Abstract
BACKGROUND
There is limited data analyzing the pharmacokinetic (PK) and pharmacodynamic (PD) properties of everolimus (EVR) between African-Americans (AA) and Caucasians (C). The purpose of this study was to determine and compare the EVR PKs and concentration associated efficacy and toxicity in AA and C adult kidney transplant recipients.
METHODS
This was a retrospective PK and PD analysis of all patients that received EVR at the Medical University of South Carolina Transplant Center between 2006 and 2012.
RESULTS
Forty-three patients received EVR (22 AA, 21 C). Baseline demographics, immunosuppression and immunologic risk were similar between races, except for pre-existing hypertension, deceased donor type, and cold ischemic time, which were higher in AA patients. PK analysis revealed AA patients received higher initial EVR doses (2.1±0.8 vs. 1.6±0.6 mg/day, p=0.036), leading to higher early EVR concentrations (EVR >6ng/mL during the 1st 60 days: 36% vs. 10%, p=0.037). Efficacy analysis demonstrated similar EVR effects on acute rejection rates (9% vs. 10%, p=0.961), chronic allograft changes (18% vs. 14%, p=0.729), and renal function, with both groups having improved creatinine clearance with EVR therapy (ΔeGFR: 27 vs. 12 mL/min/1.73 m2). Toxicity analysis demonstrated that AA patients had a trend towards higher rates of EVR discontinuation (46% vs. 19%, p=0.065) and significantly more diarrhea/GI intolerance (73% vs. 38%, p=0.022).
CONCLUSION
These results demonstrate EVR therapy is effective at preventing rejection and improving graft function in both AA and C adult renal transplant patients. Conflicting with previous mammalian Target of Rapamycin (mTOR) PK/PD analyses in AA patients, this study cohort demonstrated higher early EVR levels in the AA patients.
BACKGROUND
Over the past few decades, acute rejection rates have dramatically decreased in kidney transplantation. This is largely due to improved more sensitive techniques with HLA antibody detection and enhanced induction and maintenance immunosuppression regimens. Most transplant centers now report acute rejection rates of less than 10-15%, with one year graft survival above 90% and patient survival above 95%.(1, 2) However, despite these dramatic improvements in one year outcomes, long-term outcomes have not improved to the same degree. In particular, three, five, and 10-year graft survival rates have not kept pace with the improvements in the one year graft outcomes. One of the more important reasons attributed for this is the predominant toxicities associated with long-term immunosuppression. In particular, a major etiology is calcineurin inhibitor (CNI) induced nephrotoxicity leading to graft dysfunction and loss.(3)
mTOR inhibitors are most commonly used in combination (CNI minimization) or in place (CNI withdrawal) of CNIs to help alleviate this problematic toxicity.(4-7) The newest agent approved within this class, EVR (Zortress®, Novartis Pharmaceuticals Corporation, East Hanover, NJ) was FDA-approved for kidney transplantation in April of 2010.(8) EVR shares similar pharmacologic properties with sirolimus (Rapamune®), but considerably different pharmacokinetics. Sirolimus has a significantly longer half-life, differences in protein binding, volume of distribution, P-glycoprotein substrate activity, and metabolism.(9-11) There is limited data analyzing the PK and PD properties of EVR between AAs and Cs.(11, 12) Therefore, the purpose of this study was to determine and compare the EVR PKs and concentration associated efficacy and toxicity in AA and C adult kidney transplant recipients.
MATERIALS AND METHODS
Study Design and Patients
This was a retrospective longitudinal study of adult renal transplant recipients that received EVR as part of their immunosuppression regimen. Patients were included if transplanted between March 2006 and May 2012, were at least 18 years of age at the time of transplant, and received EVR therapy with at least one measured EVR trough concentration. Pediatrics, multi-organ transplant recipients, and those that were lost to follow-up or did not have EVR concentrations were excluded. Data collection and analysis included baseline demographics and transplant characteristics. Follow-up data collection and analysis included immunosuppressant doses and whole blood concentrations along with interacting drugs (included fluconazole and diltiazem), adverse drug events, and clinical outcomes.
