
Figure 1.
MOR BPND in the fasted state is greater in lean-BL compared with obese-BL (A). Dietary-induced weight loss increased MOR BPND in the fasted state in obese-PWL (B) and had modest normalizing effects on the difference between lean-BL and obese-PWL (C). Images show areas of significant binding for comparisons using voxel-by-voxel whole-brain analysis with a threshold for significance set at P = .02 for display purposes. Color bars relate to t scores. BSL, baseline.
Figure 2.
Robust MOR system activation in response to a standardized meal was found for lean men in several regions that regulate homeostatic, hedonic, and emotional responses (A). MOR system activation in the right temporal pole (rTP; arrow) was strongly associated with decreases in negative affect (D). Obese-BL showed minimal MOR system activation (B) in response to the standard meal and no association between rTP and negative affect (E). After diet-induced weight loss, obese-PWL exhibited modestly reinvigorated MOR system activation (C) to a standard meal; however, no relationship was found between rTP MOR response and negative affect (F). Obese-BL MOR activation was positively associated with weight regain at 1 year PWL (G). Images show areas of differences between fasted and fed for lean-BL (A) and for obese-BL and PWL (B and C) displayed at minimum thresholds of P = .005 and P = .02, respectively, in SPM8 using voxel-by-voxel whole-brain analyses. Color bars relate to t scores in these analyses.
A one-way ANOVA for self-reported fasting and difference (fed-fasted) scores of the Positive and Negative Affect Schedule and VAS hunger measurements were used to determine the differences among groups (Table 3). For display purposes (Figures 1 and 2), we chose a lower uncorrected threshold (P = .02) to illustrate that regional localization of peaks existed in areas expected to be responsive to meal consumption based on previous reports (7–9, 21). Due to the robust response of leans to a meal, we chose a more stringent threshold for display purposes (Figure 2). Table 2 shows the statistical thresholding used to confer significance, which was set at P = .005 uncorrected. Pearson's correlations were calculated between the changes in hunger, positive affect, and negative affect with %change in MOR BPND in the right temporal pole between the fasted and fed state. Analyses of extracted binding data with self-reported measures of hunger and affect were conducted in SAS version 9.3, and statistical significance was set at α = .05.
Table 3.
Self-Reported Positive/Negative Affect and Hunger-VAS in the Fasted State and the Difference Between Fasted and Fed States (Fed-Fasted)
| Affect and VAS | Lean-BL (n = 7) | Obese-BL (n = 7) | Obese-PWL (n = 6) | F | P Value |
|---|---|---|---|---|---|
| Positive affect | |||||
| Fasted | 33.0 ± 5.4 | 33.0 ± 4.5 | 32.5 ± 7.9 | 0.01 | .9855 |
| Change (fed-fasted) | 1.9 ± 4.3 | −0.9 ± 2.0 | 1.8 ± 5.5 | 0.99 | .3908 |
| Negative affect | |||||
| Fasted | 12.0 ± 1.2 | 10.7 ± 1.1 | 10.2 ± 0.4 | 6.14 | .0099a |
| Change (fed-fasted) | −1.6 ± 1.3 | 0.1 ± 1.3 | −0.2 ± 0.4 | 4.58 | .0256a |
| VAS | |||||
| Hunger | |||||
| Fasted | 51.4 ± 20.2 | 28.9 ± 21.2 | 23.8 ± 14.3 | 3.99 | .0379a |
| Change (fed-fasted) | 6.6 ± 23.0 | −10.0 ± 25.9 | −4.8 ± 9.8 | 1.11 | .3522 |
| Satisfied | |||||
| Fasted | 31.3 ± 14.1 | 19.6 ± 13.4 | 38.5 ± 22.3 | 2.14 | .148 |
| Change (fed-fasted) | −13.1 ± 21.9 | −23.0 ± 16.2 | −13.8 ± 32.2 | 0.37 | .6983 |
| Full | |||||
| Fasted | 17.0 ± 13.7 | 24.6 ± 16.4 | 37.3 ± 12.5 | 3.27 | .063 |
| Change (fed-fasted) | −27.9 ± 25.1 | −25.4 ± 21.8 | −6.2 ± 23.1 | 1.63 | .2246 |
| Can eat | |||||
| Fasted | 57.9 ± 17.4 | 51.3 ± 21.8 | 55.5 ± 18.1 | 0.21 | .8132 |
| Change (fed-fasted) | 6.4 ± 19.7 | −7.6 ± 19.0 | 11.7 ± 18.2 | 1.82 | .1929 |
| Sweet craving | |||||
| Fasted | 42.9 ± 24.3 | 34.4 ± 20.6 | 36.8 ± 26.2 | 0.24 | .7915 |
| Change (fed-fasted) | 7.1 ± 28.8 | 4.3 ± 19.0 | −1.8 ± 11.9 | 0.25 | .7851 |
| Salty craving | |||||
| Fasted | 34.4 ± 12.3 | 25.0 ± 10.4 | 31.7 ± 11.8 | 1.23 | .3159 |
| Change (fed-fasted) | −12.3 ± 23.5 | −13.9 ± 19.4 | −7.0 ± 21.7 | 0.17 | .841 |
| Savory craving | |||||
| Fasted | 52.3 ± 14.3 | 38.6 ± 12.9 | 47.3 ± 22.7 | 1.2 | .3269 |
