Abstract
Background:
Myocardial steatosis, an independent predictor of diastolic dysfunction, is frequently present in type 2 diabetes mellitus. High free fatty acid flux, hyperglycemia, and hyperinsulinemia may play a role in myocardial steatosis. There are no prior studies examining the relationship between insulin sensitivity (antilipolytic and glucose disposal actions of insulin) and cardiac steatosis.
Objective:
Using a cross-sectional study design of individuals with and without metabolic syndrome (MetSyn), we examined the relationships between cardiac steatosis and the sensitivity of the antilipolytic and glucose disposal actions of insulin.
Methods:
Pericardial fat (PF) volume, intramyocardial and hepatic fat (MF and HF) content, visceral fat (VF) and sc fat content were assessed by magnetic resonance imaging in 77 subjects (49 without MetSyn and 28 with MetSyn). In a subset of the larger cohort (n = 52), peripheral insulin sensitivity index (SI) and adipocyte insulin sensitivity (Adipo-SI) were determined from an insulin-modified frequently sampled iv glucose tolerance test. The Quantitative Insulin Sensitivity Check Index was used as a surrogate for hepatic insulin sensitivity.
Results:
Individuals with the MetSyn had significantly higher body mass index, total body fat, and MF, PF, HF, and VF content. HF and VF, but not MF, were negatively correlated with the Quantitative Insulin Sensitivity Check Index, Adipo-SI, and SI. Stepwise regression revealed that waist circumference and serum triglyceride levels independently predicted MF and PF, respectively. Adipo-SI and serum triglyceride levels independently predict HF.
Conclusion:
Myocardial steatosis is unrelated to hepatic, adipocyte, or peripheral insulin sensitivity. Although it is frequently observed in insulin-resistant subjects, further studies are necessary to identify and delineate pathogenic mechanisms that differentially affect cardiac and hepatic steatosis.
Cardiovascular complications due to hypertensive and ischemic heart disease are a leading cause of morbidity and mortality in type 2 diabetes mellitus (1). Left ventricular diastolic dysfunction, a harbinger of heart failure, is an early abnormality in individuals with glucose intolerance and obesity (2–4). Myocardial steatosis, an independent predictor of diastolic dysfunction (5) is frequently present in metabolic syndrome (MetSyn) and type 2 diabetes mellitus (5–11). However, the relationship between myocardial steatosis and MetSyn has not yet been firmly established.
Increased free fatty acid (FFA) delivery and uptake in excess of FA oxidative capacity leads to accumulation of triglycerides (TGs) and other toxic lipids such as ceramides in the myocardium (12, 13). Albumin-bound circulating FFAs and FFAs resulting from lipoprotein lipase-mediated hydrolysis of lipoproteins are the principal sources of fatty acid in the heart (14). Combined hyperglycemia and hyperinsulinemia acutely increase myocardial TG content (15). High FFA flux secondary to reduced suppression of lipolysis in adipose tissue, elevated hepatic lipoprotein synthesis, hyperglycemia, and hyperinsulinemia are characteristic of insulin resistance (16). These findings suggest a causal role for insulin resistance in cardiac steatosis. A few small studies, mainly in women, have examined the relationship between insulin resistance and cardiac steatosis with equivocal findings (7, 17, 18). Insulin sensitivity in these studies was assessed using less than robust, surrogate fasting or post-oral glucose tolerance test (OGTT)-derived measures of insulin sensitivity that reflect glucoregulatory actions of insulin. There are no prior studies examining the relationship between antilipolytic actions of insulin and cardiac steatosis.
In this study, using a cross-sectional design of individuals with and without MetSyn, we examined the relationships between cardiac, hepatic, muscle, pericardial, and visceral steatosis and insulin sensitivity of both the antilipolytic and glucose disposal actions of insulin. To that end, we show that hepatic but not cardiac steatosis is associated with adipose tissue and peripheral insulin sensitivity.
Subjects and Methods
Study design and subjects
Subjects in this study were part of an ongoing cross-sectional study conducted at the Clinical Research Center, National Institutes of Health (NIH), in Bethesda, Maryland (ClinicalTrials.gov Identifier: NCT00428987). The study protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases, and all procedures followed were in accordance with institutional guidelines. Written informed consent was obtained from all subjects. Participants over 18 years old with body mass index (BMI) > 18.5 kg/m2 and stable weight over the last 3 months were included in the study. The study cohort was classified as subjects with and without MetSyn according to the revised Adult Treatment Panel III (ATPIII) criteria for MetSyn (19). Exclusion criteria were: medical conditions including ischemic coronary heart disease, heart failure, prior cardiac surgery, chronic blood disease, severe respiratory insufficiency requiring oxygen therapy, and/or psychiatric conditions precluding participation in the study; weight > 204 kilograms; or any other contraindications for magnetic resonance (MR) scanning. All subjects had a baseline electrocardiogram, and individualized ramp cycle ergometer maximal stress testing was performed using a rate of load increase (10–25 W/min) adjusted according to the subject's age, sex, and activity level according to the American College of Sports Medicine standards. Fifteen patients received medications for hypertension (angiotensin-converting enzyme inhibitors, 26%; angiotensin receptor blockers, 47%; β-blockers, 20%; and thiazide diuretics, 33%); 25 were on hypolipidemic agents (statins, 80%; fibrates, 4%; and niacin, 16%); and eight subjects with type 2 diabetes mellitus received metformin (88%), insulin (50%), and sulfonylureas (25%). Four women were on oral contraceptives; all of these were in the group without MetSyn.
