Abstract
Purpose
To characterize psychological and neurocognitive function in long-term cancer survivors diagnosed during adolescence and early young adulthood (AeYA).
Methods
Six thousand one hundred ninety-two survivors and 390 siblings in the Childhood Cancer Survivor Study completed the Brief Symptom Inventory-18 and a Neurocognitive Questionnaire. Treatment and demographic predictors were examined, and associations with social attainment (employment, education, and living independently) were evaluated. Logistic regression models were used to compute odds ratios (ORs) and corresponding 95% CIs.
Results
Among survivors, 2,589 were diagnosed when AeYA (11 to 21 years old). Adjusted for current age and sex, these survivors, compared with siblings, self-reported higher rates of depression (11.7% v 8.0%, respectively; OR, 1.55; 95% CI, 1.04 to 2.30) and anxiety (7.4% v 4.4%, respectively; OR, 2.00; 95% CI, 1.17 to 3.43) and more problems with task efficiency (17.2% v 10.8%, respectively; OR, 1.72; 95% CI, 1.21 to 2.43), emotional regulation (19.1% v 14.1%, respectively; OR, 1.74; 95% CI, 1.26 to 2.40), and memory (25.9% v 19.0%, respectively; OR, 1.44; 95% CI, 1.09 to 1.89). Few differences were noted between survivors diagnosed with leukemia or CNS tumor before 11 years old versus during later adolescence, although those diagnosed with lymphoma or sarcoma during AeYA were at reduced risk for self-reported psychosocial and neurocognitive problems. Unemployment was associated with self-reports of impaired task efficiency (OR, 2.93; 95% CI, 2.28 to 3.77), somatization (OR, 2.29; 95% CI, 1.77 to 2.98), and depression (OR, 1.94; 95% CI, 1.43 to 2.63).
Conclusion
We demonstrated that risk for poor functional outcome is not limited to survivors' diagnoses in early childhood. AeYA is a critical period of development, and cancer during this period can impact neurocognitive and emotional function and disrupt vocational attainment.
INTRODUCTION
Currently, there are estimated to be more than 400,000 survivors of childhood and early young adult cancer in the United States.1,2 These survivors are at risk for significant disease- and treatment-related morbidity. Two thirds of survivors face at least one chronic health condition, including mental health and cognitive problems.3–6 Neurocognitive dysfunction has been demonstrated in more than 40% of survivors, with relatively higher rates of problems in processing speed, attention, memory, and executive function.5,7 Childhood cancer survivors treated when less than 6 years of age are reported to be at greater risk for neurocognitive problems compared with siblings.8 Although many studies have examined outcomes associated with survivors of childhood cancer, few have focused on survivors diagnosed during their adolescence or early young adulthood.
The adolescent and young adult (AYA) cancer population is typically defined as encompassing the ages of 15 to 39 years. The early years of AYA, ages 11 to 21 years, are a period of rapid development of advanced neurocognitive functions related to brain maturation; this period encompasses a developmental phase when behavioral patterns are established and engrained.9 The brain continues to grow throughout adolescence and into early young adulthood, with accelerated development of higher-order skills such as executive functions.9,10 The full extent of executive dysfunction may only become evident in adolescence and adulthood, with the onset of expectations to act more independently and use advanced planning and reasoning abilities.11 Literature from traumatic brain injury patients demonstrates that mild injury during early adolescence can result in executive dysfunction that is not typically associated with similar injury during younger childhood.12 Studies using magnetic resonance imaging of brains during early adolescence (ie, 11 to 14 years of age) have shown periods of rapid development in the dorsolateral prefrontal cortex, which is important for executive functions like abstract reasoning and problem solving.13 This early phase of adolescent development may be more vulnerable to disruption of executive functions compared with the later phase (ie, 15 to 21 years of age) or even preadolescence (ie, 6 to 10 years of age).
The aim of this study was to characterize self-reported psychological symptoms and subjective complaints of cognitive and behavioral function in long-term survivors of cancer diagnosed during the time of adolescence and early young adulthood (AeYA; ages 11 to 21 years) and to identify risk factors within this group that may guide the development of targeted interventions to reduce adverse behavioral and social outcomes.
METHODS
Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional, retrospective cohort study of individuals diagnosed between January 1, 1970, and December 31, 1986; individuals were younger than age 21 years of age and 5 or more years from diagnosis at the time of recruitment. Diagnoses included leukemia, CNS malignancies (all histologies), Hodgkin lymphoma, non-Hodgkin lymphoma, malignant kidney tumor, neuroblastoma, soft tissue sarcoma, and malignant bone tumor diagnosed and initially treated at one of 26 participating institutions. In addition, the study recruited the nearest-age sibling of a random sample of participating survivors to serve as a comparison group. The study procedure, cohort design, and characteristics have been described in detail elsewhere.14,15
The human subjects committee at each of the 26 participating institutions approved the CCSS protocol and contact documents. All study participants provided informed consent for participation in the study and for release of information from medical records. Cancer diagnosis and treatment information were obtained from the treating institution for all eligible survivors. Baseline questionnaires were collected to capture a wide variety of demographic and medical information. A follow-up questionnaire (FU2) was completed that contained self-reported assessments of neurocognitive and emotional functioning. Because of the length of this questionnaire, only a selected subset of siblings were asked to complete the neurocognitive/emotional functioning portion of the follow-up survey. The baseline and FU2 surveys and medical record abstraction form used for data collection are available at http://ccss.stjude.org. Within the context of the CCSS cohort, AeYA is defined as survivors diagnosed with cancer after 10 years but before 21 years of age. The lower limit of age 11 years was chosen because this has recently been identified as the mean age at which girls achieve Tanner stage II breast development, signaling the onset of puberty.16 Similarly, onset of secondary sex characteristics in boys has also been shown to begin by a mean age of 10 years.17 Given our aim to characterize neurocognitive and psychosocial outcomes that may be related to experiencing cancer within the neurobiological and socioemotional context of adolescence, we felt that it was prudent to capture participants within the entire peripubertal age range.
Of the 20,691 eligible 5-year childhood cancer survivors, 3,058 were lost to follow-up; 17,633 were contacted, and 14,357 participated in the baseline survey. Eleven thousand five hundred seventy-six survivors were contacted for the FU2 survey that contained the measures of interest for this study. Of these, 9,308 survivors (80.4%) participated in the FU2 survey, and 7,345 completed all questions on the measures of interest for the current analysis. Of these 7,345 survivors, survivors who had a diagnosis of kidney cancers and neuroblastoma were excluded as a result of small numbers, because these cancers are not typically seen during the AeYA time frame. Thus, 6,192 survivors were included in the final analysis. Three-hundred ninety siblings also completed the FU2 survey.
Outcome Measures
The Brief Symptom Inventory-18 (BSI-18) is an 18-item checklist that measures symptoms of emotional distress18 and has been validated in cancer survivors.19 An index score is generated for Anxiety, Depression, and Somatic Complaints; survivors with a standardized T score ≥ 63 (≥ 90th percentile) were classified as having emotional distress.18,20
The CCSS Neurocognitive Questionnaire (CCSS-NCQ) was designed to assess self-reported neurocognitive symptoms often affected by cancer therapy.21 The CCSS-NCQ contains four factors—task efficiency, emotional regulation, plan/organization, and working memory—derived from a 25-item questionnaire that asks participants to report the degree to which they experienced specific problems in these areas over the past 6 months. Raw scores were used and referenced to the sibling cohort, with scores ≥ 90th percentile of siblings classified as impaired. This threshold was used because it is also the recommended threshold in the standardization manual for the BSI-18, and this threshold was used in the validation studies of the CCSS-NCQ.