| Change (fed-fasted) | −2.3 ± 24.9 | −5.7 ± 26.9 | 5.0 ± 15.2 | 0.35 | .78 087 |
| Fatty craving | |||||
| Fasted | 45.9 ± 29.0 | 37.4 ± 12.1 | 25.8 ± 14.9 | 1.57 | .2363 |
| Change (fed-fasted) | 14.4 ± 31.1 | −3.6 ± 24.2 | 3.3 ± 11.2 | 0.99 | .3933 |
Self-reported positive/negative affect and hunger-VAS in the fasted state and the difference between fasted and fed states (fed-fasted). Bold text indicates statistically significant difference among groups.
Lean > Obese-BL and Obese-PWL.
Results
Receptor BPND in the fasted state (baseline)
Using an uncorrected threshold of significance at P = .005 for whole-brain voxel-by-voxel analyses, we found trends for greater MOR BPND after an overnight fast in lean men compared with obese men (Table 2, and Figure 1A) in areas regulating affect and emotion (temporal pole/amygdala). Greater BPND in lean volunteers compared with obese volunteers before weight loss was also apparent in areas that regulate homeostatic control of energy (thalamus) and executive function (prefrontal cortex). Because the central MOR system response to hunger and feeding may be impacted by the pathophysiology induced by chronic obesity, we also sought to determine the impact of weight loss on central MOR system responses to hunger and feeding. Receptor BPND increased in obese men PWL compared with BL in the left temporal pole, ventral striatum, thalamus, and medial frontal cortex (Figure 1B and Table 2). However, receptor BPND in obese men after weight loss had not completely recovered, as evidenced by greater receptor BPND in the right temporal pole and left frontal pole in lean men compared with obese-PWL (Figure 1C and Table 2).
MOR system activation
We also assessed the dynamic activation of the MOR system, assessed by reductions in the BPND measure from fasted to fed states, after the consumption of a standard meal in lean and obese subjects at BL and PWL. A standard mildly palatable meal elicited robust central MOR system activation (fasted-BPND > fed-BPND; Figure 2A and Table 2) in lean men in regions that modulate the homeostatic (thalamus), hedonic (accumbens/ventral striatum), emotional (temporal pole/amygdala and prefrontal cortex), and executive function (frontal pole). In contrast, obese subjects at BL showed modest MOR system activation to a standard meal (Figure 2B and Table 2) in the ventral striatum and left frontal pole. Weight loss enhanced MOR system activation to the standard meal (Figure 2C and Table 2) in the ventral striatum, thalamus, and right temporal pole/amygdala.
Affect and hunger in response to feeding
Negative affect ratings in the fasted state were higher in lean men compared with obese men before or after weight loss (Table 3). Additionally, lean men showed a larger decrease in negative affect after the consumption of the standard meal, whereas obese men showed minimal change in negative affect either before or after weight loss. Under fasted conditions lean individuals reported greater levels of hunger compared with obese before or after weight loss. The change in hunger from the fasted to the fed state was not statistically significant and may be related to individual differences in response to the specific meal as evidenced by the variability in change scores (Table 3).
Association of changes in BPND with affect and weight regain
MOR BPND data in the temporal pole region were extracted for secondary analysis with measures of affect and hunger. In lean individuals the change in MOR BPND from fasted to fed was highly correlated with changes in negative affect (Figure 2D). In contrast, no significant correlation was found between changes in MOR BPND and changes in negative affect in obese individuals either before (Figure 2E) or after weight loss (Figure 2F).
Five obese men completed the PET imaging and returned for a clinical follow-up 1 year after the completion of the VLCD intervention. In these men we found a trend for positive association between MOR activation in response to feeding and change in body mass index (BMI) at 1 year after the completion of the VLCD (Figure 2G). No statistically significant relationships were found between MOR BPND measures and self-reported measures of hunger and craving or between the magnitude of weight loss and weight regain.
