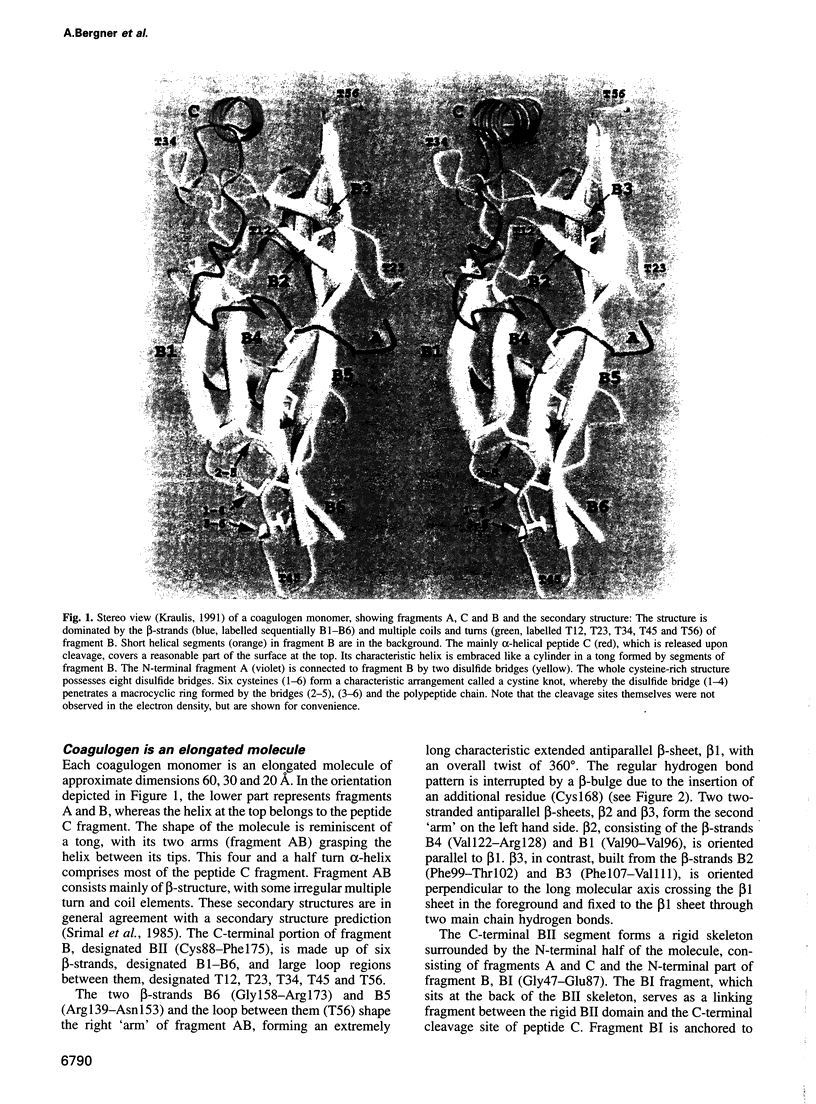
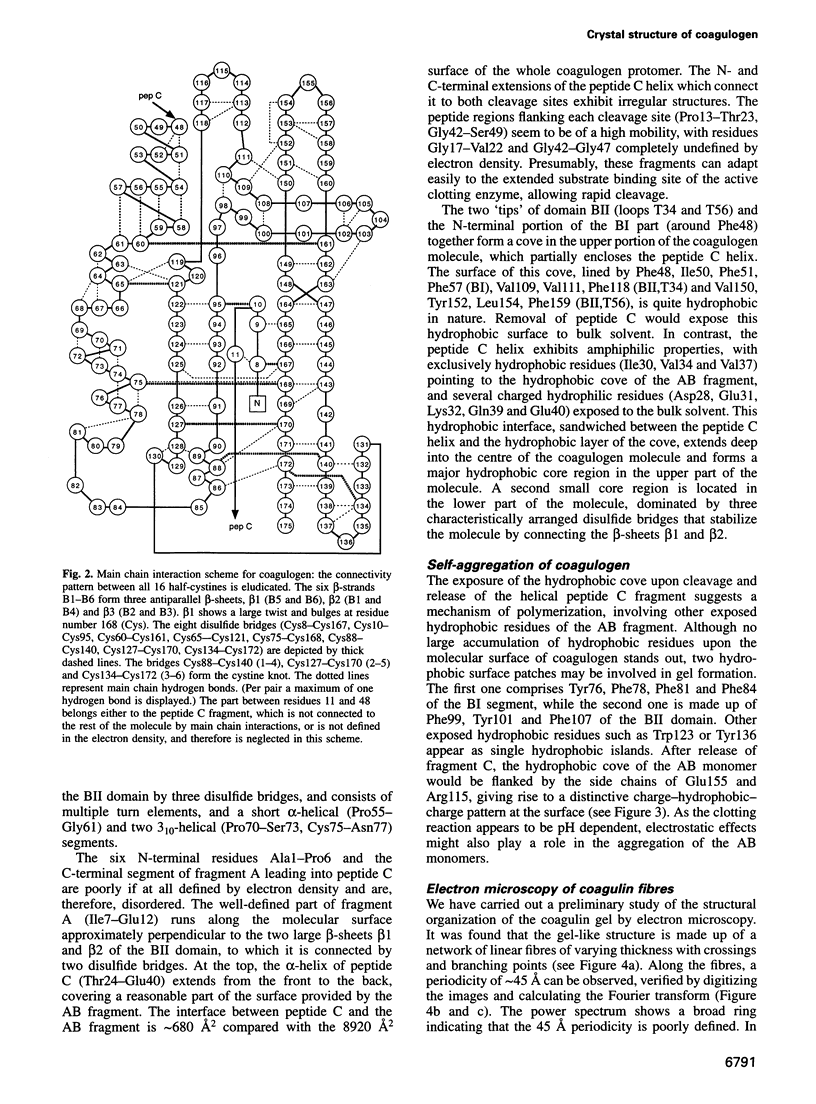
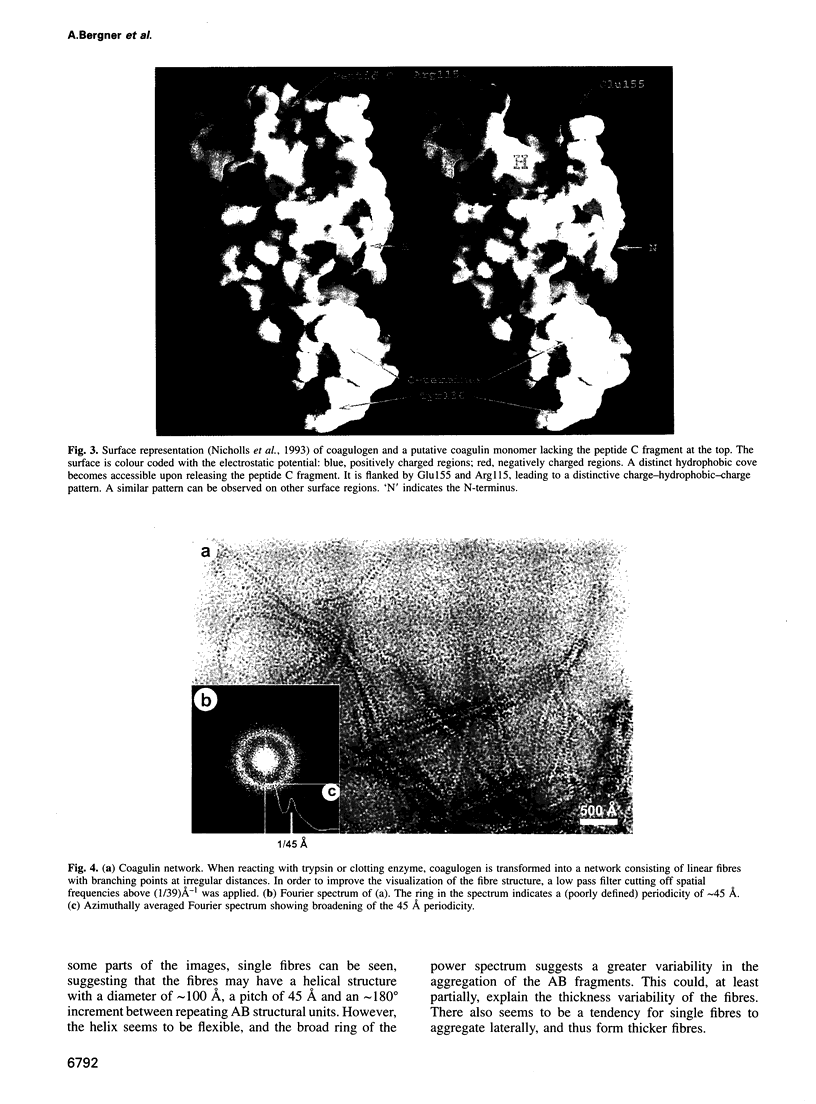
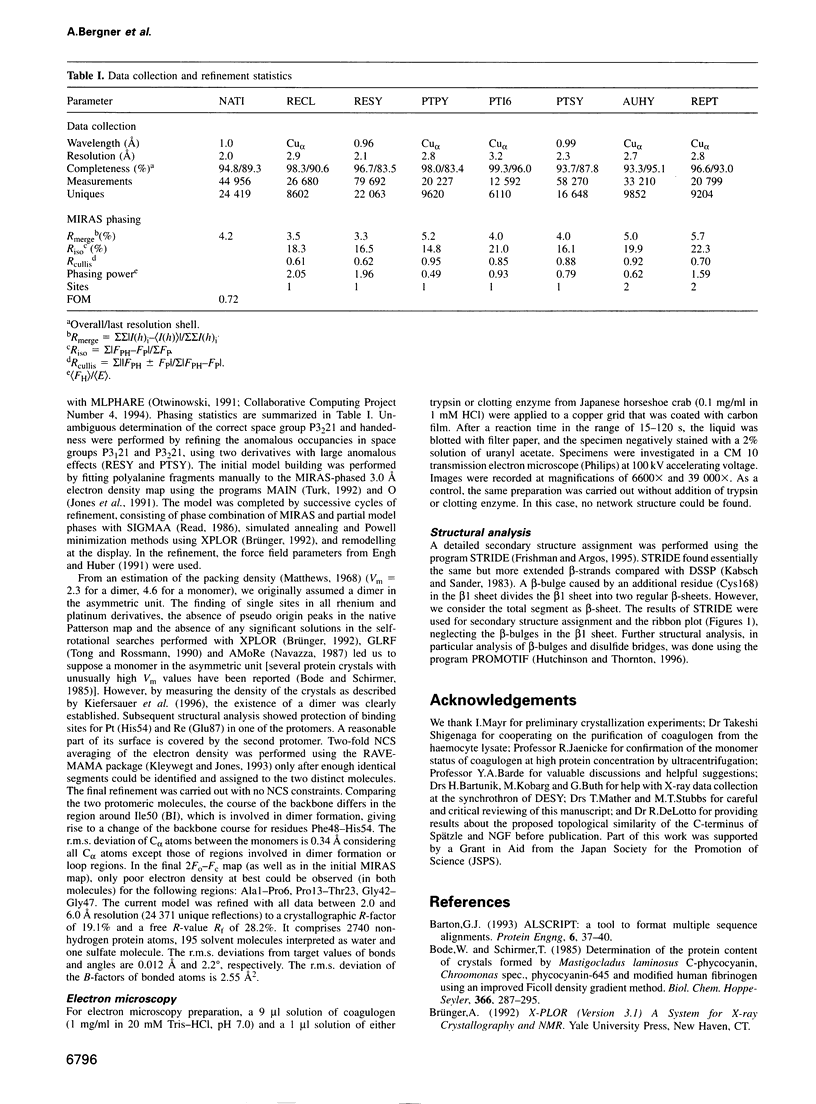
Abstract
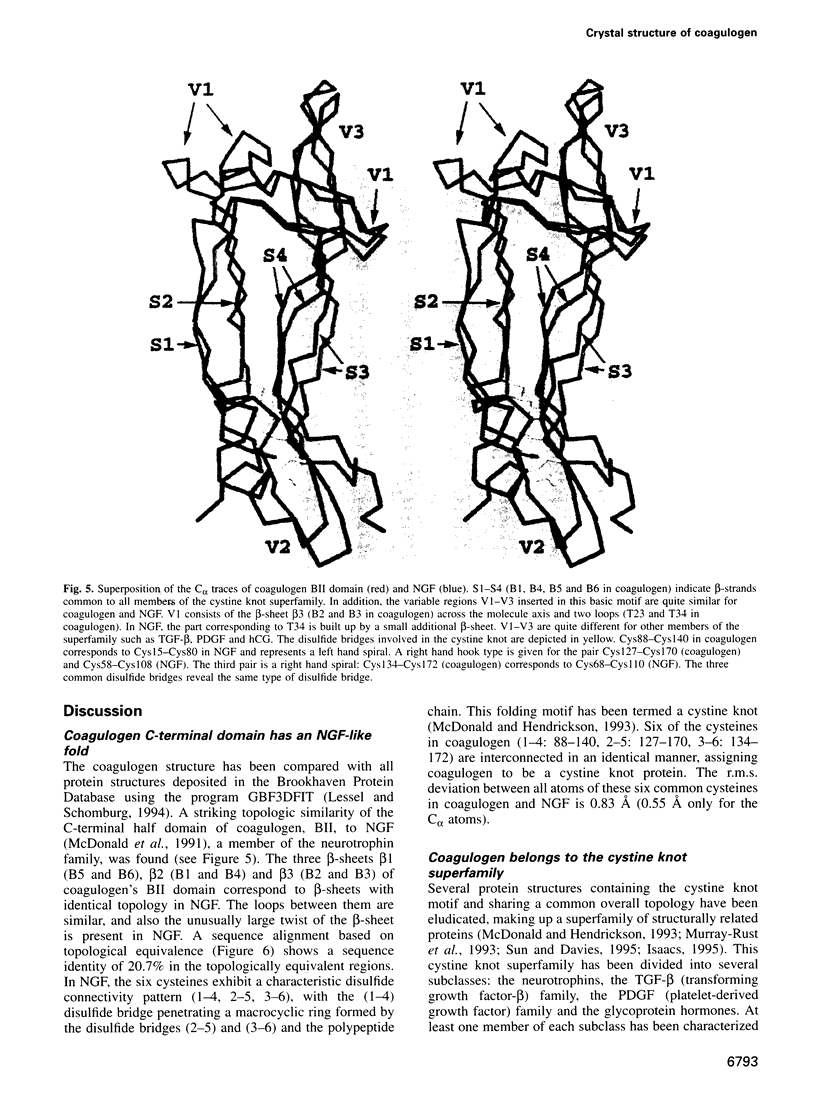
The clotting cascade system of the horseshoe crab (Limulus) is involved in both haemostasis and host defence. The cascade results in the conversion of coagulogen, a soluble protein, into an insoluble coagulin gel. The clotting enzyme excises the fragment peptide C from coagulogen, giving rise to aggregation of the monomers. The crystal structure of coagulogen reveals an elongated molecule that embraces the helical peptide C fragment. Cleavage and removal of the peptide C would expose an extended hydrophobic cove, which could interact with the hydrophobic edge of a second molecule, leading to a polymeric fibre. The C-terminal half of the coagulogen molecule exhibits a striking topological similarity to the neurotrophin nerve growth factor (NGF), providing the first evidence for a neurotrophin fold in invertebrates. Similarities between coagulogen and Spatzle, the Drosophila ligand of the receptor Toll, suggest that the neurotrophin fold might be considered more ancient and widespread than previously realized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton G. J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993 Jan;6(1):37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- Bode W., Schirmer T. Determination of the protein content of crystals formed by Mastigocladus laminosus C-phycocyanin, Chroomonas spec. phycocyanin-645 and modified human fibrinogen using an improved Ficoll density gradient method. Biol Chem Hoppe Seyler. 1985 Mar;366(3):287–295. doi: 10.1515/bchm3.1985.366.1.287. [DOI] [PubMed] [Google Scholar]