Data Analysis
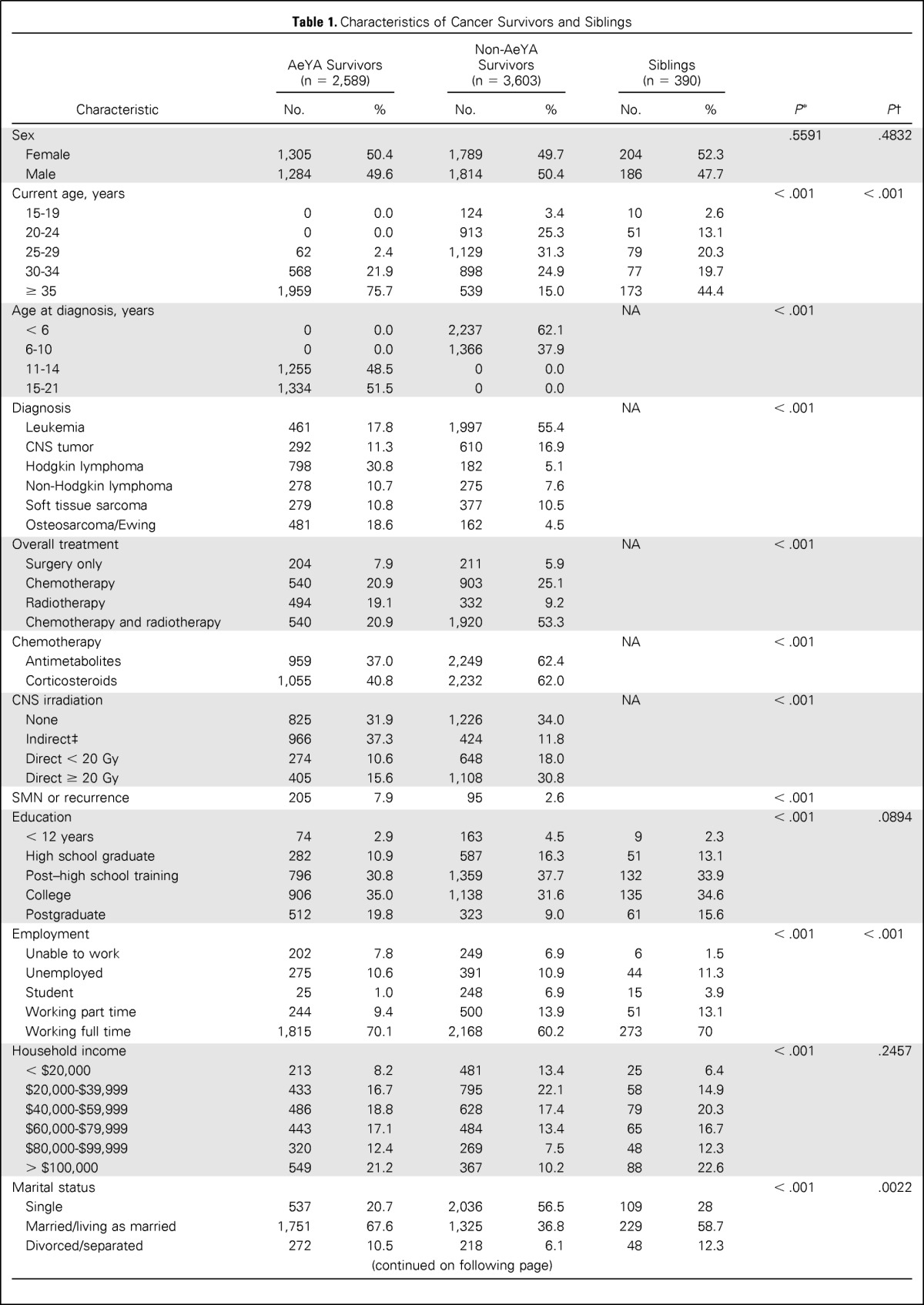
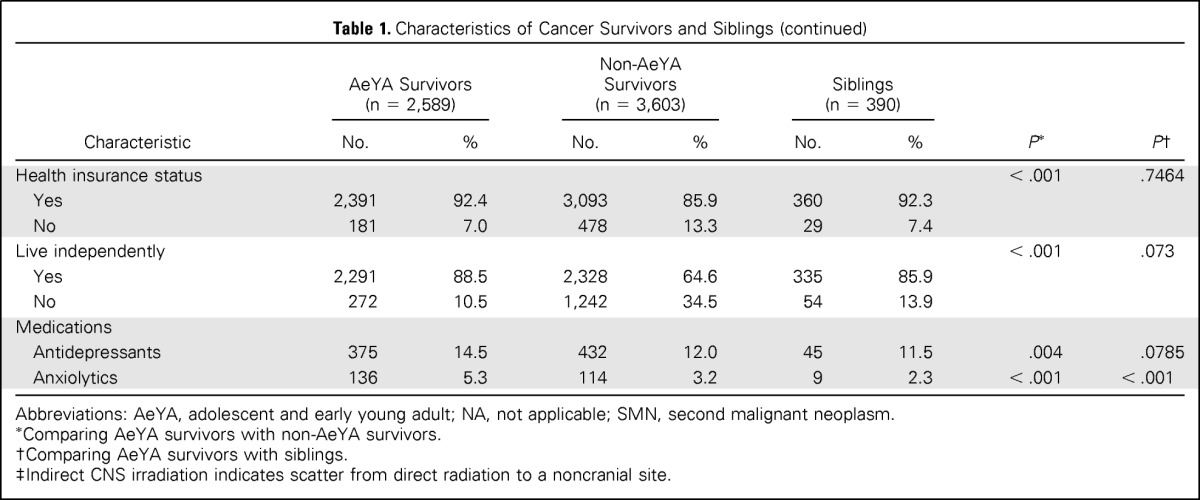
Descriptive statistics for demographic and treatment variables for adolescent survivors, nonadolescent survivors, and siblings are listed in Table 1. Differences between adolescent survivors and sibling controls were evaluated using logistic regression or generalized logistic regression within a generalized estimating equation framework with compound symmetry assumption to account for within-family correlation. The impairments in emotional and neurocognitive functions between survivors diagnosed with cancer during adolescent and sibling controls were compared using logistic regression and implemented using generalized estimating equation with compound symmetry correlation structure to account for within-family correlation. Because a larger percentage of survivors of Hodgkin lymphoma, non-Hodgkin lymphoma, soft tissue sarcomas, or bone cancers were diagnosed during adolescence (n = 1,835, 70.9%), compared with survivors of leukemia or CNS tumors (n = 753, 29.1%; Table 1), the results were stratified by grouping CNS tumors with leukemias and grouping lymphomas with sarcomas/bone tumors.
Table 1.
