Abstract
Context:
Minority communities are disproportionately affected by diabetes, and minority women are at an increased risk for glucose intolerance (dysglycemia) during pregnancy.
Objectives:
In pregnant American Indian women, the objectives of the study were to use current criteria to estimate the prevalence of first-trimester (Tr1) dysglycemia and second-trimester (Tr2) incidence of gestational diabetes mellitus (GDM) and to explore new candidate measures and identify associated clinical factors.
Design:
This was a prospective cohort study. In Tr1 we performed a 75-g, 2-hour oral glucose tolerance test (OGTT) and glycated hemoglobin (HbA1c) to determine the following: fasting insulin; homeostasis model assessment of insulin resistance; serum 1,5-anhydroglucitol; noninvasive skin autofluorescence (SCOUT). We defined dysglycemia by American Diabetes Association and Endocrine Society criteria and as HbA1c of 5.7% or greater. In Tr2 in an available subset, we performed a repeat OGTT and SCOUT.
Participants:
Pregnant American Indian women (n = 244 at Tr1; n = 114 at Tr2) participated in the study.
Outcomes:
The prevalence of dysglycemia at Tr1 and incidence of GDM at Tr2 were measured.
Results:
At Tr1, one woman had overt diabetes; 36 (15%) had impaired glucose tolerance (American Diabetes Association criteria and/or abnormal HbA1c) and 59 (24%) had GDM-Tr1 (Endocrine Society criteria). Overall, 74 (30%) had some form of dysglycemia. Associated factors were body mass index, hypertension, waist/hip circumferences, SCOUT score, fasting insulin, and homeostasis model assessment of insulin resistance. At Tr2, 114 of the Tr1 cohort underwent a repeat OGTT and SCOUT, and 26 (23%) had GDM. GDM-Tr2 was associated with increased SCOUT scores (P = .029) and Tr1 body mass index, waist/hip circumferences, diastolic blood pressure, fasting insulin, and triglyceride levels. Overall, dysglycemia at Tr1 and/or Tr2 affected 38% of the women.
Conclusions:
Dysglycemia at some point during pregnancy was common among American Indian women. It was associated with features of insulin resistance and may confer long-term health risks for mother and child.
Type 2 diabetes (T2DM) has reached epidemic proportions in the United States and disproportionately affects some minority populations. For instance, in Oklahoma, 16.2% of American Indian adults have T2DM compared with 8.4% of Caucasians (1). Similarly, the prevalence of gestational diabetes mellitus (GDM), a risk factor for type 2 diabetes and cardiovascular disease (2), continues to increase. Many women have multiple risk factors for gestational dysglycemia (defined here as any degree of abnormal glucose tolerance during pregnancy), including obesity, susceptible ethnic background (eg, American Indian), and a family history of diabetes. Because dysglycemia is usually asymptomatic, it may be undetected in many women throughout some or all of gestation. In addition, the timing of testing (early vs late pregnancy) and the criteria for diagnosing dysglycemia remain highly controversial. The lack of consensus, and the assortment of currently available diagnostic criteria, make it extremely difficult to compare incidence/prevalence among populations, and the true size of the problem and its implications among minority women remain unknown (3).
Currently, common obstetric practice is to screen for GDM at 24–28 weeks' gestation using a 1-hour, nonfasting, 50-g oral glucose tolerance test (OGTT) and then to confirm or refute abnormal values using a formal, 3-hour, 100-g OGTT (4). This protocol is based on the original recommendations of O'Sullivan and Mahan in 1964 (5), reaffirmed in 1981 (6). However, the American Diabetes Association (ADA) now recommends first-trimester screening for T2DM in women with a body mass index (BMI) of 25 kg/m2 or greater and one or more additional risk factors for diabetes (such as physical inactivity, family history of T2DM, prior GDM, or large for gestational age infant, dyslipidemia, personal history of polycystic ovarian syndrome etc). This screening is recommended at the first antenatal visit, using either a fasting blood glucose or a 75-g OGTT with standard, nonpregnant criteria (7). Given the prevalence of overweight and obesity in the United States, this recommendation covers a large proportion of women of child-bearing age. In women without overt T2DM at the first-trimester testing, the guidelines recommend the repetition of the 75-g OGTT during the second trimester, with different, outcome-based cut points to define subsequent GDM (7, 8).