Immunosuppression Regimens
Patients received induction therapy with either thymoglobulin 1.5 mg/kg IV daily for 3 to 5 doses, daclizumab 1 mg/kg IV on day 0 and day 7 post-transplant, or basiliximab 20 mg IV on day 0 and day 4 post-transplant. Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil (MMF) and corticosteroids for the vast majority of patients, with a small subset of patients receiving cyclosporine. Most patients were transitioned to EVR in place of tacrolimus several months following their transplants due to intolerance to the baseline regimen. A few patients were started on de novo EVR in combination with cyclosporine. EVR doses were adjusted to maintain a target whole blood trough concentration of 6 to 12 ng/mL when used in combination with MMF and 3 to 8 ng/mL when used in combination with a CNI. Patients that received MMF had starting doses of 1 gm PO BID and adjusted for toxicity; prednisone doses were tapered downward to a dose of 5 mg per day by month 2 to 3 after transplant.
Outcome Measures
The primary outcomes of this study were to measure and compare the PK and PD properties of EVR in AA and C adult kidney transplant patients. Clinical PK parameters captured and compared were total daily doses and 12-hour whole blood trough concentrations. Doses and concentrations were determined and compared between the two groups across the study time period by averaging these values for each patient, then taking the mean of these averages for each study cohort (AA and C). Based on linear PK principles, doses needed to achieve therapeutic EVR trough concentrations were calculated using a goal EVR trough concentration of 6 ng/mL (for example, if a patient’s mean EVR trough concentration was 4 ng/mL with a mean dose of 1.5 mg/day, then the dose needed to achieve a goal trough of 6 ng/mL would be 2.25 mg/day [6 ng/mL divided by 4 ng/mL multiplied by 1.5 mg/day]). The concentration to dose ratio (C/D ratio), a surrogate for EVR clearance, was calculated by dividing the mean trough concentration by the mean EVR dose for each patient, then averaging these across study groups. PD was determined and compared between groups for both efficacy and toxicity. Efficacy was determine by the prevention of acute rejection, preservation of renal function, and prevention of the development of chronic allograft nephropathy due to interstitial fibrosis and/or tubular atrophy (IF/TA); toxicity was determined by capturing common and severe mTOR associated toxicities, including leukopenia, GI related issues/diarrhea, proteinuria, wound healing issues, dyslipidemia, edema and infections.
Definitions
Graft failure was defined as return to chronic dialysis, re-transplantation, or death. Acute rejection had to be biopsy-proven, treated and at least Grade 1A or higher per Banff criteria.(13) All biopsies were for cause, as there was no protocol biopsies performed in these patients. Delayed graft function was defined as requiring dialysis within seven days post-transplant. EVR toxicities were defined as the following: leukopenia, WBC < 3,000 cells/mm3; proteinuria, urine protein to creatinine ratio > 800 mg/gm of creatinine; dyslipidemia, need for new lipid therapy or total cholesterol >200 mg/dL, triglycerides >150 mg/dL, or low density lipoprotein (LDL) >130 mg/dL; diarrhea/GI intolerance, documentation in the medical record of these issues; edema, documentation in the medical record or new use of loop diuretic therapy; infection, documentation in the medical record. Estimated glomerular filtration rate (eGFR) was calculated using the 4-variable MDRD equation, which has demonstrated to be one of the most accurate estimates of GFR in kidney transplant recipients.(14) For comparison of eGFR at baseline to follow-up, the last measured eGFR was carried forward to study end for each patient.
Analysis and Statistics
All values are reported as mean ± standard deviation for continuous data and percentages for categorical data. Patient demographics and outcomes were compared using the Student’s T-test test for continuous data, or the Wilcoxon rank-sum test for continuous variables that did not have a similar variance pattern as determined through Levene’s test of equality of variance. Fisher’s exact test was used to compare categorical data. For multiple comparison testing (which included comparisons for eGFR), a Bonferroni adjustment was applied to p-values in order to determine statistical significance (p<0.006). A p-value of less than 0.05 was set to represent statistical significance. Data was captured manually through review of medical records, input into MS Excel 2010 [Microsoft Corp, Seattle, WA] and SPSS [Version 20, SPSS Inc. Chicago, IL.] was used for statistical analysis.