Discussion
Here we report that chronically obese men have decreased baseline MOR BPND and smaller activation of this neurotransmitter system in response to a standard meal compared with lean men. We also show that a targeted weight-loss program increases receptor BPND and MOR system activation in obese men but does not completely restore receptor BPND or endogenous MOR system responses to feeding to the level of lean men. We also observed a strong association between meal-related MOR system activation and decreases in negative affect in lean men. This relationship did not exist in obese men before or after weight loss. In addition, we found a trend for an association between feeding-induced MOR system activation before weight loss and weight regain 1 year after the completion of the diet intervention.
μ-Opioid system response to feeding in lean and obese and the impact of weight loss
There were modest differences in the BL MOR BPND in the fasted state between lean and obese subjects before and after weight loss localized mainly in the temporal pole and frontal cortical regions, areas that are involved in the regulation of emotion and executive function, respectively (22, 23). Previous work has identified reduced MOR availability in bulimic women in the temporoinsular cortex (24), an area involved in the interface of interoceptive and emotional processing. A recent cross-sectional study found decreased MOR availability in obese women compared with lean subjects (21). Weight loss in obese subjects appeared to increase receptor BPND in homeostatic (thalamus), hedonic (ventral striatum), emotional (temporal pole), and decision-making (frontal orbital cortex) regions, consistent with the concept that weight loss and/or abstinence from normal dietary patterns restores MOR BPND.
Although some differences in BL receptor BPND were apparent, more substantial differences were found for feeding-induced MOR system activation; however, only two peaks in lean-BL after a meal reached statistical significance after correction for multiple comparisons (Table 2). After the consumption of a standardized and mildly palatable meal, lean subjects showed robust MOR system activation in regions that regulate homeostatic, hedonic, emotional, and executive function. In comparison, prior to weight loss, obese individuals showed modest MOR system activation in regions involved in hedonic and executive function. Greater feeding-induced MOR system activation was found after weight loss in the obese volunteers, suggesting that acute central MOR system response to feeding can be at least partially reestablished through diet-induced weight loss in regions regulating homeostatic, hedonic, and emotional responses to eating. Collectively this suggests that central MOR system responses to hunger and feeding is impaired in the chronically obese and that it is responsive to weight loss.
Endogenous opioid systems regulate various aspects of the energy management chain as detailed by eloquent human and animal studies (for review see reference 8). Central μ-opioid receptor-mediated mechanisms in particular have been implicated in the hedonic responses to food (9), and this is a neurochemical system integrally positioned to mediate overconsumption of palatable foods (8). The relationship between central MOR systems and affective states and stress has been investigated (25, 26); however, this is the first report to discuss the role of central MOR systems in the context of chronic obesity and subsequent weight loss.
The finding that MOR BPND and activation was only partially reversed in obese patients after the dietary weight-loss intervention raises several interesting considerations for the role of this system in obesity and weight loss. The first is that although on a VLCD, obese patients were abstaining from any highly palatable calorically dense food. Chronic consumption of palatable food, or chronic stress (27), may result in the down-regulation of central MORs in line with the reward surfeit model applied to drugs of abuse in relation to dopaminergic (28) and opioid neurotransmission (29, 30). However, the subjects were scanned before transitioning off of the VLCD and therefore should have still been abstaining from consumption of highly palatable food. In this study we used a mildly palatable, standardized meal because endogenous opioid systems modulate the gustatory and sensory aspects of food consumption (31, 32). It is possible that the activation of the MOR system may have been different if subjects were allowed to select a palatable meal of their choice because opioid systems are thought to modulate overconsumption of individually preferred food (33, 34). The lack of a more robust recovery of the central MOR response in obese-PWL may be due to the lingering excess body weight because patients were still obese (BMIPWL = 31.78 ± 1.83) despite significant weight loss. Although reestablishment of MOR availability has been shown in abstaining alcoholics (30), complete recovery of central opioid systems in obesity may require further abstinence from normal dietary patterns for the normalization of MOR system responses. It may also be that a greater amount of time at a decreased body weight is required for full recovery of central MOR systems. The amount of time living with excess body weight for chronically obese may evoke counterregulatory responses of metabolism and hormonal milieu in response to weight loss that mitigate full recovery of MOR function. Additionally, we cannot discount that genetic differences in endogenous MOR-mediated mechanisms may precede the development of obesity acting as a risk factor, although the ability of the MOR system to respond to weight loss in our current study suggests plasticity of this system.
