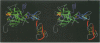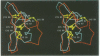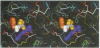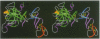Abstract
The structure of the Gla-domainless form of the human anticoagulant enzyme activated protein C has been solved at 2.8 A resolution. The light chain is composed of two domains: an epidermal growth factor (EGF)-like domain modified by a large insert containing an additional disulfide, followed by a typical EGF-like domain. The arrangement of the long axis of these domains describes an angle of approximately 80 degrees. Disulfide linked to the light chain is the catalytic domain, which is generally trypsin-like but contains a large insertion loop at the edge of the active site, a third helical segment, a prominent cationic patch analogous to the anion binding exosite I of thrombin and a trypsin-like Ca[II] binding site. The arrangement of loops around the active site partially restricts access to the cleft. The S2 and S4 subsites are much more polar than in factor Xa and thrombin, and the S2 site is unrestricted. While quite open and exposed, the active site contains a prominent groove, the surface of which is very polar with evidence for binding sites on the primed side, in addition to those typical of the trypsin class found on the non-primed side.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertina R. M., Koeleman B. P., Koster T., Rosendaal F. R., Dirven R. J., de Ronde H., van der Velden P. A., Reitsma P. H. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994 May 5;369(6475):64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- Bode W., Huber R. Natural protein proteinase inhibitors and their interaction with proteinases. Eur J Biochem. 1992 Mar 1;204(2):433–451. doi: 10.1111/j.1432-1033.1992.tb16654.x. [DOI] [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Schwager P. The refined crystal structure of bovine beta-trypsin at 1.8 A resolution. II. Crystallographic refinement, calcium binding site, benzamidine binding site and active site at pH 7.0. J Mol Biol. 1975 Nov 15;98(4):693–717. doi: 10.1016/s0022-2836(75)80005-2. [DOI] [PubMed] [Google Scholar]
- Bode W., Turk D., Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992 Apr;1(4):426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter H., Bauer M., Huber R., Lollar P., Bode W. X-ray structure of clotting factor IXa: active site and module structure related to Xase activity and hemophilia B. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9796–9800. doi: 10.1073/pnas.92.21.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981 Nov;68(5):1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B. Thrombophilia: the discovery of activated protein C resistance. Adv Genet. 1995;33:135–175. doi: 10.1016/s0065-2660(08)60333-8. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Le Bonniec B. F., Johnson A. E. Protein C activation. Methods Enzymol. 1993;222:359–385. doi: 10.1016/0076-6879(93)22024-a. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Taylor F. B., Jr, Snow T. R. Inflammation and coagulation: linked processes potentially regulated through a common pathway mediated by protein C. Thromb Haemost. 1991 Jul 12;66(1):160–165. [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Esmon C. T. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995 Jul;9(10):946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- Esmon N. L., DeBault L. E., Esmon C. T. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983 May 10;258(9):5548–5553. [PubMed] [Google Scholar]
- Evans S. V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993 Jun;11(2):134-8, 127-8. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Smudzin T. M., Walker F. J. Activated protein C-catalyzed inactivation of human factor VIII and factor VIIIa. Identification of cleavage sites and correlation of proteolysis with cofactor activity. J Biol Chem. 1991 Oct 25;266(30):20139–20145. [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Amino acid sequence of the light chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12170–12179. [PubMed] [Google Scholar]