Study procedures
Assessment of body composition and adipose tissue distribution
Body weight was measured using a digital balance (Scale-Tronix 5702; Scale-Tronix). Body composition was measured by dual-energy x-ray absorptiometry with a Lunar iDXA scanner (GE Healthcare).
MR image and spectroscopy acquisition
Cardiac MR imaging (MRI) and MR spectroscopy (MRS).
Cardiac MRI and 1H-MRS were performed using a wide-bore 3T MR scanner (MAGNETOM Verio; Siemens); a multi-channel TIM phased array coil was used for reception. Breath-hold rapid B0 mapping was used to adjust the B0 field shims (20). For cardiac MRI, retrospective electrocardiogram-gated balanced steady-state free precision cine images of the heart were acquired during breath-hold. In addition to four-chamber views, a stack of short-axis cine series was obtained to cover the entire left ventricle (LV) from the level of the mitral valve, parallel to the atrioventricular groove, to the very end point of cardiac apex with a total of 10–12 axial slices. Imaging parameters were TR/TE 48/1.3 milliseconds, flip angle 37°, matrix 256 × 192, field of view 350 mm, 5-mm slice thickness and interslice gap, and temporal resolution 35–42 milliseconds.
To perform cardiac 1H-MRS, electrocardiogram-gating and respiratory triggering was applied after setting up a breathing navigator on the dome of the diaphragm on a free-breathing scout to correct for respiratory motion. The point resolved spectroscopy (PRESS) (21) sequence was used with TE of 35 milliseconds, TR equal to R-R intervals (no less than 1.24 second), and voxel was localized within the septum at mid-diastolic quiescent period on short axis and four-chamber steady-state free precision cine images. Inversion suppression slabs across sc and pericardial fat (PF) were used to suppress signal outside the heart. The cardiac-gated spectra were collected using a cardiac trigger delay set at about 500–600 milliseconds after R-wave; a 32 averages spectrum was recorded with modest water suppression (WS), followed by another four averages water reference spectrum with the WS RF pulses nulled.
Hepatic MRS.
Hepatic fat content (HFC) was measured with single voxel MRS in an 8-mL volume in the posterior right lobe of the liver as described previously (22). A 16-channel torso coil was used, and Siemens Medical Systems provided the pulse sequences for gradient-recalled echo shimming and navigator-gated PRESS.
Musculoskeletal MRS.
A small four-channel flexible extremity receive coil was used first on the thickest part of the vastus lateralis muscle, then on the anterior tibialis and soleus muscles. The leg was aligned with the magnetic field and centered as much as possible. First, a 3D-GRE B0 field mapping image was recorded at the region of interest with TR/TE 240/2.4 milliseconds, resolution 3.3 × 3.3 × 4.7 mm, and field of view 320 × 320 × 180 mm. The B0 field was optimized with first- and second-order shims for a 150-mm-thick volume just encompassing the leg at the targeted region. In a region free of adipose tissue, a 10 × 10 × 20-mm spectroscopy volume was prescribed on out-of-phase image in the vastus lateralis, about 5 mm under sc fat. First- and second-order shims were adjusted for the size and position of the spectroscopy voxel. Two single voxel PRESS spectra were recorded at a TR/TE = 4000/24 and 1200 Hz bandwidth. One spectrum with WS including a 100-millisecond delay between WS and PRESS excitation and 24 averages was recorded. The second spectrum with the amplitude of the RF pulses of the WS sequence was set to zero and four averages. With the coil placed on the midcalf, the same procedure was used on the tibialis anterior. Finally, the water B0 optimization and recording of the suppressed and nonwater-suppressed spectra were repeated on the soleus muscle.
Visceral fat imaging.
A T1-weighted turbo-spin-echo axial image of the abdomen at the level of L4–L5 was obtained for visceral fat (VF) and sc fat measurement as representatives of total body fat (TBF), recording three signal averages at TR 700, echo train length 8, with effective TE 11 milliseconds, flip angle 120°, matrix 256 × 180, readout field of view 400–500 mm (depending on the subject's girth), and phase field of view 70%.
Image analysis and postprocessing
Cardiac, hepatic, and musculoskeletal TG content.