Central opioid response and affect
Given that MOR availability and activation in the temporal pole was identified in all of our analyses and the role this region plays in affective and emotional regulation (23), we extracted binding data from this region for secondary analysis with measures of affect and hunger. With the exception of differences in reported hunger in the fasted state, no significant changes in self-reported hunger or craving were reported for lean or obese men. Furthermore, changes in self-reported hunger and craving were not associated with MOR system activation in response to the test meal. This may be an effect of the test meal and variability inherent in VAS rankings of hunger and craving (12). The capacity for physiological and psychological stimuli to activate the central MOR system has been consistently linked to changes in affect and mood (25, 35) and with a recent report linking MOR availability with affect and pathological eating (21).
We also saw that lean men showed a robust inverse association between the reduction in negative affect after feeding and feeding-induced MOR system activation in the right temporal pole in lean subjects (Figure 2D). In contrast, obese men did not show a relationship between central MOR system activation in response to a meal and changes in affect at BL or PWL (Figure 2, E and F), suggesting that affective response is not linked to MOR system activation in response to a standardized meal in the obese. This may set the stage for compensatory overeating in an attempt to reduce negative affect (36) because mood and affective regulation have been implicated in the efficacy of naltrexone for cessation of smoking (37) and alcohol consumption (38).
We also found a trend indicating an association between MOR system activation for obese-BL and weight regain 1 year PWL (Figure 2G). Because obese do not show a relationship between MOR activation and decreases in negative affect, food, as a coping strategy (39), may not be as effective at decreasing the negative affect as in lean individuals, setting the stage for overconsumption of palatable food in an attempt to reduce negative affect.
Several limitations should be noted in the current study, First is that the small sample size requires lenient statistical thresholding for whole-brain analyses; however, our results are in line with animal and human studies (8, 21). Additionally, we cannot determine whether obese individuals were using food to mitigate stress and reduce negative affect, which subsequently led to obesity, or whether the pathophysiology of chronic obesity altered the relationship between MOR system function and affective changes induced by food. This is the first report linking endogenous central MOR system responses to food with divergent affective changes in lean and obese men, and greater MOR activation before weight loss related to greater weight regain. The relationship between negative affect and feeding-induced MOR system activation is of interest because stress alters food selection (5, 40) and food has the capacity to mitigate stress and produce pleasant feelings (39). Because mood and affective regulation have been implicated in the efficacy of naltrexone for cessation of smoking (37) and alcohol consumption (38), our preliminary findings suggest the MOR system may be a valuable part of targeted interventions for those at risk of recidivism following weight-loss.
Acknowledgments
We thank the technologists in the positron emission tomography center in the Department of Nuclear Medicine and the Department of Radiology at the University of Michigan for their contribution to this work, and we also thank Dr Tiffany Love for her helpful input on the manuscript. We also thank Health Management Resources, Inc (Boston, MA), which generously provided 800 solutions meal replacement.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK092322; the Brain and Behavior Research Foundation National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to P.R.B.); National Institute of Diabetes and Digestive and Kidney Diseases Grants P30DK089503, P30DK092926 (Michigan Center for Diabetes Translational Research), DK089503, DK020572, and National Center for Research Resources Grant UL1RR024986; the Robert C. and Veronica Adkins Foundation (to C.F.B.); the A. Alfred Taubman Medical Institute (to C.F.B. and J.-K.Z.); The Phil Jenkins Foundation (to J.-K.Z.); and Blue Care Network of Michigan.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPND
- nondisplaceable binding potential
- BL
- baseline
- BMI
- body mass index
- %change
- percentage change
- MOR
- μ-opioid receptor
- PET
- positron emission tomography
- PWL
- post weight loss
- VLCD
- very low-calorie diet.