- Foster D. C., Yoshitake S., Davie E. W. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D., Davie E. W. Characterization of a cDNA coding for human protein C. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4766–4770. doi: 10.1073/pnas.81.15.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome K., Esmon C. T. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994 Oct 21;269(42):26486–26491. [PubMed] [Google Scholar]
- Griffith M. J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. J Biol Chem. 1982 Jul 10;257(13):7360–7365. [PubMed] [Google Scholar]
- Grinnell B. W., Hermann R. B., Yan S. B. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology. 1994 Apr;4(2):221–225. doi: 10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Walls J. D., Gerlitz B. Glycosylation of human protein C affects its secretion, processing, functional activities, and activation by thrombin. J Biol Chem. 1991 May 25;266(15):9778–9785. [PubMed] [Google Scholar]
- Guinto E. R., Ye J., Le Bonniec B. F., Esmon C. T. Glu192-->Gln substitution in thrombin yields an enzyme that is effectively inhibited by bovine pancreatic trypsin inhibitor and tissue factor pathway inhibitor. J Biol Chem. 1994 Jul 15;269(28):18395–18400. [PubMed] [Google Scholar]
- Heeb M. J., Gruber A., Griffin J. H. Identification of divalent metal ion-dependent inhibition of activated protein C by alpha 2-macroglobulin and alpha 2-antiplasmin in blood and comparisons to inhibition of factor Xa, thrombin, and plasmin. J Biol Chem. 1991 Sep 15;266(26):17606–17612. [PubMed] [Google Scholar]
- Hermans J. M., Stone S. R. Interaction of activated protein C with serpins. Biochem J. 1993 Oct 1;295(Pt 1):239–245. doi: 10.1042/bj2950239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly R. D., Foster D. C. Resistance to inhibition by alpha-1-anti-trypsin and species specificity of a chimeric human/bovine protein C. Biochemistry. 1994 Feb 22;33(7):1876–1880. doi: 10.1021/bi00173a034. [DOI] [PubMed] [Google Scholar]
- Kalafatis M., Rand M. D., Mann K. G. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994 Dec 16;269(50):31869–31880. [PubMed] [Google Scholar]
- Le Bonniec B. F., Esmon C. T. Glu-192----Gln substitution in thrombin mimics the catalytic switch induced by thrombomodulin. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7371–7375. doi: 10.1073/pnas.88.16.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMMEN E. F., THOMAS W. R., SEEGERS W. H. Activation of purified prothrombin to autoprothrombin I or autoprothrombin II (platelet cofactor II or autoprothrombin II-A). Thromb Diath Haemorrh. 1960 Dec 15;5:218–249. [PubMed] [Google Scholar]
- Marlar R. A., Neumann A. Neonatal purpura fulminans due to homozygous protein C or protein S deficiencies. Semin Thromb Hemost. 1990 Oct;16(4):299–309. doi: 10.1055/s-2007-1002683. [DOI] [PubMed] [Google Scholar]
- Mesters R. M., Heeb M. J., Griffin J. H. A novel exosite in the light chain of human activated protein C essential for interaction with blood coagulation factor Va. Biochemistry. 1993 Nov 30;32(47):12656–12663. doi: 10.1021/bi00210a014. [DOI] [PubMed] [Google Scholar]
- Mesters R. M., Heeb M. J., Griffin J. H. Interactions and inhibition of blood coagulation factor Va involving residues 311-325 of activated protein C. Protein Sci. 1993 Sep;2(9):1482–1489. doi: 10.1002/pro.5560020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesters R. M., Houghten R. A., Griffin J. H. Identification of a sequence of human activated protein C (residues 390-404) essential for its anticoagulant activity. J Biol Chem. 1991 Dec 25;266(36):24514–24519. [PubMed] [Google Scholar]
- Murakawa M., Okamura T., Kamura T., Kuroiwa M., Harada M., Niho Y. A comparative study of partial primary structures of the catalytic region of mammalian protein C. Br J Haematol. 1994 Mar;86(3):590–600. doi: 10.1111/j.1365-2141.1994.tb04791.x. [DOI] [PubMed] [Google Scholar]