Characteristics of Cancer Survivors and Siblings
| Characteristic | AeYA Survivors (n = 2,589) |
Non-AeYA Survivors (n = 3,603) |
Siblings (n = 390) |
P* | P† | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Sex | .5591 | .4832 | ||||||
| Female | 1,305 | 50.4 | 1,789 | 49.7 | 204 | 52.3 | ||
| Male | 1,284 | 49.6 | 1,814 | 50.4 | 186 | 47.7 | ||
| Current age, years | < .001 | < .001 | ||||||
| 15-19 | 0 | 0.0 | 124 | 3.4 | 10 | 2.6 | ||
| 20-24 | 0 | 0.0 | 913 | 25.3 | 51 | 13.1 | ||
| 25-29 | 62 | 2.4 | 1,129 | 31.3 | 79 | 20.3 | ||
| 30-34 | 568 | 21.9 | 898 | 24.9 | 77 | 19.7 | ||
| ≥ 35 | 1,959 | 75.7 | 539 | 15.0 | 173 | 44.4 | ||
| Age at diagnosis, years | NA | < .001 | ||||||
| < 6 | 0 | 0.0 | 2,237 | 62.1 | ||||
| 6-10 | 0 | 0.0 | 1,366 | 37.9 | ||||
| 11-14 | 1,255 | 48.5 | 0 | 0.0 | ||||
| 15-21 | 1,334 | 51.5 | 0 | 0.0 | ||||
| Diagnosis | NA | < .001 | ||||||
| Leukemia | 461 | 17.8 | 1,997 | 55.4 | ||||
| CNS tumor | 292 | 11.3 | 610 | 16.9 | ||||
| Hodgkin lymphoma | 798 | 30.8 | 182 | 5.1 | ||||
| Non-Hodgkin lymphoma | 278 | 10.7 | 275 | 7.6 | ||||
| Soft tissue sarcoma | 279 | 10.8 | 377 | 10.5 | ||||
| Osteosarcoma/Ewing | 481 | 18.6 | 162 | 4.5 | ||||
| Overall treatment | NA | < .001 | ||||||
| Surgery only | 204 | 7.9 | 211 | 5.9 | ||||
| Chemotherapy | 540 | 20.9 | 903 | 25.1 | ||||
| Radiotherapy | 494 | 19.1 | 332 | 9.2 | ||||
| Chemotherapy and radiotherapy | 540 | 20.9 | 1,920 | 53.3 | ||||
| Chemotherapy | NA | < .001 | ||||||
| Antimetabolites | 959 | 37.0 | 2,249 | 62.4 | ||||
| Corticosteroids | 1,055 | 40.8 | 2,232 | 62.0 | ||||
| CNS irradiation | NA | < .001 | ||||||
| None | 825 | 31.9 | 1,226 | 34.0 | ||||
| Indirect‡ | 966 | 37.3 | 424 | 11.8 | ||||
| Direct < 20 Gy | 274 | 10.6 | 648 | 18.0 | ||||
| Direct ≥ 20 Gy | 405 | 15.6 | 1,108 | 30.8 | ||||
| SMN or recurrence | 205 | 7.9 | 95 | 2.6 | < .001 | |||
| Education | < .001 | .0894 | ||||||
| < 12 years | 74 | 2.9 | 163 | 4.5 | 9 | 2.3 | ||
| High school graduate | 282 | 10.9 | 587 | 16.3 | 51 | 13.1 | ||
| Post–high school training | 796 | 30.8 | 1,359 | 37.7 | 132 | 33.9 | ||
| College | 906 | 35.0 | 1,138 | 31.6 | 135 | 34.6 | ||
| Postgraduate | 512 | 19.8 | 323 | 9.0 | 61 | 15.6 | ||
| Employment | < .001 | < .001 | ||||||
| Unable to work | 202 | 7.8 | 249 | 6.9 | 6 | 1.5 | ||
| Unemployed | 275 | 10.6 | 391 | 10.9 | 44 | 11.3 | ||
| Student | 25 | 1.0 | 248 | 6.9 | 15 | 3.9 | ||
| Working part time | 244 | 9.4 | 500 | 13.9 | 51 | 13.1 | ||
| Working full time | 1,815 | 70.1 | 2,168 | 60.2 | 273 | 70 | ||
| Household income | < .001 | .2457 | ||||||
| < $20,000 | 213 | 8.2 | 481 | 13.4 | 25 | 6.4 | ||
| $20,000-$39,999 | 433 | 16.7 | 795 | 22.1 | 58 | 14.9 | ||
| $40,000-$59,999 | 486 | 18.8 | 628 | 17.4 | 79 | 20.3 | ||
| $60,000-$79,999 | 443 | 17.1 | 484 | 13.4 | 65 | 16.7 | ||
| $80,000-$99,999 | 320 | 12.4 | 269 | 7.5 | 48 | 12.3 | ||
| > $100,000 | 549 | 21.2 | 367 | 10.2 | 88 | 22.6 | ||
| Marital status | < .001 | .0022 | ||||||
| Single | 537 | 20.7 | 2,036 | 56.5 | 109 | 28 | ||
| Married/living as married | 1,751 | 67.6 | 1,325 | 36.8 | 229 | 58.7 | ||
| Divorced/separated | 272 | 10.5 | 218 | 6.1 | 48 | 12.3 | ||
| Health insurance status | < .001 | .7464 | ||||||
| Yes | 2,391 | 92.4 | 3,093 | 85.9 | 360 | 92.3 | ||
| No | 181 | 7.0 | 478 | 13.3 | 29 | 7.4 | ||
| Live independently | < .001 | .073 | ||||||
| Yes | 2,291 | 88.5 | 2,328 | 64.6 | 335 | 85.9 | ||
| No | 272 | 10.5 | 1,242 | 34.5 | 54 | 13.9 | ||
| Medications | ||||||||
| Antidepressants | 375 | 14.5 | 432 | 12.0 | 45 | 11.5 | .004 | .0785 |
| Anxiolytics | 136 | 5.3 | 114 | 3.2 | 9 | 2.3 | < .001 | < .001 |
Abbreviations: AeYA, adolescent and early young adult; NA, not applicable; SMN, second malignant neoplasm.
Comparing AeYA survivors with non-AeYA survivors.
Comparing AeYA survivors with siblings.
Indirect CNS irradiation indicates scatter from direct radiation to a noncranial site.
The comparison between survivors diagnosed during childhood when less than 11 years of age and those diagnosed at age 11 to 21 years with respect to emotional distress and neurocognitive impairment was done using multiple logistic regression, and the results were stratified by diagnosis groups. The covariates considered in the models included current age, sex, and treatment exposures, which are typically associated with neurocognitive impairment. Although age at diagnosis is corrected with current age (r = 0.79), the variables are not confounded, and both have potential to offer unique predictive ability to the models. A comparison of all possible combinations of predictors was done, and the best model based on minimum Akaike Information Criterion (AIC) was selected. Current age and sex were forced into all models because they are known to be associated with outcomes. Among the other variables, for those that were not significant at the P = .05 level in this model, the least significant predictor was removed and the new model was considered acceptable if the increase in AIC value was less than 10 units.22 This procedure was continued until the final model, with all insignificant factors (change in AIC value of < 10) removed, was obtained. The same approach for model selection, described earlier, was used to compare survivors diagnosed during ages 11 to 14 years to those diagnosed during the ages of 15 to 21 years, and the results, stratified by diagnosis groups, are reported in Appendix Table A1 (online only). In a similar manner, the impact of emotional and neurocognitive function on current employment, educational attainment, and living independently was examined. Odd ratios (ORs) and 95% CIs were calculated for variables retained in the final models. All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC), and two-sided statistical inferences were used throughout the analyses.
RESULTS
Characteristics of the adolescent and nonadolescent survivors and sibling controls are listed in Table 1. AeYA survivors were similar to siblings in sex and current age, although they were significantly less likely to be married (P = .02) or employed (P < .001). When AeYA survivors were compared with non-AeYA cancer survivors, non-AeYA survivors were less likely to be married (P < .001), employed (P < .001), or live independently (P < .001).