Spectra were preprocessed and analyzed by time-domain fitting with AMARES (advanced method for accurate, robust and efficient spectral fitting of MRS data) (23) in jMRUI (java MR user interface) (24). The water peaks in non-water-suppressed spectra were set to a reference frequency of 4.7 ppm; in WS spectra, the reference frequency was set to creatine at 3.01 ppm or in liver to choline at 3.24 ppm. Resonances were fitted as Gaussian line shapes. First-order phase was fixed at zero, and zero-order phase was presumed equal for all resonances. A time domain-windowing function was applied to the measured data and model functions to improve fitting of closely spaced resonances (25).
LV morphology and function.
Cine images were transferred to a dedicated postprocessing workstation, and semiautomatic segmentation of the LV was performed using QMASS software (version 7.2; Medis). LV ejection fraction (EF), end diastolic volume (EDV), end systolic volume (ESV), stroke volume (SV), cardiac output (COP), and LV mass (LVM) were estimated. Apart from EF, all variables were indexed to body surface area (BSA) to have EDVI, ESVI, SVI, COPI, and LVMI, respectively.
Measurement of PF volume.
PF volume (PFV) measurement was obtained using Osirx 5.1 software. Contours were manually drawn on every other slice of short axis cine image at end systole and then interpolated to the middle slices. A seed point was manually selected based on signal intensity; three-dimensional region growing was calculated; and finally, each slice was carefully revised and corrected using interactive two-dimensional region growing. PF mass was calculated by multiplying PFV by fat density (0.9196 g/mL).
Subcutaneous and visceral adipose tissue.
Subcutaneous and visceral adipose tissue volumes were assessed using threshold image segmentation AW software (GE Healthcare). Bright pixels corresponding to adipose tissue were defined automatically after setting up an appropriate threshold value. Segmentation was corrected manually if needed, based on visual inspection; the volume of fat depots was found by calculating the number of pixels per square centimeter multiplied by slice thickness.
Measures of insulin sensitivity
The insulin-modified frequently sampled iv glucose tolerance test (FSIVGTT) was performed at 8 am after a 12-hour fast, as previously described (26). Plasma glucose, insulin, C-peptide, and FFA concentrations were measured as previously described (26). The peripheral insulin sensitivity index (SI) was determined from the minimal model using data from the insulin-modified FSIVGTT as previously described (version 6.02; MinMOD Millenium) (26) (purchased from Dr Richard Bergman, Keck School of Medicine of University of Southern California, Los Angeles). The homeostasis model assessment index of insulin resistance (HOMA-IR) and Quantitative Insulin Sensitivity Check Index (QUICKI) were calculated as defined previously from fasting glucose and insulin values (27). QUICKI = 1/(log[I0] + log[G0]), where I0 is fasting insulin (μU/mL) and G0 is fasting glucose (mg/dL). HOMA-IR = G0*I0/22.5, where I0 is fasting insulin (μU/mL) and G0 is fasting glucose (mmol/L). QUICKI and HOMA-IR reflect the measure of hepatic insulin sensitivity/resistance (27). Plasma FFA concentrations decline exponentially during FSIVGTT, and the decay rate is proportional to the increase in insulin concentration. The FFA suppression index (minutes per microunit per milliliter, multiplied by −1 × 105), a measure of adipocyte insulin sensitivity, was expressed as the decay rate of plasma FFA concentrations between 16 and 60 minutes divided by the increment in insulin concentration, as previously described (28, 29). A higher FFA suppression index suggests greater adipocyte insulin sensitivity.
Determination of resting energy expenditure and intake and aerobic fitness
Indirect calorimetry for the determination of resting energy expenditure was performed in the morning after an overnight fast using a metabolic cart (TrueOne 2400 Metabolic Cart; ParvoMedics). Peak VO2 (mL·kg−1·min−1) was determined with a maximal graded exercise test on an upright or recumbent cycle ergometer (Lode 906900 or Lode 929900). Gas exchange and electrocardiogram were measured during the exercise test by a metabolic cart.
Laboratory assays
Routine assays for serum lipids, plasma glucose, insulin, and glycosylated hemoglobin (HbA1c) were performed in the Department of Laboratory Medicine at the Clinical Center, NIH. FFAs were measured with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Wako Chemicals USA, Inc) and run on a COBAS FARA-II analyzer (Roche Diagnostics). Plasma cytokeratin 18 (CK-18) fragment levels were measured by M30-Apoptosense ELISA kit (PEVIVA). N-terminal probrain natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hs-CRP), and soluble intracellular adhesion molecule-1 (sICAM-1) were measured in plasma by ELISA.
Statistical analyses
After testing for normality, data were logarithm-transformed where appropriate. P < .05 was considered to represent statistical significance. Data are presented as means ± SD or median (interquartile range). A mixed model was used to compare groups (with and without MetSyn) after adjusting for potential confounders. Relationships between variables of interest were assessed by linear regression analyses using Pearson's correlation coefficient. Stepwise multiple regression analysis was used to identify independent predictors of tissue-specific fat content. The statistical software JMP, version 8.1 (SAS Institute Inc), was used for data analysis.