- Neuenschwander P. F., Morrissey J. H. Alteration of the substrate and inhibitor specificities of blood coagulation factor VIIa: importance of amino acid residue K192. Biochemistry. 1995 Jul 11;34(27):8701–8707. doi: 10.1021/bi00027a020. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K., Padmanabhan K. P., Tulinsky A., Park C. H., Bode W., Huber R., Blankenship D. T., Cardin A. D., Kisiel W. Structure of human des(1-45) factor Xa at 2.2 A resolution. J Mol Biol. 1993 Aug 5;232(3):947–966. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Whinna H. C., Church F. C. A comparison of three heparin-binding serine proteinase inhibitors. J Biol Chem. 1992 May 5;267(13):8795–8801. [PubMed] [Google Scholar]
- Reitsma P. H., Bernardi F., Doig R. G., Gandrille S., Greengard J. S., Ireland H., Krawczak M., Lind B., Long G. L., Poort S. R. Protein C deficiency: a database of mutations, 1995 update. On behalf of the Subcommittee on Plasma Coagulation Inhibitors of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995 May;73(5):876–889. [PubMed] [Google Scholar]
- Rezaie A. R., Esmon C. T. Contribution of residue 192 in factor Xa to enzyme specificity and function. J Biol Chem. 1995 Jul 7;270(27):16176–16181. doi: 10.1074/jbc.270.27.16176. [DOI] [PubMed] [Google Scholar]
- Rezaie A. R., Esmon C. T. Conversion of glutamic acid 192 to glutamine in activated protein C changes the substrate specificity and increases reactivity toward macromolecular inhibitors. J Biol Chem. 1993 Sep 25;268(27):19943–19948. [PubMed] [Google Scholar]
- Rezaie A. R., Esmon C. T. Tryptophans 231 and 234 in protein C report the Ca(2+)-dependent conformational change required for activation by the thrombin-thrombomodulin complex. Biochemistry. 1995 Sep 26;34(38):12221–12226. doi: 10.1021/bi00038a016. [DOI] [PubMed] [Google Scholar]
- Rezaie A. R., Mather T., Sussman F., Esmon C. T. Mutation of Glu-80-->Lys results in a protein C mutant that no longer requires Ca2+ for rapid activation by the thrombin-thrombomodulin complex. J Biol Chem. 1994 Feb 4;269(5):3151–3154. [PubMed] [Google Scholar]
- Rivard G. E., David M., Farrell C., Schwarz H. P. Treatment of purpura fulminans in meningococcemia with protein C concentrate. J Pediatr. 1995 Apr;126(4):646–652. doi: 10.1016/s0022-3476(95)70369-1. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Curriden S., Lawrence D., Griffin J. H., Loskutoff D. J. Activated protein C stimulates the fibrinolytic activity of cultured endothelial cells and decreases antiactivator activity. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1121–1125. doi: 10.1073/pnas.82.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander C., Schneider R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins. 1991;9(1):56–68. doi: 10.1002/prot.340090107. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Scully M. F., Toh C. H., Hoogendoorn H., Manuel R. P., Nesheim M. E., Solymoss S., Giles A. R. Activation of protein C and its distribution between its inhibitors, protein C inhibitor, alpha 1-antitrypsin and alpha 2-macroglobulin, in patients with disseminated intravascular coagulation. Thromb Haemost. 1993 May 3;69(5):448–453. [PubMed] [Google Scholar]
- Seegers W. H., Novoa E., Henry R. L., Hassouna H. I. Relationship of "new" vitamin K-dependent Protein C and "old" autoprothrombin II-a. Thromb Res. 1976 May;8(5):543–552. doi: 10.1016/0049-3848(76)90236-x. [DOI] [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Stenflo J., Fernlund P. Amino acid sequence of the heavy chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12180–12190. [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Specificity of activated human protein C. Biochem J. 1985 Sep 1;230(2):497–502. doi: 10.1042/bj2300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayalakshmi J., Padmanabhan K. P., Mann K. G., Tulinsky A. The isomorphous structures of prethrombin2, hirugen-, and PPACK-thrombin: changes accompanying activation and exosite binding to thrombin. Protein Sci. 1994 Dec;3(12):2254–2271. doi: 10.1002/pro.5560031211. [DOI] [PMC free article] [PubMed] [Google Scholar]