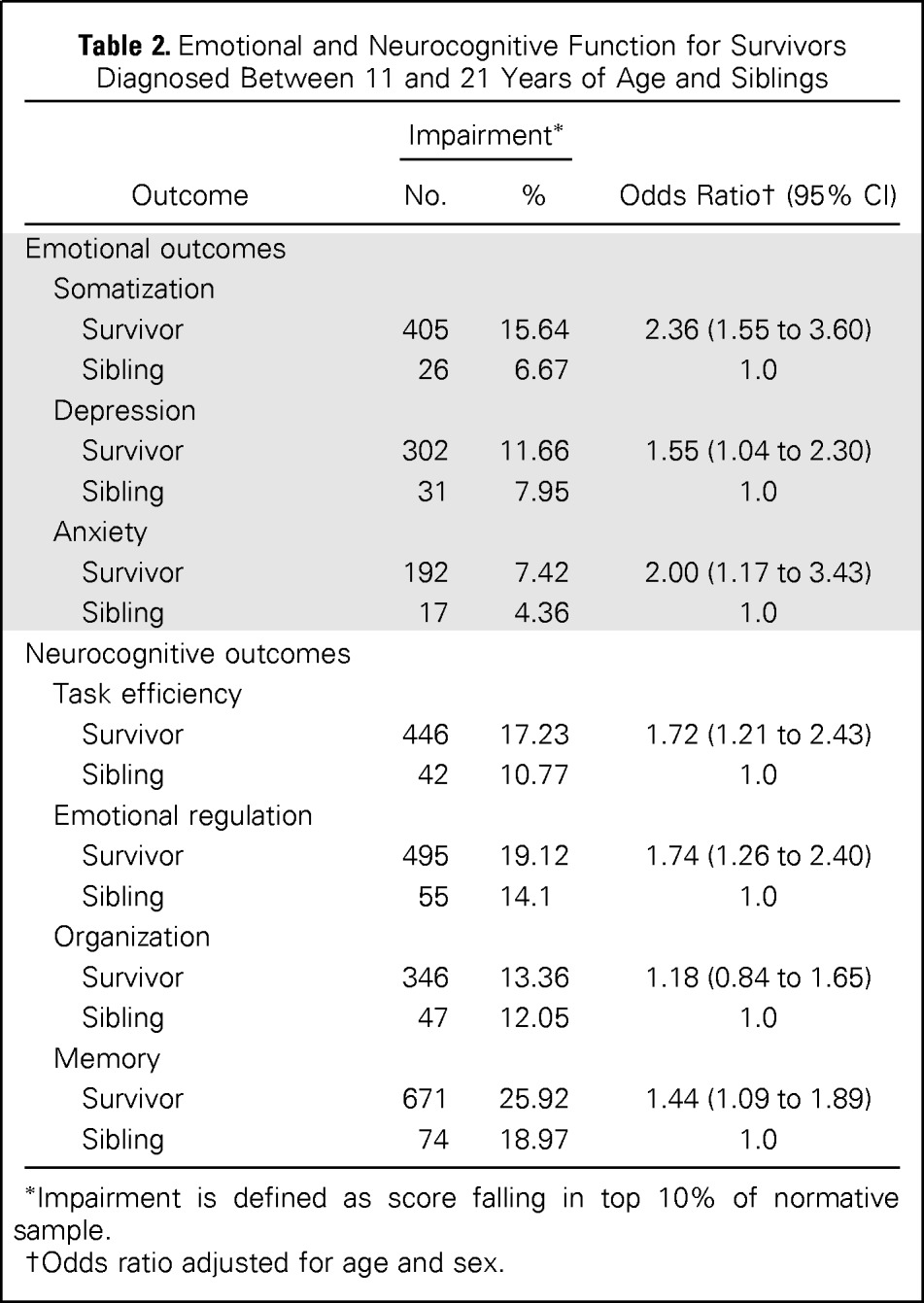
Rates of impairment in self-reported emotional and neurocognitive outcomes are listed for AeYA survivor and sibling cohorts in Table 2. After adjusting for current age and sex, survivors diagnosed as adolescents self-reported greater emotional distress, including anxiety (OR, 2.00; 95% CI, 1.17 to 3.43), somatization (OR, 2.36; 95% CI, 1.55 to 3.60), and depression (OR, 1.55; 95% CI, 1.04 to 2.30), compared with siblings. AeYA survivors also self-reported higher rates of neurocognitive problems than siblings in task efficiency (OR, 1.72; 95% CI, 1.21 to 2.43), emotional regulation (OR, 1.74; 95% CI, 1.26 to 2.40), and memory (OR, 1.44; 95% CI, 1.09 to 1.89).
Table 2.
Emotional and Neurocognitive Function for Survivors Diagnosed Between 11 and 21 Years of Age and Siblings
| Outcome | Impairment* |
Odds Ratio† (95% CI) | |
|---|---|---|---|
| No. | % | ||
| Emotional outcomes | |||
| Somatization | |||
| Survivor | 405 | 15.64 | 2.36 (1.55 to 3.60) |
| Sibling | 26 | 6.67 | 1.0 |
| Depression | |||
| Survivor | 302 | 11.66 | 1.55 (1.04 to 2.30) |
| Sibling | 31 | 7.95 | 1.0 |
| Anxiety | |||
| Survivor | 192 | 7.42 | 2.00 (1.17 to 3.43) |
| Sibling | 17 | 4.36 | 1.0 |
| Neurocognitive outcomes | |||
| Task efficiency | |||
| Survivor | 446 | 17.23 | 1.72 (1.21 to 2.43) |
| Sibling | 42 | 10.77 | 1.0 |
| Emotional regulation | |||
| Survivor | 495 | 19.12 | 1.74 (1.26 to 2.40) |
| Sibling | 55 | 14.1 | 1.0 |
| Organization | |||
| Survivor | 346 | 13.36 | 1.18 (0.84 to 1.65) |
| Sibling | 47 | 12.05 | 1.0 |
| Memory | |||
| Survivor | 671 | 25.92 | 1.44 (1.09 to 1.89) |
| Sibling | 74 | 18.97 | 1.0 |
Impairment is defined as score falling in top 10% of normative sample.
Odds ratio adjusted for age and sex.
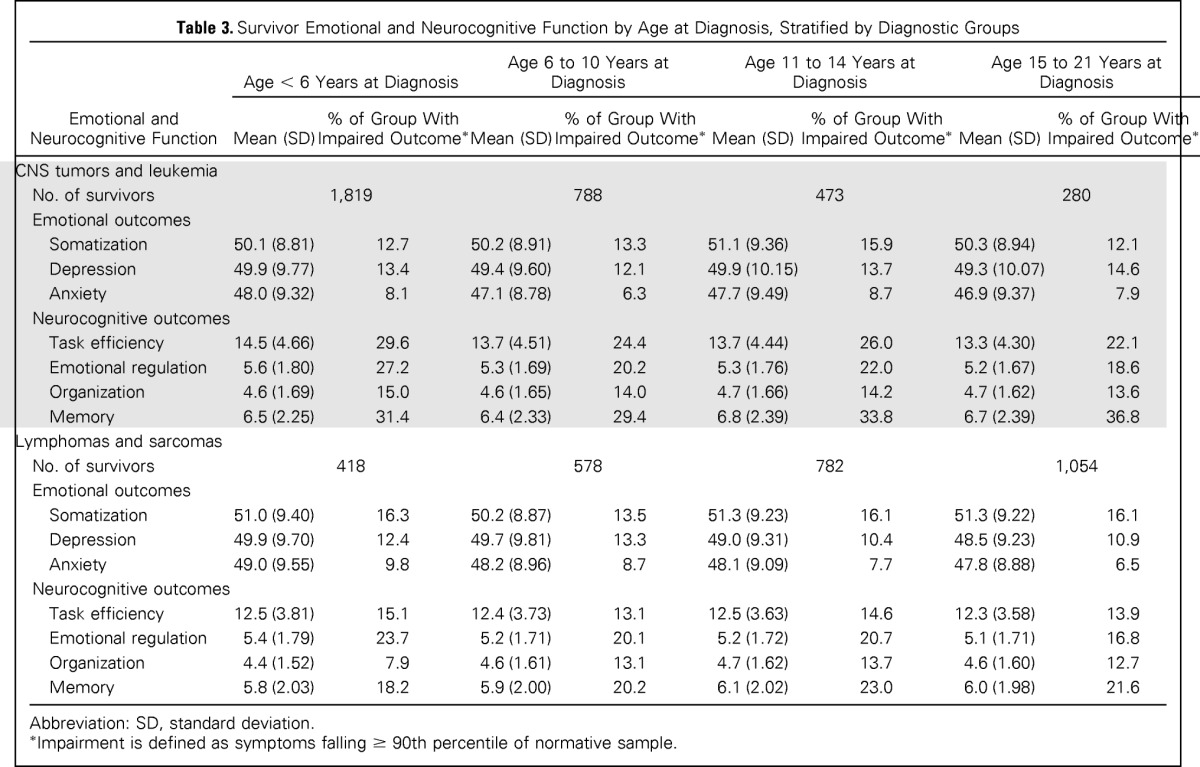
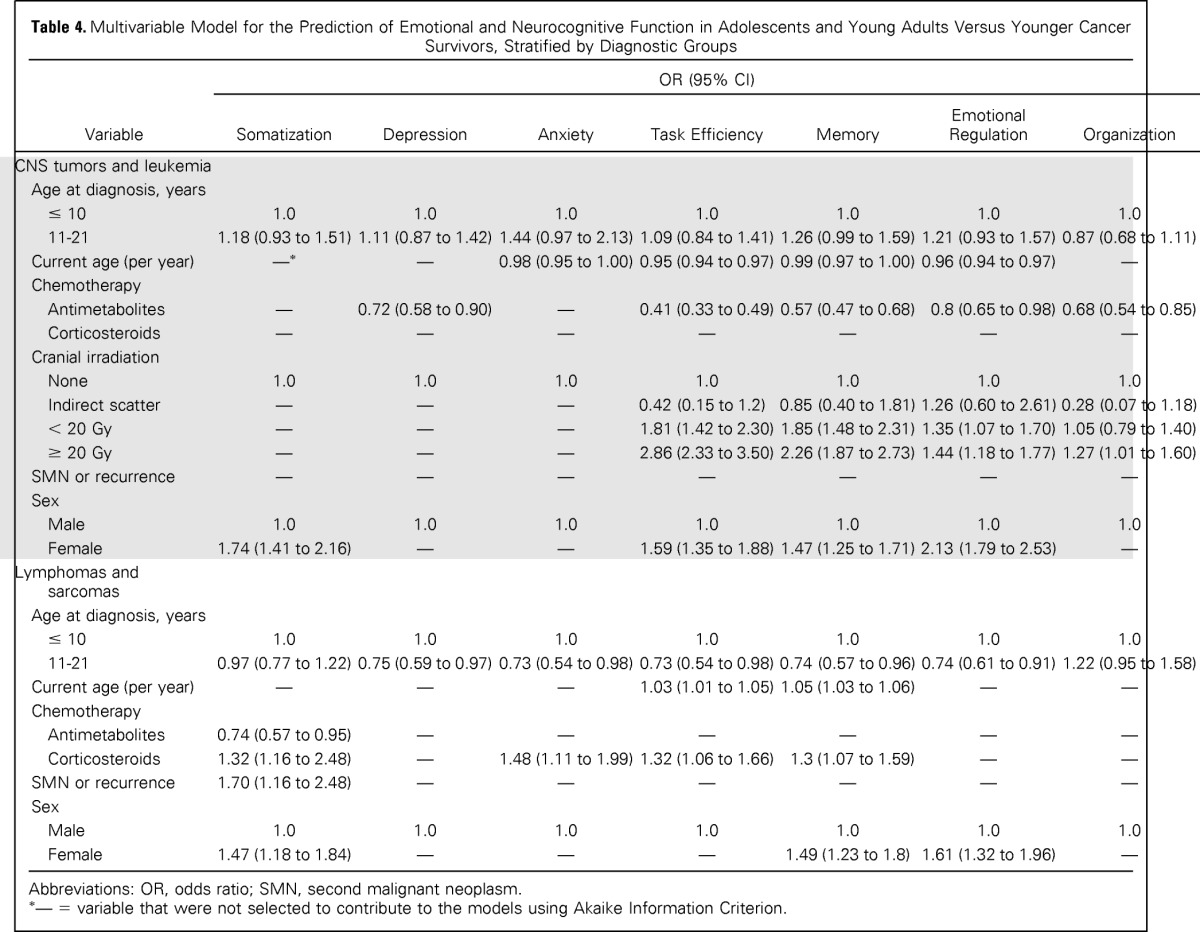
The frequencies of self-reported impairment among survivors by age at diagnosis are listed in Table 3. Historically, children and adolescents diagnosed with leukemia received antimetabolite therapy and cranial radiation therapy (CRT), whereas adolescents diagnosed with lymphomas, sarcomas, or bone tumors did not receive CRT. This, combined with the difference in frequency by age, justified the stratification by groups to account for differences in both age at diagnosis and in the prevalence of CNS-directed therapies. In multivariable models, survivors diagnosed with CNS tumors/leukemia during AeYA did not differ from survivors diagnosed when less than 11 years of age in self-reported emotional distress or neurocognitive function (Table 4). For lymphoma/sarcoma survivors, diagnosis during AeYA was associated with a lower risk for self-reported emotional distress and neurocognitive dysfunction.