Whereas the ADA recommends the use of glycated hemoglobin (HbA1c) for prediabetes screening in nonpregnant adults (7), it is somewhat unclear in the guidelines whether HbA1c should be used to diagnose prediabetes or impaired glucose tolerance (IGT) at the first trimester. For the purpose of the present study, we included an abnormal first-trimester HbA1c (≥5.7%) within our broad definition of dysglycemia. In addition, it is unclear how pregnant women with mild dysglycemia or IGT should be approached clinically. For the purpose of this study, which was initiated prior to the publication of the current Endocrine Society guidelines (9), we assessed all available nondiabetic women [by first-trimester (Tr1) screening] at the second trimester for incident GDM. The Endocrine Society guidelines recommend universal screening for GDM in the first trimester using fasting blood glucose only and, if negative [ie, fasting blood glucose (BG) < 5.1 mmol/L], following in the second trimester with a 75-g OGTT, similar to ADA/International Association of Diabetes and Pregnancy Study Groups (IADPSG) (9). They recommend using the same second-trimester cut points as the ADA, but they define an additional category of overt diabetes in higher, diabetic-range blood glucose categories (fasting BG ≥ 7.0 mmol/L and/or 2 h BG ≥ 11.1 mmol/L) (9). As aforementioned, our study was conducted prior to these guidelines being published, and our analyses based on those diagnostic criteria were added for informative purposes.
In this study, we screened universally for dysglycemia at the first trimester of pregnancy using a 75-g, 2-hour OGTT and HbA1c (with diagnostic thresholds as defined above) while assessing the utility of serum 1,5-anhydroglucitol (1,5-AG) [an index of short term antecedent glycemia (10, 11)] and skin autofluorescence (SCOUT), a noninvasive measure of skin autofluorescence [which correlates with skin content of advanced glycation end-products (AGEs) (12–15)], a marker of abnormal glucose metabolism. We also evaluated an insulin resistance index[homeostasis model assessment of insulin resistance (HOMA-IR)] as a potential first-trimester predictor of future dysglycemia. During the second trimester, we rescreened all available women from the first-trimester cohort to define the incidence of second trimester GDM (GDM-Tr2) by ADA/IADPSG/Endocrine Society criteria (9).
Materials and Methods
Study methods and subjects
This is a prospective, cohort study among pregnant American Indian women. Participants were registered tribal members recruited at the Choctaw Nation Health Services Authority Obstetric Clinic (Talihina, Oklahoma). Institutional Review Boards at the University of Oklahoma and the Choctaw Nation approved the study prior to its initiation. Participants were American Indian pregnant women (≤13 gestational weeks of pregnancy). Exclusion criteria included the following: any personal history of type 1 or type 2 diabetes; more than 13 gestational weeks of pregnancy; any factor that could limit research protocol adherence such as severe morning sickness, severe chronic illness, renal impairment (serum creatinine > 132 μmol/L), significant cardiac or hepatic impairment, cancer, history of blood transfusion within 3 months, and hemoglobinopathies.
When the study was initially designed, the target sample size was 450 participants, which would result in an estimated margin of error of ±3.3% for the prevalence of dysglycemia in the first trimester, assuming the true prevalence is 15% and a two-sided .05 α-level, and to within ±2.8% assuming the true prevalence is 10%. With a sample size of 250 (reduced due to budget limitations), the estimated margin of error is ±4.4%, assuming the true prevalence is 15%, and is ±3.7%, assuming the true prevalence is 10%.
Patient demographics, prepregnancy weight, medical history and concomitant medication use, smoking before and during pregnancy, physical activity, and family history of diabetes were recorded. Prior adverse pregnancy events were noted, including the occurrence of dystocia and preeclampsia. Current weight and height and waist and hip circumferences were measured and recorded at the first trimester visit along with systolic and diastolic blood pressures. At delivery, birth weight, mode and timing of delivery, the baby's Apgar score and any neonatal complications (eg, birth injury, hypoglycemia, hyperbilirubinemia, congenital malformations, preeclampsia/eclampsia, and stillbirth) were recorded.