Results
Metabolic characteristics and fat distribution in study subjects
We first examined differences in cardiac fat content, LV function, metabolic characteristics, fat distribution, and markers of inflammation and endothelial function in individuals with and without MetSyn. Subjects with the MetSyn were older, hyperglycemic, insulin-resistant, and dyslipidemic (Table 1). Individuals with the MetSyn had higher BMI, TBF, and VF content (Tables 1 and 2). Hepatic fat (HF), but not skeletal muscle intramyocellular (IMCL) content, was higher in MetSyn (Table 2). Consistent with this, serum alanine aminotransferase levels were significantly higher in individuals with MetSyn. However, serum concentrations of the CK-18 fragment, a biomarker of nonalcoholic fatty liver disease, were not different between the groups. sVCAM-1, a marker of endothelial function, and hs-CRP, a marker for inflammation, were not significantly different between the groups (Table 1). In the overall cohort, NT-proBNP levels were higher in women compared to men. Age- and gender-adjusted serum NT-proBNP levels were lower in MetSyn subjects.
Table 1.
Clinical and Metabolic Characteristics of Patients With and Without the MetSyn
| MetSyn Present | MetSyn Absent | P Value | |
|---|---|---|---|
| n | 28 | 49 | |
| Age, y | 57.1 ± 8.4 | 47.4 ± 13 | .001 |
| Female, % (n) | 43 (12) | 59 (29) | |
| History of type 2 diabetes, % (n) | 29 (8) | 0 (0) | |
| History of IFG, % (n) | 32 (9) | 4 (2) | |
| History of hypertension, % (n) | 43 (12) | 6 (3) | |
| History of smoking, % (n) | 11 (3) | 16 (8) | |
| Maximal oxygen consumption (VO2 max), mL·kg−1·min−1 | 19.6 ± 8.5 | 23.4 ± 9.3 | .003 |
| Resting energy expenditure, kcal/d | 1725 ± 322 | 1632 ± 360 | .03 |
| Systolic BP, mm Hg | 124 ± 11 | 123 ± 12 | .57 |
| Diastolic BP, mm Hg | 72 ± 8 | 71 ± 7 | .55 |
| Mean arterial BP, mm Hg | 90 ± 8 | 88 ± 7 | .49 |
| BMI, kg/m2 | 31 (29, 38) | 28 (24, 37) | <.001 |
| WC, cm | 106 ± 16 | 94 ± 16 | .003 |
| TBF, % | 41 (32, 47) | 33 (27, 47) | <.001 |
| Metabolic parameters | |||
| Fasting glucose, mg/dL | 110 ± 27 | 89 ± 7 | <.001 |
| Fasting insulin, μU/mL | 14 (7, 22) | 5 (2,10) | <.001 |
| HbA1c, % | 5.9 ± 0.7 | 5.5 ± 0.4 | <.001 |
| QUICKI | 0.315 ± 0.052 | 0.382 ± 0.052 | <.001 |
| ALT, U/L | 44 ± 22 | 31 ± 9 | .01 |
| AST, U/L | 25 ± 13 | 21 ± 6 | .59 |
| Lipids | |||
| Total cholesterol, mg/dL | 183 ± 37 | 180 ± 36 | .76 |
| LDL, mg/dL | 105 ± 35 | 100 ± 34 | .80 |
| HDL, mg/dL | 43 ± 10 | 60 ± 18 | <.001 |
| TG, mg/dL | 180 ± 91 | 88 ± 53 | <.001 |
| FFA, μEq/L | 633 ± 315 | 598 ± 252 | .61 |
| Inflammation and endothelial function biomarkers | |||
| hs-CRP, μg/mL | 2.75 ± 2.76 | 3.49 ± 5.71 | .51 |
| CK-18, U/L | 194 ± 153 | 164 ± 73 | .35 |
| sICAM-1, μg/mL | 0.289 ± 0.077 | 0.254 ± 0.061 | .007 |
| sVCAM, μg/mL | 0.427 ± 0.133 | 0.403 ± 0.1 | .25 |
| NT-proBNP, pg/mL | 157 ± 124 | 254 ± 367 | .20 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; IFG, impaired fasting glucose; sVCAM, soluble vascular cell adhesion molecule-1. Data are presented as mean ± SD or median (interquartile range) as appropriate. Some comparisons used log-transformed data. Comparisons are adjusted for age, race, and gender.
Table 2.