Table 3.
Survivor Emotional and Neurocognitive Function by Age at Diagnosis, Stratified by Diagnostic Groups
| Emotional and Neurocognitive Function | Age < 6 Years at Diagnosis |
Age 6 to 10 Years at Diagnosis |
Age 11 to 14 Years at Diagnosis |
Age 15 to 21 Years at Diagnosis |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | % of Group With Impaired Outcome* | Mean (SD) | % of Group With Impaired Outcome* | Mean (SD) | % of Group With Impaired Outcome* | Mean (SD) | % of Group With Impaired Outcome* | |
| CNS tumors and leukemia | ||||||||
| No. of survivors | 1,819 | 788 | 473 | 280 | ||||
| Emotional outcomes | ||||||||
| Somatization | 50.1 (8.81) | 12.7 | 50.2 (8.91) | 13.3 | 51.1 (9.36) | 15.9 | 50.3 (8.94) | 12.1 |
| Depression | 49.9 (9.77) | 13.4 | 49.4 (9.60) | 12.1 | 49.9 (10.15) | 13.7 | 49.3 (10.07) | 14.6 |
| Anxiety | 48.0 (9.32) | 8.1 | 47.1 (8.78) | 6.3 | 47.7 (9.49) | 8.7 | 46.9 (9.37) | 7.9 |
| Neurocognitive outcomes | ||||||||
| Task efficiency | 14.5 (4.66) | 29.6 | 13.7 (4.51) | 24.4 | 13.7 (4.44) | 26.0 | 13.3 (4.30) | 22.1 |
| Emotional regulation | 5.6 (1.80) | 27.2 | 5.3 (1.69) | 20.2 | 5.3 (1.76) | 22.0 | 5.2 (1.67) | 18.6 |
| Organization | 4.6 (1.69) | 15.0 | 4.6 (1.65) | 14.0 | 4.7 (1.66) | 14.2 | 4.7 (1.62) | 13.6 |
| Memory | 6.5 (2.25) | 31.4 | 6.4 (2.33) | 29.4 | 6.8 (2.39) | 33.8 | 6.7 (2.39) | 36.8 |
| Lymphomas and sarcomas | ||||||||
| No. of survivors | 418 | 578 | 782 | 1,054 | ||||
| Emotional outcomes | ||||||||
| Somatization | 51.0 (9.40) | 16.3 | 50.2 (8.87) | 13.5 | 51.3 (9.23) | 16.1 | 51.3 (9.22) | 16.1 |
| Depression | 49.9 (9.70) | 12.4 | 49.7 (9.81) | 13.3 | 49.0 (9.31) | 10.4 | 48.5 (9.23) | 10.9 |
| Anxiety | 49.0 (9.55) | 9.8 | 48.2 (8.96) | 8.7 | 48.1 (9.09) | 7.7 | 47.8 (8.88) | 6.5 |
| Neurocognitive outcomes | ||||||||
| Task efficiency | 12.5 (3.81) | 15.1 | 12.4 (3.73) | 13.1 | 12.5 (3.63) | 14.6 | 12.3 (3.58) | 13.9 |
| Emotional regulation | 5.4 (1.79) | 23.7 | 5.2 (1.71) | 20.1 | 5.2 (1.72) | 20.7 | 5.1 (1.71) | 16.8 |
| Organization | 4.4 (1.52) | 7.9 | 4.6 (1.61) | 13.1 | 4.7 (1.62) | 13.7 | 4.6 (1.60) | 12.7 |
| Memory | 5.8 (2.03) | 18.2 | 5.9 (2.00) | 20.2 | 6.1 (2.02) | 23.0 | 6.0 (1.98) | 21.6 |
Abbreviation: SD, standard deviation.
Impairment is defined as symptoms falling ≥ 90th percentile of normative sample.
Table 4.
