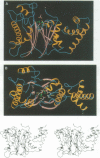
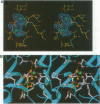
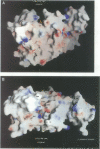
Abstract
Protein phosphatase 2C (PP2C) is a Mn2+- or Mg2+-dependent protein Ser/Thr phosphatase that is essential for regulating cellular stress responses in eukaryotes. The crystal structure of human PP2C reveals a novel protein fold with a catalytic domain composed of a central beta-sandwich that binds two manganese ions, which is surrounded by alpha-helices. Mn2+-bound water molecules at the binuclear metal centre coordinate the phosphate group of the substrate and provide a nucleophile and general acid in the dephosphorylation reaction. Our model presents a framework for understanding not only the classical Mn2+/Mg2+-dependent protein phosphatases but also the sequence-related domains of mitochondrial pyruvate dehydrogenase phosphatase, the Bacillus subtilus phosphatase SpoIIE and a 300-residue domain within yeast adenyl cyclase. The protein architecture and deduced catalytic mechanism are strikingly similar to the PP1, PP2A, PP2B family of protein Ser/Thr phosphatases, with which PP2C shares no sequence similarity, suggestive of convergent evolution of protein Ser/Thr phosphatases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barford D., Flint A. J., Tonks N. K. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994 Mar 11;263(5152):1397–1404. [PubMed] [Google Scholar]
- Barford D., Jia Z., Tonks N. K. Protein tyrosine phosphatases take off. Nat Struct Biol. 1995 Dec;2(12):1043–1053. doi: 10.1038/nsb1295-1043. [DOI] [PubMed] [Google Scholar]
- Barton G. J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993 Jan;6(1):37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Bork P., Brown N. P., Hegyi H., Schultz J. The protein phosphatase 2C (PP2C) superfamily: detection of bacterial homologues. Protein Sci. 1996 Jul;5(7):1421–1425. doi: 10.1002/pro.5560050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewis N. D., Street A. J., Prescott A. R., Cohen P. T. PPX, a novel protein serine/threonine phosphatase localized to centrosomes. EMBO J. 1993 Mar;12(3):987–996. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Field J., Ballester R., Chester N., Young D., Wigler M. Mutational mapping of RAS-responsive domains of the Saccharomyces cerevisiae adenylyl cyclase. Mol Cell Biol. 1990 Jun;10(6):2539–2543. doi: 10.1128/mcb.10.6.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995 Dec 27;377(3):421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Donella Deana A., Mac Gowan C. H., Cohen P., Marchiori F., Meyer H. E., Pinna L. A. An investigation of the substrate specificity of protein phosphatase 2C using synthetic peptide substrates; comparison with protein phosphatase 2A. Biochim Biophys Acta. 1990 Feb 19;1051(2):199–202. doi: 10.1016/0167-4889(90)90194-i. [DOI] [PubMed] [Google Scholar]
- Duncan L., Alper S., Arigoni F., Losick R., Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995 Oct 27;270(5236):641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- Egloff M. P., Cohen P. T., Reinemer P., Barford D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J Mol Biol. 1995 Dec 15;254(5):942–959. doi: 10.1006/jmbi.1995.0667. [DOI] [PubMed] [Google Scholar]
- Evans S. V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993 Jun;11(2):134-8, 127-8. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- Feger G., De Vendittis E., Vitelli A., Masturzo P., Zahn R., Verrotti A. C., Kavounis C., Pal G. P., Fasano O. Identification of regulatory residues of the yeast adenylyl cyclase. EMBO J. 1991 Feb;10(2):349–359. doi: 10.1002/j.1460-2075.1991.tb07956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J., Huang H. B., Kwon Y. G., Greengard P., Nairn A. C., Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995 Aug 31;376(6543):745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- Griffith J. P., Kim J. L., Kim E. E., Sintchak M. D., Thomson J. A., Fitzgibbon M. J., Fleming M. A., Caron P. R., Hsiao K., Navia M. A. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995 Aug 11;82(3):507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Horton J. R., LeMaster D. M. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): a vehicle for direct determination of three-dimensional structure. EMBO J. 1990 May;9(5):1665–1672. doi: 10.1002/j.1460-2075.1990.tb08287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kim Y., Huang J., Cohen P., Matthews H. R. Protein phosphatases 1, 2A, and 2C are protein histidine phosphatases. J Biol Chem. 1993 Sep 5;268(25):18513–18518. [PubMed] [Google Scholar]
- Kissinger C. R., Parge H. E., Knighton D. R., Lewis C. T., Pelletier L. A., Tempczyk A., Kalish V. J., Tucker K. D., Showalter R. E., Moomaw E. W. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995 Dec 7;378(6557):641–644. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- Klumpp S., Hanke C., Donella-Deana A., Beyer A., Kellner R., Pinna L. A., Schultz J. E. A membrane-bound protein phosphatase type 2C from Paramecium tetraurelia. Purification, characterization, and cloning. J Biol Chem. 1994 Dec 30;269(52):32774–32780. [PubMed] [Google Scholar]
- Lawson J. E., Niu X. D., Browning K. S., Trong H. L., Yan J., Reed L. J. Molecular cloning and expression of the catalytic subunit of bovine pyruvate dehydrogenase phosphatase and sequence similarity with protein phosphatase 2C. Biochemistry. 1993 Sep 7;32(35):8987–8993. doi: 10.1021/bi00086a002. [DOI] [PubMed] [Google Scholar]