Fat Distribution and Cardiac Function in Patients With and Without the MetSyn
| MetSyn Present | MetSyn Absent | P Value | |
|---|---|---|---|
| n | 28 | 49 | |
| Cardiac fat | |||
| Intramyocardial fat, % | 0.953 ± 0.651 | 0.602 ± 0.328 | .04 |
| PF, cm3 | 193 ± 85 | 108 ± 52 | <.0001 |
| Abdominal fat distribution | |||
| Total fat volume, cm3 | 708 ± 287 | 525 ± 260 | <.001 |
| Subcutaneous fat volume, cm3 | 454 (378, 696) | 350 (277, 584) | .04 |
| VF volume, cm3 | 169 (101, 240) | 58 (38, 94) | <.001 |
| HF, % | 6.97 ± 6.63 | 2.49 ± 4.56 | .001 |
| Musculoskeletal fat, arbitrary units | 2.103 ± 1.825 | 2.367 ± 4.012 | .07 |
| LV function | |||
| EDVI | 54 ± 17 | 63 ± 12 | <.001 |
| ESVI | 21 ± 8 | 25 ± 7 | .003 |
| EF, % | 62 ± 6 | 62 ± 6 | .84 |
| SVI | 34 ± 13 | 38 ± 7 | <.001 |
| LVMI | 51 ± 11 | 56 ± 11 | .15 |
Data are expressed as mean ± SD or median (interquartile range), as appropriate. Some comparisons used log-transformed data. Comparisons are adjusted for age and gender.
Cardiac fat and LV function of study subjects
Myocardial fat content (MFC) and PFV were higher in MetSyn patients (Table 2). BSA-indexed ESV (ESVI) and EDV (EDVI) were lower in MetSyn compared with the control group (Table 2). LV EF and LVM indexed to BSA (LVMI) did not differ between the groups. Although 43% of the subjects with MetSyn had a history of hypertension and/or were on antihypertensive medications, systolic blood pressure (BP), diastolic BP, and mean arterial BP were not significantly different between the groups (Table 1). There were no significant relationships between measures of LV function and MFC or PFV (Supplemental Table 1). The significant differences in cardiac fat content and parameters of LV function persisted even after adjustment for the use of medications for dyslipidemia, hypertension, and diabetes. In addition, the differences in outcomes (fat distribution and cardiac function parameters) listed in Table 2 remain significant (except for intramyocardial fat, P = .09), even after excluding the diabetic subjects.
Relationships between cardiac, hepatic, visceral, and skeletal muscle fat contents and metabolic parameters
Univariate correlational analysis is presented in Supplemental Table 1. BMI and waist circumference (WC) were positively related to all fat depots. TBF was positively associated with all of the fat depots except MFC. Serum FFA levels were unrelated to tissue-specific fat content. However, serum TG levels were positively related to PF, HFC, and VF but not MFC or IMCL. HbA1c was positively correlated with HFC, IMCL, and VF, but not MFC or PF. QUICKI, a surrogate marker for insulin sensitivity, was negatively associated with all the fat depots except MFC. CK-18 levels positively correlated with HFC but not the other fat depots.
Stepwise regression revealed that WC independently predicted MFC and IMCL. WC and serum TG levels were independent predictors of HFC and VF, whereas serum TG levels alone predicted PF.
Relationships between cardiac fat and measures of insulin sensitivity
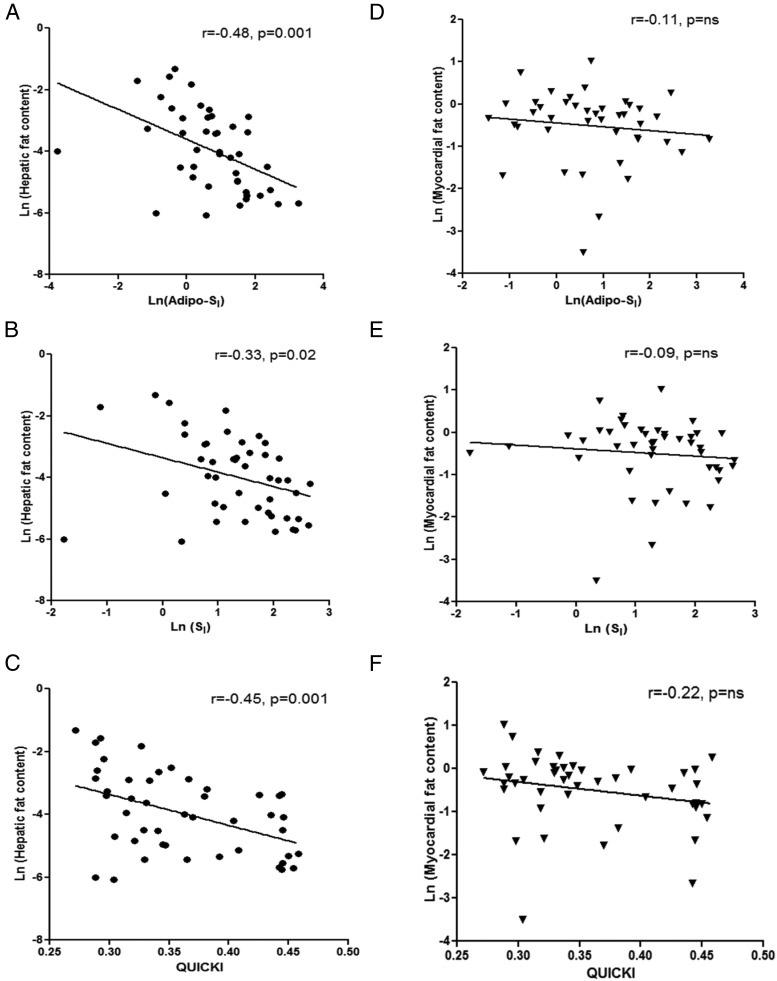
Insulin-modified FSIVGTT was performed on a smaller subset of the cohort (n = 50) with no diabetic subjects. The clinical characteristics of this subset of subjects are shown in Supplemental Table 2. Hepatic, adipocyte, and peripheral insulin sensitivity was assessed by QUICKI, FFA suppression index, and SI, respectively. HFC, IMCL, and VF were negatively related to insulin sensitivity (QUICKI, FFA suppression index, and SI) (Figure 1) (Supplemental Table 3). Similarly, PF tended to negatively correlate with insulin sensitivity (P = .08 to .11). However, MFC was unrelated to QUICKI, FFA suppression index, or SI. These relationships were not affected by smoking status (interaction between the respective insulin SI and smoking status of P > .10).