Multivariable Model for the Prediction of Emotional and Neurocognitive Function in Adolescents and Young Adults Versus Younger Cancer Survivors, Stratified by Diagnostic Groups
| Variable | OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Somatization | Depression | Anxiety | Task Efficiency | Memory | Emotional Regulation | Organization | |
| CNS tumors and leukemia | |||||||
| Age at diagnosis, years | |||||||
| ≤ 10 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 11-21 | 1.18 (0.93 to 1.51) | 1.11 (0.87 to 1.42) | 1.44 (0.97 to 2.13) | 1.09 (0.84 to 1.41) | 1.26 (0.99 to 1.59) | 1.21 (0.93 to 1.57) | 0.87 (0.68 to 1.11) |
| Current age (per year) | —* | — | 0.98 (0.95 to 1.00) | 0.95 (0.94 to 0.97) | 0.99 (0.97 to 1.00) | 0.96 (0.94 to 0.97) | — |
| Chemotherapy | |||||||
| Antimetabolites | — | 0.72 (0.58 to 0.90) | — | 0.41 (0.33 to 0.49) | 0.57 (0.47 to 0.68) | 0.8 (0.65 to 0.98) | 0.68 (0.54 to 0.85) |
| Corticosteroids | — | — | — | — | — | — | — |
| Cranial irradiation | |||||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Indirect scatter | — | — | — | 0.42 (0.15 to 1.2) | 0.85 (0.40 to 1.81) | 1.26 (0.60 to 2.61) | 0.28 (0.07 to 1.18) |
| < 20 Gy | — | — | — | 1.81 (1.42 to 2.30) | 1.85 (1.48 to 2.31) | 1.35 (1.07 to 1.70) | 1.05 (0.79 to 1.40) |
| ≥ 20 Gy | — | — | — | 2.86 (2.33 to 3.50) | 2.26 (1.87 to 2.73) | 1.44 (1.18 to 1.77) | 1.27 (1.01 to 1.60) |
| SMN or recurrence | — | — | — | — | — | — | — |
| Sex | |||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.74 (1.41 to 2.16) | — | — | 1.59 (1.35 to 1.88) | 1.47 (1.25 to 1.71) | 2.13 (1.79 to 2.53) | — |
| Lymphomas and sarcomas | |||||||
| Age at diagnosis, years | |||||||
| ≤ 10 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 11-21 | 0.97 (0.77 to 1.22) | 0.75 (0.59 to 0.97) | 0.73 (0.54 to 0.98) | 0.73 (0.54 to 0.98) | 0.74 (0.57 to 0.96) | 0.74 (0.61 to 0.91) | 1.22 (0.95 to 1.58) |
| Current age (per year) | — | — | — | 1.03 (1.01 to 1.05) | 1.05 (1.03 to 1.06) | — | — |
| Chemotherapy | |||||||
| Antimetabolites | 0.74 (0.57 to 0.95) | — | — | — | — | — | — |
| Corticosteroids | 1.32 (1.16 to 2.48) | — | 1.48 (1.11 to 1.99) | 1.32 (1.06 to 1.66) | 1.3 (1.07 to 1.59) | — | — |
| SMN or recurrence | 1.70 (1.16 to 2.48) | — | — | — | — | — | — |
| Sex | |||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.47 (1.18 to 1.84) | — | — | — | 1.49 (1.23 to 1.8) | 1.61 (1.32 to 1.96) | — |
Abbreviations: OR, odds ratio; SMN, second malignant neoplasm.
— = variable that were not selected to contribute to the models using Akaike Information Criterion.
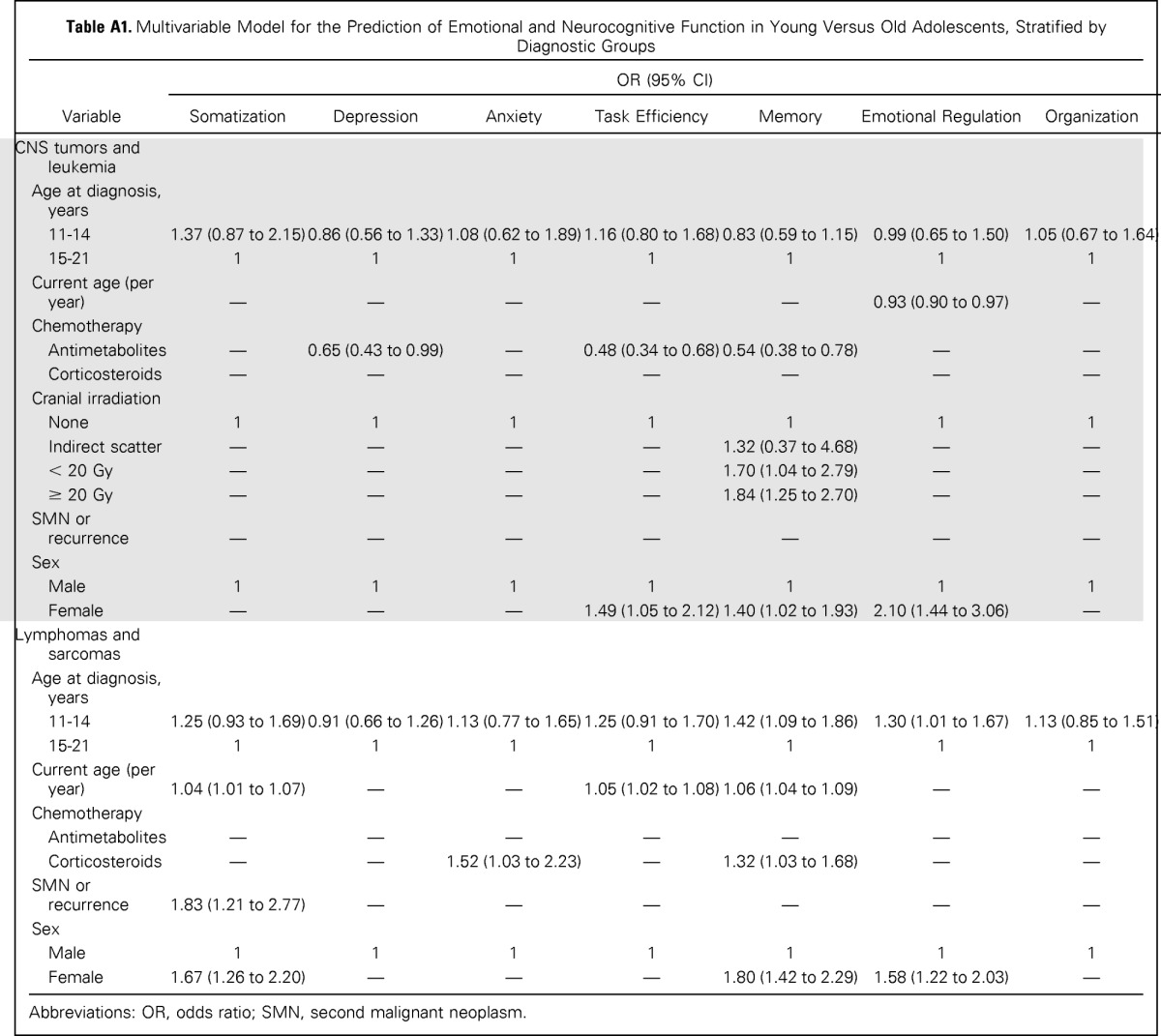
Stratified analyses were also conducted within AeYA subgroups (ie, those diagnosed during the first part of AeYA [11 to 14 years] v those diagnosed during the later part of AeYA [15 to 21 years]). Within the CNS tumor/leukemia group, multivariable models revealed no differences between early and late AeYA diagnosis (Appendix Table A1). However, in the lymphoma/sarcoma group, those diagnosed during early adolescence demonstrated significantly higher risk for self-reported memory (OR, 1.42; 95% CI, 1.09 to 1.86) and emotional regulation (OR, 1.30; 95% CI, 1.01 to 1.67) problems compared with those diagnosed during late AeYA.