- Casanova J., Furriols M., McCormick C. A., Struhl G. Similarities between trunk and spätzle, putative extracellular ligands specifying body pattern in Drosophila. Genes Dev. 1995 Oct 15;9(20):2539–2544. doi: 10.1101/gad.9.20.2539. [DOI] [PubMed] [Google Scholar]
- Daopin S., Li M., Davies D. R. Crystal structure of TGF-beta 2 refined at 1.8 A resolution. Proteins. 1993 Oct;17(2):176–192. doi: 10.1002/prot.340170207. [DOI] [PubMed] [Google Scholar]
- Daopin S., Piez K. A., Ogawa Y., Davies D. R. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992 Jul 17;257(5068):369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- Frishman D., Argos P. Knowledge-based protein secondary structure assignment. Proteins. 1995 Dec;23(4):566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Keith F. J. Regulation of translation and proteolysis during the development of embryonic dorso-ventral polarity in Drosophila. Homology of easter proteinase with Limulus proclotting enzyme and translational activation of Toll receptor synthesis. Biochim Biophys Acta. 1992 Oct 20;1132(3):290–296. doi: 10.1016/0167-4781(92)90163-t. [DOI] [PubMed] [Google Scholar]
- Hinck A. P., Archer S. J., Qian S. W., Roberts A. B., Sporn M. B., Weatherbee J. A., Tsang M. L., Lucas R., Zhang B. L., Wenker J. Transforming growth factor beta 1: three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor beta 2. Biochemistry. 1996 Jul 2;35(26):8517–8534. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- Hutchinson E. G., Thornton J. M. PROMOTIF--a program to identify and analyze structural motifs in proteins. Protein Sci. 1996 Feb;5(2):212–220. doi: 10.1002/pro.5560050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs N. W. Cystine knots. Curr Opin Struct Biol. 1995 Jun;5(3):391–395. doi: 10.1016/0959-440x(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Iwanaga S. Primitive coagulation systems and their message to modern biology. Thromb Haemost. 1993 Jul 1;70(1):48–55. [PubMed] [Google Scholar]
- Iwanaga S. The limulus clotting reaction. Curr Opin Immunol. 1993 Feb;5(1):74–82. doi: 10.1016/0952-7915(93)90084-6. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. Crystal structure of human chorionic gonadotropin. Nature. 1994 Jun 9;369(6480):455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Lessel U., Schomburg D. Similarities between protein 3-D structures. Protein Eng. 1994 Oct;7(10):1175–1187. doi: 10.1093/protein/7.10.1175. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Chao M. V. Structural determinants of neurotrophin action. J Biol Chem. 1995 Aug 25;270(34):19669–19672. doi: 10.1074/jbc.270.34.19669. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Hendrickson W. A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993 May 7;73(3):421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Lapatto R., Murray-Rust J., Gunning J., Wlodawer A., Blundell T. L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991 Dec 5;354(6352):411–414. doi: 10.1038/354411a0. [DOI] [PubMed] [Google Scholar]