After initial screening, 244 participants returned for the first-trimester OGTT, performed after an 8- to 12-hour fast and preceded by 3 days on a high-carbohydrate diet (>150 g/d) with regular physical activity. A fasting venous blood sample was obtained, and then the participant drank a solution containing 75 g of anhydrous glucose, followed by a 2-hour glucose measurement. Any nausea or vomiting was recorded, and if the participant vomited the glucose solution, testing was aborted. Any woman with a first-trimester fasting blood glucose of 7 mmol/L or greater and/or a 2-hour blood glucose of 11.1 mmol/L or greater was diagnosed with overt diabetes mellitus per ADA and Endocrine Society guidelines (7, 9), referred for treatment, and excluded from second-trimester testing. Any woman with a fasting BG of 5.5–6.9 mmol/L and/or a 2-hour glucose of 7.8–11 mmol/L was considered dysglycemic and classified as having IGT or prediabetes (7) per ADA standards. We also defined women with an HbA1c of 5.7%–6.4% as dysglycemic. Furthermore, we later added the new Endocrine Society guidelines (9) to define first-trimester GDM (GDM-Tr1) if the fasting blood glucose was 5.1–6.9 mmol/L and reanalyzed our data for comparative purposes between the ADA and Endocrine Society criteria. We also measured fasting insulin, lipids, and HbA1c at the first-trimester visit. No active intervention (dietary or pharmacological) was implemented prior to the second-trimester visit, and unless specifically diagnosed with diabetes and excluded from the study, participants were blinded to the results. Hence, no change in diet or level of activity was implemented as part of our protocol. In an available subset (n = 114), in whom overt T2DM had been excluded at the first trimester, a repeat 75-g OGTT was performed at 24–28 weeks with the addition of a 1-hour glucose measurement. Cut points for a positive diagnosis were as stipulated by ADA/IADPSG standards [later endorsed by The Endocrine Society (9)]. Women were considered to have GDM-Tr2 if they had any of the following: fasting BG of 5.1 mmol/L or greater, 1-hour BG of 10.0 mmol/L or greater, or 2-hour BG of 8.5 mmol/ L or greater (7).
Measurements
HbA1c was measured at the first trimester only using clinical laboratory-based HPLC in a Clinical Laboratory Improvement Amendments-certified laboratory, using a Diabetes Control and Complications Trial-standardized reference assay (7). This measurement was not repeated at the second trimester visit.
Serum 1,5-AG was measured at the first trimester using a colorimetric assay (GlycoMark; Toyota Tsusho America, Inc) on a Roche autoanalyzer. This Food and Drug Administration-approved high through-put assay was performed at the University of Oklahoma Clinical Laboratory using frozen samples shipped from the primary study site. The assay is stable for unfrozen serum samples for up to 1 week at 4°C and for up to three freeze-thaw cycles. Intra- and interassay coefficients of variation are less than 4% (10, 11, 16).
Screening for diabetes using noninvasive skin fluorescence spectroscopy (SFS) has recently been proposed (12–15). The SCOUT diabetes score device (VeraLight) uses SFS for noninvasive measurement of biomarkers of diabetes in the skin, including fluorescent AGEs (eg, pentosidine, cross-lines) and indicators of cell metabolism and oxidative stress (nicotinamide adenine dinucleotide hydroxide and flavine adenine dinucleotide) (17, 18). The device illuminates the skin of the volar forearm with low-intensity light at near-UV and visible wavelengths and is therefore without risk to mother or fetus. Because skin tone varies among subjects due to melanin and hemoglobin concentrations and light scattering, the intensity and duration of the excitation light is automatically adjusted by the SCOUT device for each subject, compensating for attenuation of excitation light and emitted fluorescence. The measured optical signals are then analyzed for fluorescence related to the development of prediabetes and diabetes and a SCOUT diabetes score (SDS) is produced (14, 15). SFS measurements do not require fasting or blood handling. The score output by the SDS device is reported on a scale of 0–100, with higher SDS values indicating higher disease probability (14, 15). Each subject received duplicate skin fluorescence measures (same arm) at the first trimester and the means of these values were used for data analyses. SCOUT measures were repeated at the time of the second-trimester OGTT.
Finally, HOMA-IR was calculated at the first trimester using the HOMA2 calculator as published by the University of Oxford Diabetes Trials Unit (19), and 25-hydroxyvitamin D levels were measured at the University of Oklahoma Clinical Laboratory using HPLC/tandem mass spectrometry (20).
Statistical analysis
Descriptive statistics were used for summary purposes. Exact 95% confidence intervals were estimated for the prevalence of various categories of dysglycemia. Demographic and clinical characteristics were compared between normal and abnormal glucose groups using a two-sample t test for continuous variables, a χ2 test (or Fisher's exact test if at least 20% of the expected frequency counts were less than 5) for categorical variables, and a Cochran-Armitage trend test for ordinal categorical variables. All analyses were performed using SAS version 9.3 (SAS Institute) with an overall two-sided α-level of .05
Results
Patient characteristics
A total of 310 women were screened. Of these, 47 did not meet eligibility criteria (notably, six because of a prior history of diabetes), resulting in 259 eligible patients. Of those, 244 completed the first-trimester visit. Baseline participant characteristics and measurements at the first study visit are summarized in Table 1. Of the 244, 12 (5%) had a prior history of GDM, 38 (15%) had a family history of GDM, and 198 (81%) reported a family history of diabetes. Of the 244, 182 completed the second-trimester visit, among whom 114 had a complete set of fasting, 1-hour, and 2-hour blood glucose measurements and a SCOUT measurement, allowing ultimate inclusion in the analysis. A total of 68 participants did not undergo the 1-hour blood glucose measurement due to a miscommunication regarding the protocol-specified procedures at one of the study centers. The studied subset (n = 114) did not differ significantly from the cohort studied only at the first trimester (n = 130) by any of the criteria listed in Table 1.