References
- 1. Friedman J. A war on obesity, not the obese. Science (New York, NY). 2003;299:856–858. [DOI] [PubMed] [Google Scholar]
- 2. Larsen J, van Strien T, Eisinga R, Engels R. Gender differences in the association between alexithymia and emotional eating in obese individuals. J Psychosom Res. 2006;60:237–243. [DOI] [PubMed] [Google Scholar]
- 3. Slochower J, Kaplan S, Mann L. The effects of life stress and weight on mood and eating. Appetite. 1981;2:115–125. [DOI] [PubMed] [Google Scholar]
- 4. Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66:511–515. [DOI] [PubMed] [Google Scholar]
- 5. Zellner D, Loaiza S, Gonzalez Z, et al. Food selection changes under stress. Physiol Behav. 2006;87:789–793. [DOI] [PubMed] [Google Scholar]
- 6. Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. [DOI] [PubMed] [Google Scholar]
- 7. Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. [DOI] [PubMed] [Google Scholar]
- 8. Gosnell B, Levine A. Reward systems and food intake: role of opioids. Int J Obes. (2005) 2009;33(suppl 2):8. [DOI] [PubMed] [Google Scholar]
- 9. Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rothberg A, McEwen L, Fraser T, Burant C, Herman W. The impact of a managed care obesity intervention on clinical outcomes and costs: a prospective observational study. Obesity (Silver Spring, Md). 2013;\21(11):2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott D, Stohler C, Koeppe R, Zubieta J-K. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse (New York, NY). 2007;61:707–714. [DOI] [PubMed] [Google Scholar]
- 12. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 13. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personal Soc Psychol. 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 14. Dannals R, Ravert H, Frost J, Wilson A, Burns H, Wagner H. Radiosynthesis of an opiate receptor binding radiotracer: [11C]carfentanil. Int J Appl Radiat Isotopes. 1985;36:303–306. [DOI] [PubMed] [Google Scholar]
- 15. Jewett D. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28:733–734. [DOI] [PubMed] [Google Scholar]
- 16. Minoshima S, Koeppe R, Mintun M, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- 17. Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 18. Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. [DOI] [PubMed] [Google Scholar]
- 19. Quelch DR, Katsouri L, Nutt DJ, Parker CA, Tyacke RJ. Imaging endogenous opioid peptide release with [11C]carfentanil and [3H]diprenorphine: influence of agonist-induced internalization. J Cereb Blood Flow Metab. 2014;34:1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1:195–206. [DOI] [PubMed] [Google Scholar]
- 21. Karlsson HK, Tuominen L, Tuulari JJ, et al. Obesity Is Associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. [DOI] [PubMed] [Google Scholar]
- 23. Olson I, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. [DOI] [PubMed] [Google Scholar]
- 24. Bencherif B, Guarda A, Colantuoni C, Ravert H, Dannals R, Frost J. Regional μ-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–1351. [PubMed] [Google Scholar]
- 25. Zubieta J-K, Ketter T, Bueller J, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. [DOI] [PubMed] [Google Scholar]
- 26. Barfield E, Moser V, Hand A, Grisel J. β-Endorphin modulates the effect of stress on novelty-suppressed feeding. Front Behav Neurosci. 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Umberg E, Pothos E. Neurobiology of aversive states. Physiol Behav. 2011;104:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang G, Volkow N, Fowler J, et al. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. [DOI] [PubMed] [Google Scholar]
- 29. Weerts E, Wand G, Kuwabara H, et al. Positron emission tomography imaging of μ- and δ-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcohol Clin Exp Res. 2011;35:2162–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heinz A, Reimold M, Wrase J, et al. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005;62:57–64. [DOI] [PubMed] [Google Scholar]
- 31. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. [DOI] [PubMed] [Google Scholar]
- 32. Glass M, Billington C, Levine A. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. [DOI] [PubMed] [Google Scholar]
- 33. Gosnell B, Krahn D, Majchrzak M. The effects of morphine on diet selection are dependent upon baseline diet preferences. Pharmacol Biochem Behav. 1990;37:207–212. [DOI] [PubMed] [Google Scholar]
- 34. Glass M, Grace M, Cleary J, Billington C, Levine A. Potency of naloxone's anorectic effect in rats is dependent on diet preference. Am J Physiol. 1996;271:21. [DOI] [PubMed] [Google Scholar]
- 35. Zubieta JK, Smith YR, Bueller JM, et al. Regional μ opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. [DOI] [PubMed] [Google Scholar]
- 36. Koob G, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2005;8:1442–1444. [DOI] [PubMed] [Google Scholar]
- 37. Walsh Z, Epstein A, Munisamy G, King A. The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis. 2008;27:65–72. [DOI] [PubMed] [Google Scholar]
- 38. Kranzler H, Armeli S, Feinn R, Tennen H. Targeted naltrexone treatment moderates the relations between mood and drinking behavior among problem drinkers. J Consult Clin Psychol. 2004;72:317–327. [DOI] [PubMed] [Google Scholar]
- 39. Taut D, Renner B, Baban A. Reappraise the situation but express your emotions: impact of emotion regulation strategies on ad libitum food intake. Front Psychol. 2012;3:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oliver G, Wardle J, Gibson E. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. [DOI] [PubMed] [Google Scholar]