RESULTS
Between March 2006 and May 2012, 1,065 adult solitary kidney transplants were performed at our institution; of which, 48 (5%) received EVR therapy. Four patients did not have EVR blood concentrations measured and one patient was lost to follow-up. These patients were excluded for this analysis, leaving 43 patients in the final study population (22 AAs and 21 C patients). Patients were converted to EVR an average of 7±10 months after transplant, which was similar between groups (6±9 mos in AAs, 8±11 mos in Cs; p=0.522). Eighty-one percent of patients in this cohort were on a CNI-free regimen of EVR, MMF and prednisone. This was also similar when comparing between groups (86% in AAs, 76% in Cs; p=0.457). Table 1 displays the baseline characteristics for each group. AA patients were more likely to have pre-existing hypertension and receive a deceased donor transplant. Demographics, including age, gender, weight and BMI were similar between groups. Immunologic characteristics (HLA matches and PRA) were also similar, with AA patients have a trend toward longer cold ischemic times (CIT); this is likely related to this group having less living donor transplants. Baseline immunosuppression was very similar between groups, as was the use of drugs known to interact with EVR (either fluconazole or diltiazem).
Table 1.
Baseline Demographics and Transplant Characteristics
| Characteristic | AA (n=22) | C (n=21) | p-Value |
|---|---|---|---|
| Age (years±SD) | 50±13 | 52±15 | 0.717 |
| Male Gender | 82% | 57% | 0.078 |
| Pre-Existing Diabetes | 50% | 43% | 0.639 |
| Pre-Existing Hypertension | 100% | 81% | 0.032 |
| Baseline Weight(kg±SD) | 96±23 | 86±20 | 0.156 |
| Baseline Body Mass Index (kg/m2±SD) | 30±6 | 28±6 | 0.301 |
| Donor Type Standard Criteria | 73% | 38% | 0.040 |
| Extended Criteria | 14% | 14% | |
| Living | 14% | 48% | |
| Human Leukocyte Antigen Matches | 2.2±1.6 | 2.7±1.9 | 0.321 |
| Panel Reactive Antibodies (%±SD) | 15±29 | 12±28 | 0.695 |
| Cold Ischemic Time (min±SD) | 1075±540 | 726±667 | 0.066 |
| Induction IL-2RA | 41% | 52% | 0.739 |
| Alemtuzumab | 5% | 5% | |
| rATG | 55% | 43% | |
| De novo EVR | 14% | 10% | 0.674 |
| EVR Conversion | 86% | 90% | |
| Baseline CNI Tacrolimus | 96% | 86% | 0.272 |
| Cyclosporine | 5% | 14% | |
| Developed Delayed Graft Function | 32% | 19% | 0.337 |
| Receiving Interacting Drug | 27% | 19% | 0.523 |
Pharmacokinetic Analysis
PK analysis was conducted in all patients included in this study. Table 2 displays the EVR pharmacokinetic properties and compares these between AAs and Cs. Because cyclosporine (CyA) is known to interact with EVR by decreasing it’s clearance, the PK parameters for each group were calculated for the entire cohort (left side of Table 2) and for those that did not receive CyA (right side of Table 2). Four patients (1 in the AA group and 3 in the C group) received CyA. PK parameters were similar in both analyses; the non-CyA patients have slightly lower EVR doses and C/D ratios, consistent with the known drug-drug interaction between CyA and EVR. The mean number of EVR trough concentrations included in this analysis was similar between groups (8±5 in AA [range 2-15] vs 8±5 in C [range 2-15]; p=0.95). AA patients were more likely to have elevated EVR whole blood trough concentrations, especially during the first 30 and 60 days of therapy. Overall mean EVR concentrations trended towards being higher in AA patients, with this group having fewer patients with low EVR trough concentrations. AAs were started on higher doses at the initiation of EVR (2.1±0.8 vs. 1.6±0.6, p=0.036); mean doses over the course of follow-up were numerically higher but statistical similar between groups (2.6±1.1 vs. 2.2±0.7, p=0.152). There was a trend towards AA patients requiring lower calculated doses to achieve target EVR trough concentrations of 6 ng/mL (3.4±1.8 vs. 4.5±2.5, p=0.094). The mean steady-state C/D ratios were similar between groups (2.5±1.6 in AA vs. 2.0 ±1.7 in C; p=0.36). In both groups, the calculated required doses needed to achieve therapeutic EVR concentrations are significantly higher than the mean starting dose and the currently recommended starting dose per the Zortress® labeling and product information (0.75 mg twice daily).(8) Doses and EVR trough concentrations were similar between patients on a CNI-minimization, compared to a CNI-free regimen, both within the first 60-days and during the entire follow-up period.