Table 1.
Baseline Characteristics and Measurements at the First Study Visit of Participants (n = 259)
| Mean (SD) | Range | |
|---|---|---|
| Demographic characteristics | ||
| Age, y | 24.9 (4.8) | 15.3–38.5 |
| Clinical factors characteristics | ||
| Prepregnancy weight, kg | 76.1 (19.8) | 41.8–132.7 |
| Current weight, kg | 78.3 (19.0) | 43.1–131.5 |
| BMI, kg/m2 | 29.0 (6.7) | 17.4–49.8 |
| Waist circumference, cm | 95.5 (15.5) | 64.5–135.0 |
| Systolic blood pressure, mm Hg | 118.0 (11.7) | 86–160 |
| Diastolic blood pressure, mm Hg | 73.5 (8.7) | 45–98 |
| SCOUT score (processed) | 39.5 (7.0) | 25.7–70.1 |
| Fasting blood glucose, mmol/d | 4.8 (0.5) | 2.9–8.1 |
| 2-hour blood glucose, mmol/dL | 5.6 (1.3) | 2.2–9.9 |
| HbA1c, % | 5.2 (0.3) | 4.2–6.1 |
| Fasting insulin, μU/mLa | 11.9 (1.9) | 2–199.4 |
| HOMA-IR | 3.1 (4.1) | 0.3–46.8 |
| Serum 1,5-AG, μg/mL | 17.9 (4.4) | 3.1–26.9 |
| Total cholesterol, mmol/L | 4.2 (0.8) | 2.3–7.1 |
| HDL-cholesterol, mmol/L | 1.5 (0.3) | 0.6–2.7 |
| LDL-cholesterol, mmol/L | 2.3 (0.6) | 0.7–4.2 |
| Triglycerides, mmol/La | 0.9 (0.02) | 0.2–2.7 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Geometric means (SD).
First-trimester glycemic status
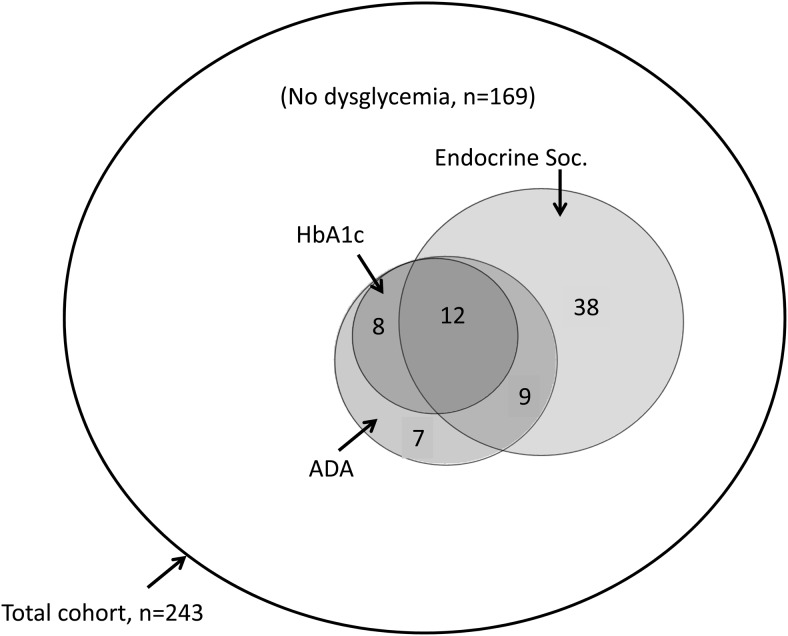
First-trimester fasting blood glucose, OGTT, and HbA1c data were analyzed in 244 participants to define the prevalence of dysglycemia or overt diabetes by either ADA or Endocrine Society criteria. The study was originally designed to use ADA criteria alone, but during the final phases of data collection, The Endocrine Society guidelines were published. The prevalence of dysglycemia by each set of criteria are summarized in Table 2. One woman had undiagnosed T2DM (overt diabetes) [0.4%, 95% confidence interval (CI) 0.0%–2.3%]. Using ADA criteria including elevated HbA1c (5.7%–6.4%), 36 (15%) were dysglycemic (95% CI 10%–19%), and 207 (85%) were euglycemic. Among the 36 dysglycemic subjects, five were classified by fasting glucose alone, 16 by HbA1c alone, and 11 by 2-hour OGTT value alone; the remainder had more than one abnormal value. Using the Endocrine Society criteria at the first trimester, 59 had GDM-Tr1 (24%, 95% CI 18%–30%). However, among the 184 participants classified as normal based on The Endocrine Society criteria, 15 were found to be dysglycemic based on an abnormal HbA1c (n = 8) and/or abnormal 2-hour blood glucose value on the OGTT (n = 8). Overall, this suggests that the addition of the abnormal HbA1c (5.7%–6.4%) or OGTT criteria to The Endocrine Society fasting blood glucose diagnostic threshold would offer a higher sensitivity for detection of dysglycemia. If patients with an abnormal HbA1c (5.7%–6.4%) who do not meet The Endocrine Society criteria for GDM-Tr1 (n = 8) are combined with those who are identified with first-trimester dysglycemia (n = 59), the prevalence of first-trimester dysglycemia