- Meitinger T., Meindl A., Bork P., Rost B., Sander C., Haasemann M., Murken J. Molecular modelling of the Norrie disease protein predicts a cystine knot growth factor tertiary structure. Nat Genet. 1993 Dec;5(4):376–380. doi: 10.1038/ng1293-376. [DOI] [PubMed] [Google Scholar]
- Mittl P. R., Priestle J. P., Cox D. A., McMaster G., Cerletti N., Grütter M. G. The crystal structure of TGF-beta 3 and comparison to TGF-beta 2: implications for receptor binding. Protein Sci. 1996 Jul;5(7):1261–1271. doi: 10.1002/pro.5560050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Rust J., McDonald N. Q., Blundell T. L., Hosang M., Oefner C., Winkler F., Bradshaw R. A. Topological similarities in TGF-beta 2, PDGF-BB and NGF define a superfamily of polypeptide growth factors. Structure. 1993 Oct 15;1(2):153–159. doi: 10.1016/0969-2126(93)90029-g. [DOI] [PubMed] [Google Scholar]
- Muta T., Oda T., Iwanaga S. Horseshoe crab coagulation factor B. A unique serine protease zymogen activated by cleavage of an Ile-Ile bond. J Biol Chem. 1993 Oct 5;268(28):21384–21388. [PubMed] [Google Scholar]
- Muta T., Seki N., Takaki Y., Hashimoto R., Oda T., Iwanaga A., Tokunaga F., Iwanaga S. Purified horseshoe crab factor G. Reconstitution and characterization of the (1-->3)-beta-D-glucan-sensitive serine protease cascade. J Biol Chem. 1995 Jan 13;270(2):892–897. [PubMed] [Google Scholar]