Figure 1.
Linear correlations between indices of insulin sensitivity (Adipo-SI, SI, and QUICKI), HF (A–C), and myocardial fat (D–F). Pearson correlation coefficient (r) and corresponding P values are shown.
Discussion
The relationship between myocardial steatosis and measures of insulin sensitivity has not been thoroughly investigated. The results of our study suggest that myocardial steatosis is unrelated to hepatic, adipocyte, or peripheral insulin sensitivity. In contrast, the PF depot, similar to fat content in liver, skeletal muscle, and visceral adipose tissue, is negatively related to insulin sensitivity.
We first examined the differences in fat content in various tissue depots using MRS in a larger cohort (n = 77) of subjects with and without MetSyn. As expected, individuals with MetSyn were insulin-resistant and dyslipidemic. In addition to patients having a higher BMI and TBF, hepatic steatosis and visceral adiposity was prominent in MetSyn. Patients with MetSyn had lower cardiovascular fitness and elevated markers of endothelial dysfunction. Thus, our cohort of subjects with MetSyn showed typical characteristics of the syndrome. MFC and PFV were approximately 1.5- to 1.8-fold higher in MetSyn. Although within normal limits, both EDVI and ESVI were reduced in MetSyn. In contrast to other tissue-specific fat depots, the striking finding in our analyses was the lack of a relationship of MFC to various metabolic parameters. Univariate relationships between individual fat depots (HF, VF, IMCL, and PF) and metabolic measures (TG, HbA1c, and QUICKI) were similar in magnitude, whereas MFC has no significant relationship to any of the metabolic parameters. Likewise, except for MFC, all other fat depots are inter-related. Stepwise regression analyses suggest that plasma TG levels independently predict PF, HF, and VF, but not MFC. Lower aerobic fitness was a strong independent predictor of MFC in nonobese individuals (30). In contrast, in our study, aerobic fitness as expected was negatively associated with VF and IMCL content but not MFC.
Our results are consistent with prior reports of elevated MFC and PF in MetSyn (6, 31). Our cohort was older (mean age, 57 y), included women (53%), and was ethnically diverse. In the study by Gaborit et al (31), which included both men and women, PF, but not MFC, was related to fasting glucose, insulin, and HOMA-IR—a finding similar to our study. Likewise, TG levels were strongly correlated with PF but not MFC in that study. These reports together with our findings suggest that: 1) mechanisms mediating intramyocardial fat accumulation may be different than fat accretion in other tissues such as liver and skeletal muscle; and 2) factors modulating hepatic steatosis and visceral adiposity, such as plasma TG, also affect PF depot but not MFC.
Elegant studies in rodents have contributed to much of the current understanding of the mechanisms mediating myocardial steatosis. Oxidative metabolism of glucose and FFA is the primary source for ATP in cardiomyocytes. However, in states of insulin resistance such as obesity and type 2 diabetes mellitus, increased uptake of FFA in excess of the mitochondrial oxidative capacity has been proposed as a primary mechanism leading to cardiac steatosis (32, 33). Cardiac-specific overexpression of genes involved in FFA metabolism such as lipoprotein lipase and fatty acid transporters leads to lipid accumulation in cardiomyocytes (34, 35). These studies suggest that increased FFA delivery and uptake by cardiomyocytes play a role in cardiac steatosis, at least in rodents. In insulin-resistant states, impaired insulin suppression of lipolysis is a major abnormality in FFA metabolism (36).