increased to 27% (n = 67) in our cohort, and if subjects dysglycemic by any criterion, including OGTT, are included, 30% are affected. Figure 1 shows the extent of agreement/disagreement among the criteria: note that HbA1c data were included within the ADA definition, but data for HbA1c alone are also shown for illustrative purposes. Table 3 summarizes sensitivity, specificity, positive predictive value, and negative predictive value of each set of criteria individually and combined.
Table 2.
Prevalence of Dysglycemia at the First Trimester Using American Diabetes Association and Endocrine Society Criteria and Incidence of Second-Trimester GDM Using ADA/IADPSG/Endocrine Society Criteria
| Glycemic Category | First Trimester (n = 244) |
|||
|---|---|---|---|---|
| ADA/HbA1c Criteria |
Endocrine Society Criteria |
|||
| Count | Percentage (95% CI) | Count | Percentage (95% CI) | |
| Overt diabetes: FBG ≥ 7 mmol/L or 2-h OGGT ≥ 11 mmol/L or HbA1c ≥ 6.5% | 1 | 0.4% (0.0%–2.3%) | 1 | 0.4% (0.0%–2.3%) |
| IGT or GDM-Tr1a | 36 | 14.8% (10.3–19.2%) | 59 | 24.2% (18.1%–30.0%) |
| Normal glucose tolerance | 207 | 84.8% | 184 | 75.4% |
| Glycemic Category | Second Trimester (n = 114) |
|||
|---|---|---|---|---|
| Count | Percentage (95% CI) | |||
| GDM-Tr2 FBG ≥ 5.1 mmol/L or 1-hour OGTT ≥ 10.0 mmol/L or 2-hour OGTT ≥ 8.5 mmol/L | 26 | 22.8% (15.5%–31.6%) | ||
| Normoglycemia | 88 | 77.2% | ||
Abbreviation: FBG, fasting blood glucose. Data are from an epidemiological study in dysglycemia among pregnant Oklahoma American Indian Women.
Modified ADA criteria for first-trimester IGT: FBG of 5.6–7 mmol/L or 2-h BG of 7.8–11.0 mmol/L or HbA1c of 5.7%–6.4%; Endocrine Society criteria for GDM-Tr1: FBG of 5.1–7.0 mmol/L.
Figure 1.
Venn diagram summarizing the agreement/disagreement among ADA, The Endocrine Society, and HbA1c definitions of dysglycemia at the first trimester in 243 American Indian women without overt diabetes. Numbers displayed inside the circles represent the number of women who were positive based on each criterion. Circles are drawn proportional in size to the sample size. A total of 169 women were negative based on all criteria. Overall, approximately 30% of women had dysglycemia by some definition at the first trimester.
Table 3.
Predictive Properties of First-Trimester Dysglycemia According to ADA, Endocrine Society (ES), and HbA1c Definitions Relative to Second-Trimester Gestational Diabetes Status
| First-Trimester Criterion | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| ADA | 0.23 | 0.95 | 0.60 | 0.81 |
| ES | 0.58 | 0.83 | 0.50 | 0.87 |
| HbA1c | 0.15 | 0.97 | 0.57 | 0.79 |
| Composite (ES+HbA1c)a | 0.62 | 0.82 | 0.50 | 0.88 |
| Composite (ES+HbA1c+ADA)a | 0.62 | 0.82 | 0.50 | 0.88 |
Note that HbA1c of 5.7%–6.4% are included in the ADA guidelines.
Positive on any of Endocrine Society or HbA1c criteria with or without ADA.