Table 2.
EVR Pharmacokinetic Properties for the Entire Cohort and for those that Received Tacrolimus CNI Therapy
| Characteristic | Entire Study Cohort | CyA Patients Removed | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| AA | C | p-Value | AA | C | p-Value | |
|
| ||||||
| (n=22) | (n=21) | (n=21) | (n=18) | |||
|
| ||||||
| Mean EVR Level 1st 30 Days | 4.7±4.1 | 2.9±2.0 | 0.071 | 4.4±3.9 | 2.7±1.8 | 0.082 |
|
| ||||||
| EVR Levels 1st 30 Days of Therapy | ||||||
| >3 ng/mL | 68% | 43% | 0.129 | 67% | 39% | 0.083 |
| >6 ng/mL | 36% | 5% | 0.011 | 33% | 0% | 0.007 |
| >8 ng/mL | 23% | 0% | 0.020 | 19% | 0% | 0.051 |
|
| ||||||
| Mean EVR Level 1st 60 Days | 4.8±3.6 | 3.2±2.2 | 0.075 | 4.6±3.6 | 2.9±1.8 | 0.068 |
|
| ||||||
| EVR Levels 1st 60 days of Therapy | ||||||
| >3 ng/mL | 68% | 43% | 0.129 | 67% | 39% | 0.083 |
| >6 ng/mL | 36% | 10% | 0.037 | 33% | 6% | 0.032 |
| >8 ng/mL | 23% | 5% | 0.089 | 19% | 0% | 0.051 |
|
| ||||||
| Average EVR Levels | ||||||
| <3 ng/mL | 18% | 62% | 0.003 | 19% | 67% | 0.003 |
| <6 ng/mL | 59% | 76% | 0.042 | 62% | 83% | 0.138 |
| <8 ng/mL | 82% | 92% | 0.413 | 86% | 94% | 0.370 |
|
| ||||||
| Starting EVR Dose (mg/day) | 2.1±0.8 | 1.6±0.6 | 0.036 | 2.0±0.8 | 1.6±0.5 | 0.044 |
| Mean EVR Dose (mg/day) | 2.6±1.1 | 2.2±0.7 | 0.152 | 2.6±1.1 | 2.2±0.8 | 0.194 |
| Mean Concentration-to-Dose Ratio | 2.5±1.6 | 2.0±1.7 | 0.360 | 2.4±1.6 | 1.9±1.7 | 0.376 |
Clinical Outcomes
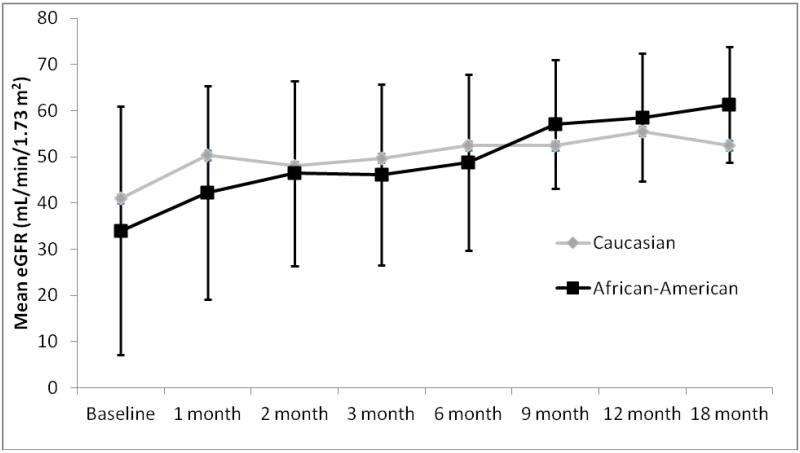
Primary outcomes are displayed in Table 3 and Figure 1. The two groups were similar, both in terms of EVR therapy efficacy (preventing the development of acute rejection and chronic allograft nephropathy) and EVR toxicities. Overall acute rejection rates, both before (5% vs. 0%, p=0.323) and after (9% vs. 10%, p=0.961) EVR initiation were low and similar between AA and C patients, respectively. In terms of histologic outcomes, the two groups were similar with regards to number of biopsies performed, all of which were for cause (2.1±1.3 biopsies in AA [range 0-5] vs 1.8±1.2 in C [range 0-4]; p=0.403). A noteworthy number of patients in both groups had chronic allograft nephropathy (IF/TA) at the time of EVR initiation (23% AA vs. 14% C; p=0.477), with fewer AA patients having IF/TA after EVR conversion (23% as baseline, 18% after EVR conversion in AA patients vs. 14% at baseline, 14% after EVR conversion in C patients). Toxicity analysis revealed comparable outcomes between groups for most toxicities, although AA patients were significantly more likely to have diarrhea/GI intolerances following EVR initiation (73% vs. 38%, p=0.022). Rates of EVR discontinuation trended to being higher in AA patients (46% vs. 19%, p=0.065), but reasons for discontinuation was similar between groups. Estimated GFRs (eGFR) following conversion to EVR are depicted in figure 1 (baseline eGFR is at the time of EVR initiation) and demonstrate that both groups had similar eGFRs throughout the entire study period which significantly improved from the time patients were converted to EVR to end of follow-up in both AAs (34±13 mL/min/1.73 m2 vs. 61±14 mL/min/1.73 m2, p=0.013) and Cs (41±27 vs. 53±13 mL/min/1.73 m2, p=0.021).