We used the FFA suppression index measured during FSIVGTT as a measure of adipocyte insulin sensitivity. Impaired insulin-mediated suppression of FFA leads to “spillover” of fat from adipose tissue to liver, resulting in hepatic steatosis (37). As demonstrated in other studies, adipose insulin sensitivity was negatively associated with hepatic (38) and VF content (39). However, the novel finding from our study is that MFC was unrelated to adipose tissue insulin sensitivity, unlike other sites of fat deposition. In contrast, PF tended to negatively correlate with FFA suppression index. These results suggest that in insulin-resistant states, mechanisms mediating PF accretion may be similar to ectopic fat accumulation in liver and visceral adiposity. Increased hepatic FFA delivery due to inadequate insulin-mediated suppression of FFA contributes to increased TG production. Consistent with this, plasma TG levels were negatively associated with adipocyte insulin sensitivity and positively related with HF and VF. However, plasma TGs were unrelated to MFC. Thus, it appears that adipocyte insulin resistance and FFA/TG levels may not play a major role in cardiac steatosis in humans.
In our study, QUICKI and SI, measures of hepatic and peripheral insulin sensitivity, respectively, were unrelated to MFC. Few studies have systematically examined the relationship between insulin sensitivity/resistance and MFC. Utz et al (17), using a composite insulin SI derived from OGTT in 29 women, reported a higher MFC in insulin-resistant women. However, other smaller studies in women using insulin sensitivity indices derived from OGTT report no relationship between insulin sensitivity and MFC (18, 40). Using a more robust measure of insulin sensitivity, in a larger, ethnically diverse study population that includes men and women, we show that insulin sensitivity as it relates to glucose disposal is negatively related to HF, IMCL, and VF, but not to MFC. These studies together suggest that insulin resistance is not an independent determinant of myocardial steatosis in men and women.
Our study has several strengths and limitations. We used robust measures of insulin sensitivity to examine the relationship between antilipolytic and glucoregulatory actions of insulin and cardiac steatosis in a diverse cohort. Concurrent measurement of fat content in liver, skeletal muscle, visceral tissue, and pericardium has provided novel insights in the differential associations of tissue-specific fat and insulin sensitivity. Additionally, the use of higher magnetic field (3T) and advanced shimming tools provided robust and sensitive MRS methods for noninvasive in vivo fat measurements. Furthermore, the use of a large-bore MR scanner facilitated the ability to successfully perform such measurements simultaneously in various organs and a wide range of BMIs. Among the limitations, the cross-sectional nature of the study precludes any causal inferences related to the pathogenesis of myocardial steatosis. Although our study sample size is larger than previous studies, these findings need confirmation in a larger cohort. Hyperinsulinemic euglycemic clamp is the “gold standard” to directly assess insulin sensitivity; however, it is laborious and time consuming, limiting its feasibility in large cross-sectional studies. Albeit an indirect measure of insulin sensitivity, SI derived from FSIVGTT has been used in large epidemiological studies (27, 41). Finally, selection of our cohort is based on criteria (ATPIII) for MetSyn, and thus the group by definition is heterogeneous and includes individuals with type 2 diabetes. The definition of metabolic “syndrome” is a subject of much controversy (42). Although, MetSyn describes a set of premorbid conditions, it appears that insulin resistance is a significant link between the components of the MetSyn. However, our study design precludes us from assessing independent effects of hyperglycemia, dyslipidemia, and hypertension on MFC. Nonetheless, it is interesting that MFC was unrelated to insulin sensitivity, even in a heterogeneous cohort that included insulin-resistant patients with diabetes, dyslipidemia, and hypertension.
In summary, we report that in contrast to hepatic steatosis, myocardial steatosis is unrelated to antilipolytic and glucoregulatory actions of insulin in humans without existing cardiovascular disease. Additional studies are necessary to identify and delineate pathogenic mechanisms that differentially affect cardiac and hepatic steatosis.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Trial Registration: Clinicaltrials.gov NCT00428987.
Disclosure Summary: All authors state that they have no conflicts of interest.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- BSA
- body surface area
- CK-18
- cytokeratin 18
- COP
- cardiac output
- EDV
- end diastolic volume
- EDVI
- EDV index
- EF
- ejection fraction
- ESV
- end systolic volume
- ESVI
- ESV index
- FFA
- free fatty acid
- FSIVGTT
- frequently sampled iv glucose tolerance test
- HbA1c
- glycosylated hemoglobin
- HF
- hepatic fat
- HFC
- HF content
- HOMA-IR
- homeostasis model assessment index of insulin resistance
- hs-CRP
- high-sensitivity C-reactive protein
- IMCL
- intramyocellular
- LV
- left ventricle
- LVM
- LV mass
- LVMI
- LVM index
- MetSyn
- metabolic syndrome
- MFC
- myocardial fat content
- MR
- magnetic resonance
- MRI
- MR imaging
- MRS
- MR spectroscopy
- NT-proBNP
- N-terminal probrain natriuretic peptide
- OGTT
- oral glucose tolerance test
- PF
- pericardial fat
- PFV
- PF volume
- PRESS
- point resolved spectroscopy
- QUICKI
- Quantitative Insulin Sensitivity Check Index
- SI
- sensitivity index
- sICAM-1
- soluble intracellular adhesion molecule-1
- SVI
- stroke volume index
- TBF
- total body fat
- TG
- triglyceride
- VF
- visceral fat
- WC
- waist circumference
- WS
- water suppression.