Factors associated with dysglycemia at the first trimester
Factors positively associated with first-trimester dysglycemia using any criteria included the following: prepregnancy weight and BMI; waist and hip circumference; elevated blood pressure; fasting insulin; and HOMA-IR (all P < .05). Furthermore, using the ADA criteria, additional positive predictive factors were as follows: personal history of GDM; family history of T2DM; and higher triglyceride levels (P < .05). SCOUT scores were significantly higher in women with vs without GDM-Tr1 (42.4 ± 8.1 vs 38.6 ± 6.4; P < .05). However, there was no correlation between SCOUT score and glycemic status when using the ADA/HbA1c criteria. Serum 1,5-AG was not a significant predictor using either criteria (P > .05). These data are summarized in Supplemental Tables 1 and 2.
Second-trimester glycemic status
The second-trimester visit was completed by 182 patients, of whom 114 had complete fasting blood glucose, 1-hour blood glucose, and 2-hour blood glucose measures to allow classification of GDM-Tr2 status and inclusion in the data analysis. This subset was compared with the group of patients who completed only the first-trimester visit and did not differ significantly by any of the criteria listed in Table 1. Of those 114 participants, 104 (91%) had a prior normal glucose tolerance based on the ADA criteria, whereas 10 (9%) were dysglycemic. As aforementioned, this study was conducted prior to the publication of The Endocrine Society guidelines. Hence, we assessed all available nondiabetic women at the second trimester for incident GDM-Tr2; the findings are summarized in Table 2. Overall, the incidence of second-trimester GDM in our cohort was 23% (95% CI 16%–32%) based on the fasting, 1-hour, and 2-hour OGTT results (Table 2). Of the 104 who had normal glucose tolerance testing at the first trimester, 20 (19%) developed GDM-Tr2. Of 10 participants with first-trimester dysglycemia by ADA criteria, six (60%) developed GDM-Tr2. Among women with first-trimester dysglycemia as defined by The Endocrine Society criteria (n = 30 completers), 15 (50%) developed GDM-Tr2.
Among the 26 subjects who met the definition of GDM-Tr2, 15 (58%) were diagnosed by fasting blood glucose alone, three (12%) by 1-hour blood glucose alone, two (8%) by both fasting and 1-hour blood glucose, and three (12%) by both fasting and 2-hour blood glucose. Only two participants (8%) were diagnosed by the 2-hour blood glucose alone. Therefore, 92% were diagnosed based on an abnormal fasting and/or 1-hour blood glucose.
At the second trimester, SCOUT scores were significantly elevated in women with vs without GDM-Tr2 (43.7 ± 7.5 vs 38.8 ± 7.7; mean ± SD; P = .029).
Finally, first-trimester clinical factors that were significantly associated with a higher probability of GDM-Tr2 were higher weight and BMI, presence of hypertension, greater waist and hip circumference, higher diastolic blood pressure, higher fasting insulin, higher total cholesterol level, and higher triglyceride levels (all P < .05).
Pregnancy outcomes
Pregnancy outcome data were available for 100 participants who completed both the first- and second-trimester visits and who were delivered at the study center. Birth and pregnancy complications were recorded. There were no cases of preeclampsia or dystocia among women with first-trimester or second-trimester dysglycemia. Duration of pregnancy, Apgar scores, and the rate of complications were similar to those of euglycemic women when the ADA criteria were used for the analysis. On the other hand, when The Endocrine Society criteria were used for the first-trimester definition of GDM-Tr1, dysglycemic women had a slightly shorter duration of pregnancy and newborns had a slightly lower Apgar score (P < .05). Women who developed GDM-Tr2 had a slightly shorter duration of pregnancy compared to women with no GDM (P = .01) (Supplemental Table 3).
Discussion
In our study, we were able to assess the prevalence of dysglycemia at the first trimester of pregnancy in women from a minority population that is particularly affected by the diabetes epidemic. In doing so, we were surprised to find a very low prevalence of undiagnosed T2DM (0.4%). On the other hand, using two published sets of early pregnancy screening criteria, with the inclusion of an abnormal HbA1c, the prevalence of milder dysglycemia (IGT or GDM-Tr1) was high: 15% using ADA criteria and/or abnormal HbA1c (5.7%–6.4%); 24% using the 2013 Endocrine Society criteria for diagnosing GDM-Tr1; and 30% by any definition. Our data also suggest that a combination of a fasting blood glucose (using Endocrine Society criteria) and HbA1c at the first trimester may increase the sensitivity of screening and could have merit because a full OGTT may not be tolerated due to morning sickness. The less stringent Endocrine Society criteria identified most women with abnormal values by other criteria; however, 15 participants (6%) with fasting glucose less than 5.1 mmol/L had an abnormal HbA1c level (n = 8) or an elevated 2-hour glucose on the OGTT (n = 8) and were thus potentially misclassified as normoglycemic by The Endocrine Society criteria. We therefore suggest the addition of an abnormal HbA1c level (5.7%–6.4%) to those criteria to enhance sensitivity while still avoiding the OGTT.