- Muta T., Tokunaga F., Nakamura T., Morita T., Iwanage S. Limulus clotting factor C: lipopolysaccharide-sensitive serine protease zymogen. Methods Enzymol. 1993;223:336–345. doi: 10.1016/0076-6879(93)23054-q. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iwanaga S., Harada T., Niwa M. A clottable protein (coagulogen) from amoebocyte lysate of Japanese horseshoe crab (Tachypleus tridentatus). Its isolation and biochemical properties. J Biochem. 1976 Nov;80(5):1011–1021. doi: 10.1093/oxfordjournals.jbchem.a131357. [DOI] [PubMed] [Google Scholar]
- Noguti T., Adachi-Yamada T., Katagiri T., Kawakami A., Iwami M., Ishibashi J., Kataoka H., Suzuki A., Go M., Ishizaki H. Insect prothoracicotropic hormone: a new member of the vertebrate growth factor superfamily. FEBS Lett. 1995 Dec 4;376(3):251–256. doi: 10.1016/0014-5793(95)01296-8. [DOI] [PubMed] [Google Scholar]
- Oefner C., D'Arcy A., Winkler F. K., Eggimann B., Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921–3926. doi: 10.1002/j.1460-2075.1992.tb05485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Axis determination. Proteolytic generation of a morphogen. Curr Biol. 1994 Aug 1;4(8):755–757. doi: 10.1016/s0960-9822(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Smith C. L., DeLotto R. A common domain within the proenzyme regions of the Drosophila snake and easter proteins and Tachypleus proclotting enzyme defines a new subfamily of serine proteases. Protein Sci. 1992 Sep;1(9):1225–1226. doi: 10.1002/pro.5560010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., DeLotto R. Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature. 1994 Apr 7;368(6471):548–551. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- Srimal S., Miyata T., Kawabata S., Miyata T., Iwanaga S. The complete amino acid sequence of coagulogen isolated from Southeast Asian horseshoe crab, Carcinoscorpius rotundicauda. J Biochem. 1985 Aug;98(2):305–318. doi: 10.1093/oxfordjournals.jbchem.a135283. [DOI] [PubMed] [Google Scholar]
- Stein D., Nüsslein-Volhard C. Multiple extracellular activities in Drosophila egg perivitelline fluid are required for establishment of embryonic dorsal-ventral polarity. Cell. 1992 Feb 7;68(3):429–440. doi: 10.1016/0092-8674(92)90181-b. [DOI] [PubMed] [Google Scholar]
- Stein D., Roth S., Vogelsang E., Nüsslein-Volhard C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell. 1991 May 31;65(5):725–735. doi: 10.1016/0092-8674(91)90381-8. [DOI] [PubMed] [Google Scholar]
- Sun P. D., Davies D. R. The cystine-knot growth-factor superfamily. Annu Rev Biophys Biomol Struct. 1995;24:269–291. doi: 10.1146/annurev.bb.24.060195.001413. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Iwanaga S. Limulus test for detecting bacterial endotoxins. Methods Enzymol. 1993;223:358–364. doi: 10.1016/0076-6879(93)23057-t. [DOI] [PubMed] [Google Scholar]
- Tong L. A., Rossmann M. G. The locked rotation function. Acta Crystallogr A. 1990 Oct 1;46(Pt 10):783–792. doi: 10.1107/s0108767390005530. [DOI] [PubMed] [Google Scholar]
- Wu H., Lustbader J. W., Liu Y., Canfield R. E., Hendrickson W. A. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure. 1994 Jun 15;2(6):545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]