Table 3.
Clinical Outcomes Analysis
| Characteristic | AA (n=22) | C (n=21) | p-Value |
|---|---|---|---|
|
| |||
| EFFICACY ANALYSIS | |||
|
| |||
| Rejection Before EVR Conversion | 5% | 0% | 0.323 |
|
| |||
| Chronic Allograft Nephropathy Before EVR Conversion |
23% | 14% | 0.477 |
|
| |||
| Rejection After EVR Conversion | 9% | 10% | 0.961 |
|
| |||
| Chronic Allograft Nephropathy After EVR Conversion |
18% | 14% | 0.729 |
|
| |||
| SAFETY ANALYSIS | |||
|
| |||
| EVR Discontinued | 46% | 19% | 0.065 |
| Reason for Discontinuation | |||
| Proteinuria | 9% | 5% | 0.314 |
| Infection | 14% | 0% | |
| Rejection | 9% | 5% | |
| Wound Complication | 5% | 5% | |
| Anemia | 9% | 0% | |
|
| |||
| EVR Held at Any Time | 14% | 14% | 0.951 |
|
| |||
| Dose Modification of EVR for Toxicity | 9% | 10% | 0.961 |
|
| |||
| Leukopenia | 36% | 33% | 0.835 |
|
| |||
| New Onset Proteinuria (>800mg/gm of creatinine) |
23% | 5% | 0.089 |
|
| |||
| New Onset Dyslipidemia | 18% | 19% | 0.942 |
| On Lipid Lowering Therapy (New or Continued After Transplant) |
68% | 43% | 0.095 |
|
| |||
| Diarrhea/GI Intolerance | 73% | 38% | 0.022 |
|
| |||
| Edema | 55% | 38% | 0.280 |
|
| |||
| Any Infection After Conversion | 27% | 19% | 0.523 |
|
| |||
| Hospital Admission After EVR Conversion | 36% | 29% | 0.586 |
|
| |||
| Admission Likely Related to EVR | 9% | 5% | 0.578 |
Figure 1.
Estimated GFR (MDRD) Across Follow-Up Period for Each Group
*Baseline time point is at the initiation of EVR
Pharmacodynamic Analysis
Table 4 displays the pharmacodynamic analysis for each group, comparing EVR trough concentrations separately for those that either developed an efficacy failure (rejection or IF/TA) or toxicity compared to those that did not experience such an event. Overall, for both patient groups, EVR trough concentrations had limited ability to discern differences for either efficacy or toxicity. In AA patients, EVR concentrations did not appear to influence efficacy or most toxicities. AA patients that were admitted to the hospital after EVR initiation had significantly higher concentrations (overall admissions and those potentially attributable to EVR toxicity) and there was a trend towards patients having lower levels that developed edema. For C patients, there were also a few noteworthy differences. C patients that developed IF/TA had significantly higher EVR concentrations (7.4±1.4 ng/mL vs. 3.3±2.0 ng/mL, p=0.003). Additionally, C patients that developed GI intolerance or diarrhea had lower levels (2.5±0.9 ng/mL vs.4.7±2.6 ng/mL, p=0.015) and those that developed edema had higher levels (5.3±2.2 ng/mL vs.3.0±2.1 ng/mL, p=0.028). Average MMF doses were similar in AA and Cs (1417±710 mg/day vs. 1390±536 mg/day; p=0.904) as well as those that experienced diarrhea compared to those that did not (1348±725 mg/day vs. 1481±479 mg/day; p=0.555).