Although the clinical significance (short term pregnancy and long term mother and offspring outcomes) of early dysglycemia in pregnancy remains unclear, given the lack of intervention trials using the new sets of criteria, we believe that our findings are important. The Hyperglycemia and Adverse Pregnancy Outcomes and other studies suggest that even mild dysglycemia may adversely affect pregnancy outcomes, particularly fetal growth and hypertensive disorders of pregnancy (21–23) and that treating mild GDM, often simply managed with dietary changes, is worthwhile (24, 25). There is also increasing evidence linking in utero exposure to hyperglycemia and future risk for T2DM among the offspring (26).
Weight, BMI, waist circumference, and HOMA-IR were positively associated with dysglycemic status at the first trimester, whereas 1,5-AG did not correlate with glycemic status. Regarding the SCOUT measurement, there was a significant association between the first-trimester SCOUT score and an abnormal fasting blood glucose using the more liberal Endocrine Society diagnostic criteria, but no association was found when we correlated it with glycemic status using the ADA criteria.
At the second trimester, of the representative 114 participants who remained in the study and had complete data, 23% were found to have GDM-Tr2. If only those with strict euglycemia at the first-trimester testing (using ADA criteria and excluding those with IGT) were taken into consideration, the incidence of GDM was 19%. This is similar to estimates of the incidence of GDM using the new IADPSG criteria in other populations. Of note, only 8% of our cohort (two participants) were diagnosed solely on the basis of the 2-hour blood glucose: a majority had an abnormal fasting or 1-hour blood glucose. This suggests that given access to rapid blood glucose measurement, the test could be shortened to 1 hour while still retaining diagnostic value, with benefits for patient convenience and costs.
Interestingly, a higher SCOUT score was positively associated with dysglycemic status throughout our study. Whereas normal SCOUT scores in pregnancy are yet to be defined, the finding of an abnormal value at both the first and second trimesters and the absence of side effects from the procedure suggests that this measurement may have a role in the detection of dysglycemia in pregnancy. Future studies should seek to attempt and correlate the presence of AGEs and pregnancy complications.
A clear-cut finding in our study is that more than half of the women testing positive by any measure at the first trimester went on to develop GDM-Tr2. Whereas our study was not powered to detect complications of pregnancy, this finding suggests that effective counseling, education, and lifestyle modification after the abnormal first-trimester findings might help reduce the incidence of GDM-Tr2 and perhaps improve pregnancy outcomes. Overall, about 38% of our American Indian cohort exhibited dysglycemia by some measure at some stage of pregnancy, a figure that is cause for grave concern.
Finally, we found no significant correlation between glycemic status and pregnancy outcomes. The power to detect such associations with our study was inadequate due to the small sample size and rarity of adverse birth outcomes. Nevertheless, preeclampsia complicates about 5% in healthy, nondiabetic pregnancies and is increased by diabetes or GDM, so the absence of any cases in our cohort is surprising.
Strengths of our study include the opportunity to work with a population in which the epidemiology of dysglycemia in pregnancy has seldom been assessed, particularly since new guidelines have been published. We were able to investigate alternative screening tools and found that SCOUT measurements may play a role in diagnosing dysglycemia in pregnancy. Limitations of our study include the small sample size, especially at the second trimester, which limited our ability to detect associations with birth outcomes. Inferences from the study are also limited by incomplete follow-up and incomplete data capture relative to some laboratory values for some patients that may have resulted in selection bias. One other potential limitation is the question of how HbA1c is affected by race and ethnicity. Some studies have suggested that American Indians may have higher HbA1c levels than Caucasians for similar levels of glycemia (27), and this may have impacted our results. However, it is unclear whether HbA1c is affected differentially in pregnancy and whether it differs among pregnant women of different races/ethnicities for similar glycemic status. Studies with large numbers of women in diverse race and ethnicity groups are needed to answer that question.
Conclusion
In conclusion, our study showed a high prevalence of dysglycemia in a cohort of pregnant American Indian women. The significance of this, and the potential impact of treating mild, early hyperglycemia on pregnancy outcomes, remain to be elucidated in future studies.
Acknowledgments
We are grateful to the Choctaw Nation of Oklahoma for allowing us to use their facilities and resources to conduct this project. A special thanks go to Mary Ayn Tullier, RN, Charlotte Coleman (study coordinators), and Clare Kelly for assistance with the data analysis.