- Leung J., Bouvier-Durand M., Morris P. C., Guerrier D., Chefdor F., Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994 Jun 3;264(5164):1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Livingstone C. D., Barton G. J. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput Appl Biosci. 1993 Dec;9(6):745–756. doi: 10.1093/bioinformatics/9.6.745. [DOI] [PubMed] [Google Scholar]
- Maeda T., Wurgler-Murphy S. M., Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994 May 19;369(6477):242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Mann D. J., Campbell D. G., McGowan C. H., Cohen P. T. Mammalian protein serine/threonine phosphatase 2C: cDNA cloning and comparative analysis of amino acid sequences. Biochim Biophys Acta. 1992 Feb 28;1130(1):100–104. doi: 10.1016/0167-4781(92)90471-b. [DOI] [PubMed] [Google Scholar]
- Martin B. L., Graves D. J. Isotope effects on the mechanism of calcineurin catalysis: kinetic solvent isotope and isotope exchange studies. Biochim Biophys Acta. 1994 May 18;1206(1):136–142. doi: 10.1016/0167-4838(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Meyer K., Leube M. P., Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994 Jun 3;264(5164):1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Minato T., Wang J., Akasaka K., Okada T., Suzuki N., Kataoka T. Quantitative analysis of mutually competitive binding of human Raf-1 and yeast adenylyl cyclase to Ras proteins. J Biol Chem. 1994 Aug 19;269(33):20845–20851. [PubMed] [Google Scholar]
- Mol C. D., Kuo C. F., Thayer M. M., Cunningham R. P., Tainer J. A. Structure and function of the multifunctional DNA-repair enzyme exonuclease III. Nature. 1995 Mar 23;374(6520):381–386. doi: 10.1038/374381a0. [DOI] [PubMed] [Google Scholar]
- Moore F., Weekes J., Hardie D. G. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem. 1991 Aug 1;199(3):691–697. doi: 10.1111/j.1432-1033.1991.tb16172.x. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Pato M. D., Kerc E. Regulation of smooth muscle phosphatase-II by divalent cations. Mol Cell Biochem. 1991 Feb 27;101(1):31–41. doi: 10.1007/BF00238435. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., van Zyl W. H., Phizicky E. M., Broach J. R. TPD1 of Saccharomyces cerevisiae encodes a protein phosphatase 2C-like activity implicated in tRNA splicing and cell separation. Mol Cell Biol. 1994 Jun;14(6):3634–3645. doi: 10.1128/mcb.14.6.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S. Protein serine/threonine phosphatases--new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Akhavan-Niaki H., McGowan C. H., Russell P. Protein phosphatase 2C, encoded by ptc1+, is important in the heat shock response of Schizosaccharomyces pombe. Mol Cell Biol. 1994 Jun;14(6):3742–3751. doi: 10.1128/mcb.14.6.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K., Russell P. Counteractive roles of protein phosphatase 2C (PP2C) and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995 Feb 1;14(3):492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. M., Collinge M. A., Smith R. D., Horn M. A., Walker J. C. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science. 1994 Nov 4;266(5186):793–795. doi: 10.1126/science.7973632. [DOI] [PubMed] [Google Scholar]
- Stuckey J. A., Schubert H. L., Fauman E. B., Zhang Z. Y., Dixon J. E., Saper M. A. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 A and the complex with tungstate. Nature. 1994 Aug 18;370(6490):571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- Su X. D., Taddei N., Stefani M., Ramponi G., Nordlund P. The crystal structure of a low-molecular-weight phosphotyrosine protein phosphatase. Nature. 1994 Aug 18;370(6490):575–578. doi: 10.1038/370575a0. [DOI] [PubMed] [Google Scholar]
- Suck D., Oefner C., Kabsch W. Three-dimensional structure of bovine pancreatic DNase I at 2.5 A resolution. EMBO J. 1984 Oct;3(10):2423–2430. doi: 10.1002/j.1460-2075.1984.tb02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Lynch K. R., Larner J., Fox J., Yasui A., Kikuchi K., Suzuki Y., Tsuiki S. Molecular cloning of rat type 2C (IA) protein phosphatase mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1796–1800. doi: 10.1073/pnas.86.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk J., Trompeter H. I., Pettrich K. G., Cohen P. T., Campbell D. G., Mieskes G. Molecular cloning and primary structure of a protein phosphatase 2C isoform. FEBS Lett. 1992 Feb 3;297(1-2):135–138. doi: 10.1016/0014-5793(92)80344-g. [DOI] [PubMed] [Google Scholar]
- Wera S., Hemmings B. A. Serine/threonine protein phosphatases. Biochem J. 1995 Oct 1;311(Pt 1):17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A. A., Price C. W. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals. J Bacteriol. 1995 Jan;177(1):123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Van Etten R. L., Stauffacher C. V. Crystal structure of bovine heart phosphotyrosyl phosphatase at 2.2-A resolution. Biochemistry. 1994 Sep 20;33(37):11097–11105. doi: 10.1021/bi00203a006. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Dixon J. E. Protein tyrosine phosphatases: mechanism of catalysis and substrate specificity. Adv Enzymol Relat Areas Mol Biol. 1994;68:1–36. doi: 10.1002/9780470123140.ch1. [DOI] [PubMed] [Google Scholar]
- Zhuo S., Clemens J. C., Stone R. L., Dixon J. E. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J Biol Chem. 1994 Oct 21;269(42):26234–26238. [PubMed] [Google Scholar]