Table 4.
Pharmacodynamic Analysis Comparing Mean EVR Concentrations for AA and Cs
| Characteristic | AA | C | ||||
|---|---|---|---|---|---|---|
| (n=22) | (n=21) | |||||
| EFFICACY ANALYSIS (mean±SD EVR concentrations (ng/mL)) | ||||||
| No Event | Event | p-Value | No Event | Event | p-Value | |
| Acute Rejection | 5.8±3.0 | 2.8±2.3 | 0.174 | 3.8±2.4 | 4.7±3.3 | 0.633 |
| Chronic Allograft Nephropathy | 4.3±3.0 | 5.9±2.9 | 0.346 | 3.3±2.0 | 7.4±1.4 | 0.003 |
| TOXICITY ANALYSIS (mean±SD EVR concentrations (ng/mL)) | ||||||
| EVR Held | 5.5±3.1 | 6.1±2.2 | 0.743 | 3.9±2.5 | 3.8±2.1 | 0.962 |
| EVR Discontinued | 5.7±2.3 | 5.4±3.4 | 0.840 | 4.1±2.6 | 3.0±0.9 | 0.195 |
| Dose Change for Side Effect | 5.7±3.1 | 4.7±3.3 | 0.746 | 3.9±2.4 | 3.4±3.0 | 0.843 |
| Leukopenia | 5.3±3.5 | 6.1±2.1 | 0.538 | 4.1±2.5 | 3.4±2.1 | 0.553 |
| Proteinuria | 5.5±3.1 | 6.0±2.8 | 0.760 | 3.9±2.4 | 2.2±NA | 0.486 |
| Dyslipidemia | 5.6±3.3 | 5.5±1.7 | 0.965 | 4.0±2.3 | 3.1±2.7 | 0.474 |
| GI/Diarrhea | 5.0±2.5 | 5.8±3.2 | 0.577 | 4.7±2.6 | 2.5±0.9 | 0.015 |
| Wound/Healing | 5.9±2.9 | 3.9±3.1 | 0.298 | 3.9±2.5 | 3.7±2.1 | 0.263 |
| Edema | 6.8±2.9 | 4.6±2.8 | 0.090 | 3.0±2.1 | 5.3±2.2 | 0.028 |
| Any Infection | 5.6±3.2 | 5.5±2.6 | 0.937 | 3.5±2.4 | 5.3±1.8 | 0.199 |
| Hospital Admission | 4.7±2.5 | 7.1±3.3 | 0.065 | 3.6±2.2 | 4.6±2.7 | 0.368 |
| Admission Related to EVR | 5.3±3.0 | 8.1±0.1 | 0.001 | 3.9±2.4 | 2.4±NA | 0.540 |
DISCUSSION
The results of this EVR analysis in kidney transplant recipients demonstrate that AA patients appear to have similar PK properties as compared to Cs, with both groups requiring higher than currently recommended starting doses to achieve therapeutic concentrations. EVR was fairly well tolerated in these patients and has excellent efficacy in preventing acute rejection, IF/TA and improving eGFR across both AA and C patients.
There is limited data published with regards to comparing PK or PD parameters for EVR across racial or ethnic groups. One study published by Kovarik et al demonstrated black patients had approximately a 20% higher clearance of EVR.(11) However, all the patients in this study were receiving concomitant cyclosporine therapy, which is known to significantly impact EVR PK. There are no published studies comparing the racial or ethnic PK or PD of EVR when used in combination with tacrolimus or in CNI withdrawal regimens.(10, 15) It seems reasonable, given the similarity of EVR PK metabolism to other immunosuppressants, that AA patients, in general, would require higher doses to achieve similar trough concentrations.(11, 15) It is not completely apparent why this was not demonstrated within the context of the study results presented here. Certainly, drug interactions or the combination of cyclosporine and EVR would explain this discrepancy, but neither of these was more common in AA patients. One explanation could be the significantly higher incidence of diarrhea in the AA patients. Studies with tacrolimus have demonstrated that patients with diarrhea have significantly higher dose normalized concentrations, perhaps related to a disruption in enterocyte P-glycoprotein activity.(16-20) It appears the EVR is a substrate for PGP(15, 21) and thus diarrhea may impact bioavailability in a similar fashion as describe with tacrolimus. However, in the C patients, EVR trough concentrations were lower in those that had diarrhea, arguing against this as an etiology of the higher concentrations in AA patients.