References
- 1. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 2. Cosson S, Kevorkian JP. Left ventricular diastolic dysfunction: an early sign of diabetic cardiomyopathy? Diabetes Metab. 2003;29:455–466. [DOI] [PubMed] [Google Scholar]
- 3. Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. [DOI] [PubMed] [Google Scholar]
- 4. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28:612–616. [DOI] [PubMed] [Google Scholar]
- 5. Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. [DOI] [PubMed] [Google Scholar]
- 6. Granér M, Siren R, Nyman K, et al. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98:1189–1197. [DOI] [PubMed] [Google Scholar]
- 7. Iozzo P, Lautamaki R, Borra R, et al. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94:4472–4482. [DOI] [PubMed] [Google Scholar]
- 8. Kankaanpää M, Lehto HR, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–4695. [DOI] [PubMed] [Google Scholar]
- 9. McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. [DOI] [PubMed] [Google Scholar]
- 10. McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. [DOI] [PubMed] [Google Scholar]
- 11. Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. [DOI] [PubMed] [Google Scholar]
- 12. Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34(suppl 2):S371–S379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–347. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winhofer Y, Krssák M, Jankovic D, et al. Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes. 2012;61:1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368:1–19. [DOI] [PubMed] [Google Scholar]
- 17. Utz W, Engeli S, Haufe S, et al. Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart. 2011;97:1585–1589. [DOI] [PubMed] [Google Scholar]
- 18. Krššák M, Winhofer Y, Göbl C, et al. Insulin resistance is not associated with myocardial steatosis in women. Diabetologia. 2011;54:1871–1878. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 20. Shah S, Kellman P, Greiser A, Weale PJ, Zuehlsdorff S, Jerecic R. Rapid fieldmap estimation for cardiac shimming. Proc Intl Soc Mag Reson Med. 2009;17:565. [Google Scholar]
- 21. Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci. 1987;508:333–348. [DOI] [PubMed] [Google Scholar]
- 22. Ouwerkerk R, Pettigrew RI, Gharib AM. Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology. 2012;265:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. [DOI] [PubMed] [Google Scholar]
- 24. Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12:141–152. [DOI] [PubMed] [Google Scholar]
- 25. Vanhamme L, Sundin T, Van Hecke P, Van Huffel S, Pintelon R. Frequency-selective quantification of biomedical magnetic resonance spectroscopy data. J Magn Reson. 2000;143:1–16. [DOI] [PubMed] [Google Scholar]
- 26. Muniyappa R, Sachdev V, Sidenko S, et al. Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am J Physiol Endocrinol Metab. 2012;302:E218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. [DOI] [PubMed] [Google Scholar]
- 28. Zoratti R, Godsland IF, Chaturvedi N, et al. Relation of plasma lipids to insulin resistance, nonesterified fatty acid levels, and body fat in men from three ethnic groups: relevance to variation in risk of diabetes and coronary disease. Metabolism. 2000;49:245–252. [DOI] [PubMed] [Google Scholar]
- 29. Mehta SR, Godsland IF, Thomas EL, et al. Intrahepatic insulin exposure, intrahepatocellular lipid and regional body fat in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2012;97:2151–2159. [DOI] [PubMed] [Google Scholar]
- 30. Sarma S, Carrick-Ranson G, Fujimoto N, et al. Effects of age and aerobic fitness on myocardial lipid content. Circ Cardiovasc Imaging. 2013;6:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaborit B, Kober F, Jacquier A, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes (Lond). 2012;36:422–430. [DOI] [PubMed] [Google Scholar]
- 32. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 33. Leichman JG, Lavis VR, Aguilar D, Wilson CR, Taegtmeyer H. The metabolic syndrome and the heart–a considered opinion. Clin Res Cardiol. 2006;95(suppl 1):i134–i141. [DOI] [PubMed] [Google Scholar]
- 34. Duncan JG, Bharadwaj KG, Fong JL, et al. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-α transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-α activators. Circulation. 2010;121:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. [DOI] [PubMed] [Google Scholar]
- 36. Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. [DOI] [PubMed] [Google Scholar]
- 39. Bush NC, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Insulin-mediated FFA suppression is associated with triglyceridemia and insulin sensitivity independent of adiposity. J Clin Endocrinol Metab. 2012;97:4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winhofer Y, Krššák M, Wolf P, et al. Hepatic rather than cardiac steatosis relates to glucose intolerance in women with prior gestational diabetes. PLoS One. 2014;9:e91607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Howard G, O'Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. [DOI] [PubMed] [Google Scholar]
- 42. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]