This work was supported by the Oklahoma Center for the Advancement of Sciences and Technology Grant OCAST HR10–025 (to M.A.). Partial funding was provided by National Institutes of Health, National Institute of General Medical Sciences Grant 1 U54GM104938, an Institutional Development Award Program for Clinical and Translational Research to the University of Oklahoma Health Sciences Center (to J.A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADA
- American Diabetes Association
- 1,5-AG
- 1,5-anydroglucitol
- AGE
- advanced glycation end-products
- BG
- blood glucose
- BMI
- body mass index
- CI
- confidence interval
- GDM
- gestational diabetes mellitus
- GDM-Tr1
- first trimester GDM
- GDM-Tr2
- second-trimester GDM
- HbA1c
- glycated hemoglobin
- HOMA-IR
- homeostatic model assessment-insulin resistance
- IADPSG
- International Association of Diabetes and Pregnancy Study Groups
- IGT
- impaired glucose tolerance
- OGTT
- oral glucose tolerance test
- SCOUT
- skin autofluorescence
- SDS
- SCOUT diabetes score
- SFS
- skin fluorescence spectroscopy
- T2DM
- type 2 diabetes mellitus
- Tr1
- first trimester of pregnancy (<13 wk gestational age)
- Tr2
- second trimester of pregnancy (typically, women are screened for GDM at 24–28 wk).
References
- 1. Oklahoma State Department of Health. http://www.ok.gov/health/pub/boh/state08/IndicatorReportCards/diabetes%20prevalence.pdf.
- 2. Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med,. 2004;21(2):103–113. [DOI] [PubMed] [Google Scholar]
- 3. Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational diabetes mellitus: results from a survey of country prevalence and practices. J Matern Fetal Neonatal Med. 2012;25(6): p. 600–10. [DOI] [PubMed] [Google Scholar]
- 4. Committee on Practice Bulletins-Obstetrics. Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. [DOI] [PubMed] [Google Scholar]
- 5. O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 6. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7): 768–773. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Summary of revisions to the 2011 clinical practice recommendations. Diabetes Care. 2011;34(suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Association of Diabetes Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–63. [DOI] [PubMed] [Google Scholar]
- 11. McGill JB, Cole TG, Nowatzke W, et al. Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care. 2004;27(8):1859–1865. [DOI] [PubMed] [Google Scholar]
- 12. Maynard JD, Rohrscheib M, Way JF, Nguyen CM, Ediger MN. Noninvasive type 2 diabetes screening: superior sensitivity to fasting plasma glucose and A1C. Diabetes Care. 2007;30(5):1120–1124. [DOI] [PubMed] [Google Scholar]
- 13. Ediger MN, Olson BP, Maynard JD. Noninvasive optical screening for diabetes. J Diabetes Sci Technol. 2009;3(4):776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olson BP, Matter NI, Ediger MN, Hull EL, Maynard JD. Noninvasive skin fluorescence spectroscopy is comparable to hemoglobin A1c and fasting plasma glucose for detection of abnormal glucose tolerance. J Diabetes Sci Technol,. 2013;7(4):990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tentolouris N, Lathouris P, Lontou S, Tzemos K, Maynard J. Screening for HbA1c-defined prediabetes and diabetes in an at-risk Greek population: performance comparison of random capillary glucose, the ADA diabetes risk test and skin fluorescence spectroscopy. Diabetes Res Clin Pract. 2013;100(1):39–45. [DOI] [PubMed] [Google Scholar]
- 16. Nowatzke W, Sarno MJ, Birch NC, Stickle DF, Eden T, Cole TG. Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMark) and determination of reference intervals on the Hitachi 917 analyzer. Clin Chim Acta. 2004;350(1–2):201–209. [DOI] [PubMed] [Google Scholar]
- 17. Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heikal AA. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark Med. 2010;4(2):241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.dtu.ox.ac.uk/homacalculator/.
- 20. http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=17306&fromFlyOut=true.
- 21. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 22. Metzger BE, Persson B, Lowe LP, et al. Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126(6):e1545–e1552. [DOI] [PubMed] [Google Scholar]
- 23. Jensen DM, Korsholm L, Ovesen P, Beck-Nielsen H, Molsted-Pedersen L, Damm P. Adverse pregnancy outcome in women with mild glucose intolerance: is there a clinically meaningful threshold value for glucose? Acta Obstet Gynecol Scand. 2008;87(1):59–62. [DOI] [PubMed] [Google Scholar]
- 24. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. [DOI] [PubMed] [Google Scholar]
- 25. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–2292. [DOI] [PubMed] [Google Scholar]
- 27. Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]