The clinical outcomes analysis demonstrated that EVR is safe and effective at preventing acute rejection, IF/TA, and potentially improving renal function after conversion from a CNI-based regimen. This was consistent across both AA and C patients. There is no data published comparing racial differences in clinical outcomes with an EVR conversion strategy.(7, 22-25) Studies comparing sirolimus efficacy between different patient races or ethnicities have generally demonstrated good results, but most use sirolimus with CNI minimization strategies.(26, 27) One study did demonstrate increased risk of acute rejection in AA patients when sirolimus was used in a CNI withdrawal regimen.(28) The results of the data presented here suggest EVR may be effective when used in AA patients in a CNI withdrawal regimen as long as initial EVR doses are substantially higher than current product labeling recommends.
The pharmacodynamic analysis did not reveal any strong associations between EVR concentrations and either efficacy or toxicity for either AAs or Cs, although AA patients that were admitted to the hospital following EVR initiation had significantly higher concentrations. Previous studies have demonstrated that achieving an EVR concentration of at least 3 ng/mL when used in a CNI minimization regimen is important to maximize efficacy.(29-31) There is less compelling data with regards to therapeutic levels when EVR is used in CNI withdrawal regimens, but most studies aim for target troughs of 6 to 12 ng/mL.(32) There is even less data analyzing the relationship between EVR concentrations and toxicity.(30) The results of this study suggesting higher EVR concentrations in AA patients admitted to the hospital is of interest and may demonstrate the potential for a race specific EVR concentration-toxicity relationship; however, larger prospective studies are needed to confirm and further define this relationship.
There are several limitations with this study worthy of discussion. First, this was a small retrospective study and therefore has certain data elements could not be collected or analyzed. This includes having more detailed EVR time-concentration data to determine additional PK parameters, such as bioavailability, volume of distribution, AUC, and clearance. However, these parameters have been well described in previous studies(10) and were not the primary objective of this study, which was to determine and compare the clinical PK and PD parameters of EVR between AA and Cs. Therefore, the limitations of lacking these data elements do not diminish the overall results or conclusions of this analysis. Due to small numbers, some of the trends in the PD efficacy and toxicity comparisons may not be statistically different due to a lack of power, rather than there not being a true clinical difference. Therefore, we recommend that further large-scale analyses are conducted with EVR to determine if there is validity in the data results presented within this analysis. One final limitation is related to the estimation of renal function. Because a number of these patients were converted to EVR early after transplant or in the midst of an acute elevation in their serum creatinine concentrations, the baseline eGFR may be lower than actual renal function, and subsequent increases in eGFR after EVR conversion may not be completely related to EVR therapy. Nonetheless, EVR therapy did demonstrate a continued improvement in eGFR over a substantial timeframe following initiation of therapy, with low rates of acute rejection and IF/TA. This data suggests that EVR is a safe and effective therapy in both AA and C renal transplant recipients.
CONCLUSION
In summary, this data demonstrates that AA kidney transplant recipients converted to EVR based immunosuppressive therapy have similar efficacy and toxicity to C patients. Both groups of patients require significantly higher than currently recommended doses to achieve therapeutic concentrations and further studies are needed to determine if race specific EVR concentration-efficacy or toxicity relationships exist.