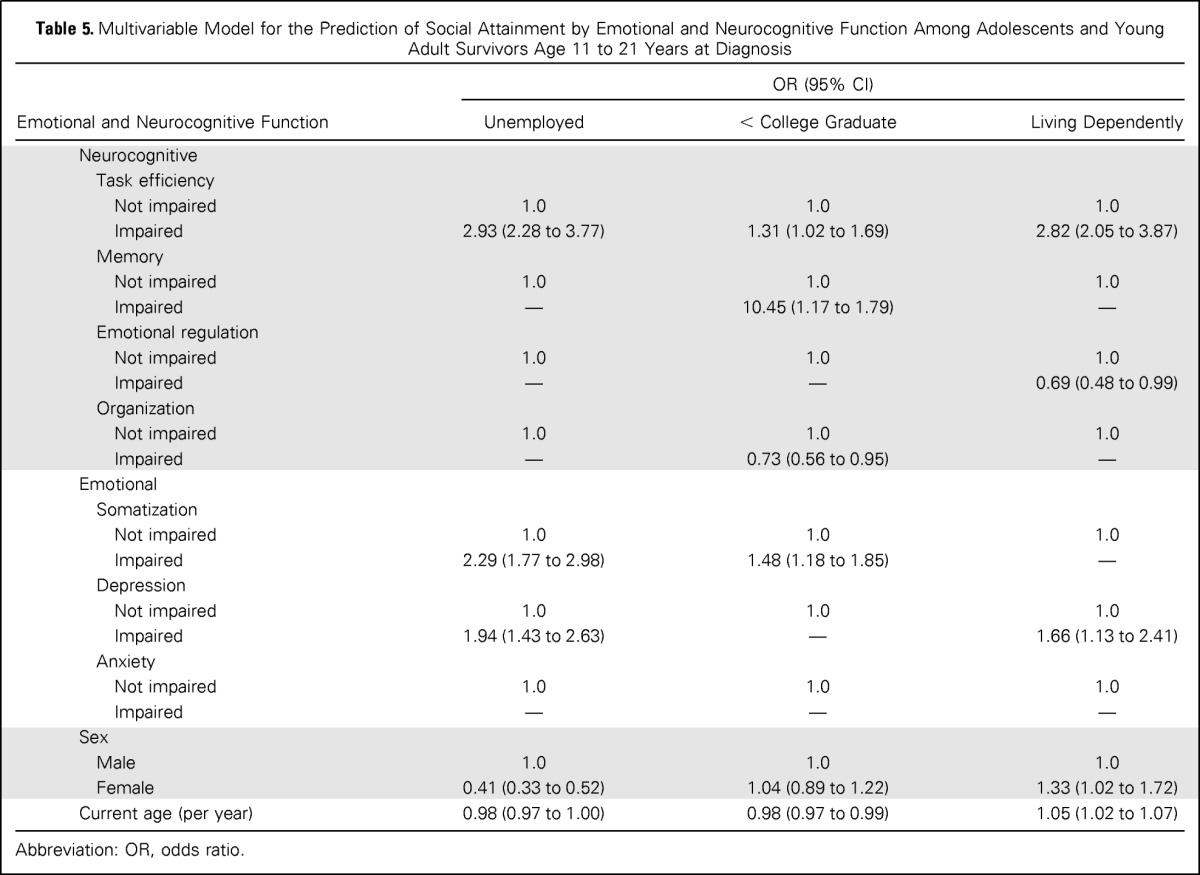
Finally, multivariable models were generated to investigate associations between social attainment and CCSS-NCQ and BSI-18 predictors (Table 5). Self-reported problems with task efficiency increased risk for unemployment (OR, 2.93; 95% CI, 2.28 to 3.77), attaining less than a college education (OR, 1.31; 95% CI, 1.02 to 1.69), and dependent living (OR, 2.82; 95% CI, 2.05 to 3.87) compared with survivors without problems. Self-reported problems with memory increased risk for achieving less than a college education (OR, 1.45; 95% CI, 1.17 to 1.79).
Table 5.
Multivariable Model for the Prediction of Social Attainment by Emotional and Neurocognitive Function Among Adolescents and Young Adult Survivors Age 11 to 21 Years at Diagnosis
| Emotional and Neurocognitive Function | OR (95% CI) |
||
|---|---|---|---|
| Unemployed | < College Graduate | Living Dependently | |
| Neurocognitive | |||
| Task efficiency | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | 2.93 (2.28 to 3.77) | 1.31 (1.02 to 1.69) | 2.82 (2.05 to 3.87) |
| Memory | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | — | 10.45 (1.17 to 1.79) | — |
| Emotional regulation | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | — | — | 0.69 (0.48 to 0.99) |
| Organization | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | — | 0.73 (0.56 to 0.95) | — |
| Emotional | |||
| Somatization | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | 2.29 (1.77 to 2.98) | 1.48 (1.18 to 1.85) | — |
| Depression | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | 1.94 (1.43 to 2.63) | — | 1.66 (1.13 to 2.41) |
| Anxiety | |||
| Not impaired | 1.0 | 1.0 | 1.0 |
| Impaired | — | — | — |
| Sex | |||
| Male | 1.0 | 1.0 | 1.0 |
| Female | 0.41 (0.33 to 0.52) | 1.04 (0.89 to 1.22) | 1.33 (1.02 to 1.72) |
| Current age (per year) | 0.98 (0.97 to 1.00) | 0.98 (0.97 to 0.99) | 1.05 (1.02 to 1.07) |
Abbreviation: OR, odds ratio.
DISCUSSION
Survivors of childhood cancers are at known risk for impaired neurocognitive functioning, leading to poor attainment of adult social milestones. Although previous studies have focused on early childhood as a period of susceptibility,23 the current study is the first to examine self-reported emotional distress and neurocognitive function in adults diagnosed with cancer during AeYA (age 11 to 21 years at diagnosis). Results demonstrate that survivors diagnosed during adolescence exhibit increased rates of self-reported emotional distress and neurocognitive dysfunction when compared with their sibling counterparts. Survivors diagnosed during AeYA were also significantly less likely than sibling controls to have attained post–high school education, to be working full time, to be married, or to be living independently, and social outcomes were related to neurocognitive symptoms.
Treatment of adolescents can be longer and more challenging than that of younger children as a result of unique developmental and psychosocial aspects of adolescence.24 Previous studies have demonstrated that a diagnosis of cancer during this critical time can be disruptive to the growth process necessary for adulthood.25 Cancer treatment during this time has the potential to interfere with adolescents' separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies. The long-term impact of disrupted development in these important areas of social, emotional, and functional autonomy is unknown, but it is reasonable to infer that protracted or delayed maturation in these areas nay be associated with persistent distress. In addition, important brain structural and functional maturation processes continue well into adolescence and early adulthood. Areas such as the prefrontal cortex, which coordinate executive functions, mature later than areas that are associated with sensory and motor tasks.26
The risk for self-reported distress and neurocognitive problems among adolescent survivors was diagnosis dependent. Survivors of lymphoma or sarcoma demonstrated lower risk for self-reported distress and neurocognitive problems when diagnosed during adolescence compared with those diagnosed earlier, whereas no such differences were apparent among survivors diagnosed with CNS tumors or leukemia. Because the leukemia/CNS tumor group was more likely to receive CRT, which is well established as a significant predictor of neurocognitive late effects, it may be that the contribution of CRT to self-reported distress and neurocognitive dysfunction is apparent in adulthood, regardless of age at which CRT is administered.
Because survivor of lymphoma and sarcoma are not treated with CRT, detection of differences related to chemotherapies may be possible. In our sample, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory for those diagnosed with lymphomas or sarcomas during adolescence. In addition, we found that those treated with steroids in younger adolescence (age 11 to 14 years) were significantly more likely than those treated in older adolescence (age 15 to 21 years) to report problems with anxiety and memory. Although the vast majority of literature examining neuropsychological late effects has focused on survivors of CNS-impacting cancers, recent findings have provided evidence that non–CNS-directed therapies may be associated with neurocognitive difficulties. For example, survivors of Hodgkin lymphoma treated with thoracic irradiation have been demonstrated to display decreased performance on measures of attention and memory function.27 Deficits were associated with indices of cardiopulmonary health. Current findings support this previous work and contribute to the notion of multiple sources of risk for neurocognitive impairment in long-term survivors of childhood cancer.
Results should be considered in light of several limitations. It is important to note that self-reports of psychological distress and neurocognitive dysfunction are likely to be intercorrelated. This can make it difficult to discern whether emotional distress is contributing to actual or perceived neurocognitive impairment (or vice versa) or if one or more additional variables underlie the emergence of difficulties in both emotional and cognitive domains. Direct assessment of neurocognitive functioning is often conducted in smaller studies, although this was not feasible in the current study. We have, however, recently demonstrated correspondence between self-reported neurocognitive functioning on the CCSS-NCQ and performance-based measures in a sample of more than 800 adult survivors of childhood cancer.28 Results indicated that the CCSS-NCQ demonstrates acceptable discriminant validity against widely used measures of neuropsychological functioning, particularly for the Memory and Task Efficiency domains.
An additional limitation was our choice to stratify results by grouping diagnoses with similar treatment and ages at diagnosis. Further stratification by disease would have limited the ability to use specific treatment as predictors as a result of reduced variance and/or confounding of treatment with diagnosis variables. Finally, we recognize that the treatment protocols that were used in the CCSS cohort are now more than 20 years old. Although previous studies have shown that the intensity of treatment has been reduced for many diagnoses, the pattern of cognitive impairment remains quite similar.7 Previous treatment protocols continue to inform about current risk of late effects. For example, patient strategies for treatment of low-risk leukemia in the 1980s are similar to strategies for standard- and high-risk leukemia, and neurocognitive deficits were still self-reported in 34% of leukemia survivors surveyed upward of 18 years after diagnosis.29 Also, CCSS participants and thousands of adult survivors of childhood cancer who were treated decades ago remain at risk for late effects, and it is critical to document cancer-related late effects in this group over time. Also, we recognize that we are unable to examine young adults in a more thorough manner because our inclusion criteria only included AeYAs diagnosed before the age of 21 years. As such, we recognize that generalization of treatment and late effects of young adult patients treated at nonpediatric tertiary cancer centers may be limited.
Limitations notwithstanding, the current study is the first, to our knowledge, to focus on self-reported neurocognitive function and psychological distress in survivors diagnosed during adolescence. The AeYA population is a group that is not well represented in outcome studies, although there is a need to identify emotional and behavioral issues that are specific to them. This study demonstrated that there are high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones. Accordingly, further follow-up with AeYA survivors is necessary. The National Comprehensive Cancer Network published guidelines in 2012 specific to the AYA population and a neuropsychological evaluation stating that, although severe neurocognitive deficits were uncommon in the survivors of AYA cancer, including CNS tumors, subtle deficits in executive function, sustained memory, and processing speed were noted in patients treated with CRT.30 The National Comprehensive Cancer Network recommended that in patients with evidence of impaired educational or vocational progress, formal neuropsychological evaluation should be completed.30 Our results suggest that these guidelines may need to be expanded to include additional diagnoses.
Appendix
Table A1.
Multivariable Model for the Prediction of Emotional and Neurocognitive Function in Young Versus Old Adolescents, Stratified by Diagnostic Groups
| Variable | OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Somatization | Depression | Anxiety | Task Efficiency | Memory | Emotional Regulation | Organization | |
| CNS tumors and leukemia | |||||||
| Age at diagnosis, years | |||||||
| 11-14 | 1.37 (0.87 to 2.15) | 0.86 (0.56 to 1.33) | 1.08 (0.62 to 1.89) | 1.16 (0.80 to 1.68) | 0.83 (0.59 to 1.15) | 0.99 (0.65 to 1.50) | 1.05 (0.67 to 1.64) |
| 15-21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Current age (per year) | — | — | — | — | — | 0.93 (0.90 to 0.97) | — |
| Chemotherapy | |||||||
| Antimetabolites | — | 0.65 (0.43 to 0.99) | — | 0.48 (0.34 to 0.68) | 0.54 (0.38 to 0.78) | — | — |
| Corticosteroids | — | — | — | — | — | — | — |
| Cranial irradiation | |||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Indirect scatter | — | — | — | — | 1.32 (0.37 to 4.68) | — | — |
| < 20 Gy | — | — | — | — | 1.70 (1.04 to 2.79) | — | — |
| ≥ 20 Gy | — | — | — | — | 1.84 (1.25 to 2.70) | — | — |
| SMN or recurrence | — | — | — | — | — | — | — |
| Sex | |||||||
| Male | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Female | — | — | — | 1.49 (1.05 to 2.12) | 1.40 (1.02 to 1.93) | 2.10 (1.44 to 3.06) | — |
| Lymphomas and sarcomas | |||||||
| Age at diagnosis, years | |||||||
| 11-14 | 1.25 (0.93 to 1.69) | 0.91 (0.66 to 1.26) | 1.13 (0.77 to 1.65) | 1.25 (0.91 to 1.70) | 1.42 (1.09 to 1.86) | 1.30 (1.01 to 1.67) | 1.13 (0.85 to 1.51) |
| 15-21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Current age (per year) | 1.04 (1.01 to 1.07) | — | — | 1.05 (1.02 to 1.08) | 1.06 (1.04 to 1.09) | — | — |
| Chemotherapy | |||||||
| Antimetabolites | — | — | — | — | — | — | — |
| Corticosteroids | — | — | 1.52 (1.03 to 2.23) | — | 1.32 (1.03 to 1.68) | — | — |
| SMN or recurrence | 1.83 (1.21 to 2.77) | — | — | — | — | — | — |
| Sex | |||||||
| Male | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Female | 1.67 (1.26 to 2.20) | — | — | — | 1.80 (1.42 to 2.29) | 1.58 (1.22 to 2.03) | — |
Abbreviations: OR, odds ratio; SMN, second malignant neoplasm.
Footnotes
Supported by Grant No. CA55727 from the National Cancer Institute, Bethesda, MD (G.T.A.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Pinki K. Prasad, Gregory T. Armstrong, Leslie L. Robison, Kevin Krull
Provision of study materials or patients: Leslie L. Robison
Collection and assembly of data: Pinki K. Prasad, Kristina K. Hardy, Deokumar Srivastava, Gregory T. Armstrong, Leslie L. Robison, Kevin Krull
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Psychosocial and Neurocognitive Outcomes in Adult Survivors of Adolescent and Early Young Adult Cancer: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Pinki K. Prasad
No relationship to disclose
Kristina K. Hardy
No relationship to disclose
Nan Zhang
No relationship to disclose
Kim Edelstein
No relationship to disclose
Deokumar Srivastava
Consulting or Advisory Role: SRA International
Lonnie Zeltzer
No relationship to disclose
Marilyn Stovall
No relationship to disclose
Nita L. Seibel
No relationship to disclose
Wendy Leisenring
Research Funding: Merck
Gregory T. Armstrong
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Kevin Krull
No relationship to disclose
REFERENCES
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meadows AT, Gordon J, Massari DJ, et al. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;2:1015–1018. doi: 10.1016/s0140-6736(81)91216-2. [DOI] [PubMed] [Google Scholar]
- 4.Kuskonmaz B, Unal S, Gumruk F, et al. The neurologic complications in pediatric acute lymphoblastic leukemia patients excluding leukemic infiltration. Leuk Res. 2006;30:537–541. doi: 10.1016/j.leukres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31:4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 10.Lerner RM, Steinberg LD. Handbook of Adolescent Psychology (ed 3) Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 11.Anderson V, Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): A 2 year follow-up. Brain Inj. 2005;19:459–470. doi: 10.1080/02699050400004823. [DOI] [PubMed] [Google Scholar]
- 12.Tonks J, Williams WH, Yates P, et al. Cognitive correlates of psychosocial outcome following traumatic brain injury in early childhood: Comparisons between groups of children aged under and over 10 years of age. Clin Child Psychol Psychiatry. 2011;16:185–194. doi: 10.1177/1359104511403583. [DOI] [PubMed] [Google Scholar]
- 13.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 14.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the childhood cancer survivor study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robison LL, Armstrong GT, Boice JD, et al. The childhood cancer survivor study: A national cancer institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksglaede L, Juul A, Olsen LW, et al. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: Data from the pediatric research in office settings network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 18.Derogatis L. Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 19.Recklitis CJ, Parsons SK, Shih MC, et al. Factor structure of the Brief Symptom Inventory–18 in adult survivors of childhood cancer: Results from the Childhood Cancer Survivor Study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Zabora J, BrintzenhofeSzoc K, Jacobsen P, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics. 2001;42:241–246. doi: 10.1176/appi.psy.42.3.241. [DOI] [PubMed] [Google Scholar]
- 21.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–2197. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnham KP, Anderson DR. Model Selection and Inference: A Practical Information-Theoretic Approach. New York, NY: Springer; 1998. p. 353. [Google Scholar]
- 23.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 24.Bleyer WA. Cancer in older adolescents and young adults: Epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38:1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 25.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konrad K, Firk C, Uhlhaas PJ. Brain development during adolescence: Neuroscientific insights into this developmental period. Dtsch Arztebl Int. 2013;110:425–431. doi: 10.3238/arztebl.2013.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol. 2012;30:3618–3624. doi: 10.1200/JCO.2012.42.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krull KR, Minoshima S, Edelmann M, et al. Regional brain glucose metabolism and neurocognitive function in adult survivors of childhood cancer treated with cranial radiation. J Nucl Med. 2014;55:1805–1810. doi: 10.2967/jnumed.114.142950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15:841–851. doi: 10.1016/S1470-2045(14)70265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10:1112–1150. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]