Acknowledgments
Support: This research was supported through a grant from Novartis Pharmaceuticals, Nutley, NJ
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN / SRTR 2010 annual data report. Department of health and human services, health resources and services administration, healthcare systems bureau, division of transplantation; rockville, MD: [accessed Dec 14, 2012]. 2011. http://srtr.transplant.hrsa.gov/annual_reports/2011/ [Google Scholar]
- 2.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Han F, Wu J, Huang H, et al. Conversion from cyclosporine to sirolimus in chronic renal allograft dysfunction: A 4-year prospective study. Exp Clin Transplant. 2011;9:42–9. [PubMed] [Google Scholar]
- 5.Sennesael JJ, Bosmans JL, Bogers JP, et al. Conversion from cyclosporine to sirolimus in stable renal transplant recipients. Transplantation. 2005;80:1578–85. doi: 10.1097/01.tp.0000184623.35773.6a. [DOI] [PubMed] [Google Scholar]
- 6.Maharaj S, Assounga AG. Conversion of cyclosporine to sirolimus before 12 months is associated with marked improvement in renal function and low proteinuria in a South African renal transplant population. Exp Clin Transplant. 2010;8:14–18. [PubMed] [Google Scholar]
- 7.Holdaas H, Rostaing L, Seron D, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: A randomized, multicenter, 24-month study. Transplantation. 2011;92:410–418. doi: 10.1097/TP.0b013e318224c12d. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous . Zortress (everolimus) package insert. Novartis Pharmaceutical Corporation; East Hanover, NJ: Oct, 2012. [Google Scholar]
- 9.Bouzas L, Hermida J, Tutor JC. Sirolimus and everolimus clearance in maintenance kidney and liver transplant recipients: Diagnostic efficiency of the concentration/dose ratio for the prediction of trough steady-state concentrations. Ups J Med Sci. 2010;115:125–130. doi: 10.3109/03009730903291026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budde K, Fritsche L, Waiser J, et al. Pharmacokinetics of the immunosuppressant everolimus in maintenance renal transplant patients. Eur J Med Res. 2005;10:169–174. [PubMed] [Google Scholar]
- 11.Kovarik JM, Hsu CH, McMahon L, et al. Population pharmacokinetics of everolimus in de novo renal transplant patients: Impact of ethnicity and comedications. Clin Pharmacol Ther. 2001;70:247–254. doi: 10.1067/mcp.2001.118022. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Fructuoso AI. Everolimus: An update on the mechanism of action, pharmacokinetics and recent clinical trials. Expert Opin Drug Metab Toxicol. 2008;4:807–819. doi: 10.1517/17425255.4.6.807. [DOI] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, et al. The banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 14.Poggio E, Wang X, Weinstein D, et al. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant. 2005;6:100–108. doi: 10.1111/j.1600-6143.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 15.Moes DJ, Press RR, den Hartigh J, et al. Population pharmacokinetics and pharmacogenetics of everolimus in renal transplant patients. Clin Pharmacokinet. 2012;51:467–480. doi: 10.2165/11599710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Lemahieu W, Maes B, Verbeke K, et al. Cytochrome P450 3A4 and p-glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant. 2005;5:1383–91. doi: 10.1111/j.1600-6143.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- 17.Eades SK, Boineau FG, Christensen ML. Increased tacrolimus levels in a pediatric renal transplant patient attributed to chronic diarrhea. Pediatr Transplant. 2001;4:63–6. doi: 10.1034/j.1399-3046.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- 18.Asano T, Nishimoto K, Hayakawa M. Increased tacrolimus trough levels in association with severe diarrhea, a case report. Transplant P. 2004;36:2096–7. doi: 10.1016/j.transproceed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Mittal N, Thompson JF, Kato T, et al. Tacrolimus and diarrhea: Pathogenesis of altered metabolism. Pediatr Transplant. 2001;5:75–9. doi: 10.1034/j.1399-3046.2001.005002075.x. [DOI] [PubMed] [Google Scholar]
- 20.Leroy S, Isapof A, Fargue S, et al. Tacrolimus nephrotoxicity: Beware of the association of diarrhea, drug interaction and pharmacogenetics. Pediatr Nephrol. 2010;25:965–69. doi: 10.1007/s00467-009-1402-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Elie V, Roussey G, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86:609–18. doi: 10.1038/clpt.2009.210. [DOI] [PubMed] [Google Scholar]
- 22.Audard V. Replacing calcineurin inhibitors with proliferation signal inhibitors after kidney transplantation: Indications, results, and disadvantages. Nephrol Ther. 2009;5:S395–9. doi: 10.1016/S1769-7255(09)73432-7. [DOI] [PubMed] [Google Scholar]
- 23.Campistol JM, de Fijter JW, Nashan B, et al. Everolimus and long-term outcomes in renal transplantation. Transplantation. 2011;92:S3–26. doi: 10.1097/TP.0b013e3182230900. [DOI] [PubMed] [Google Scholar]
- 24.Cataneo-Davila A, Zuniga-Varga J, Correa-Rotter R, et al. Renal function outcomes in kidney transplant recipients after conversion to everolimus-based immunosuppression regimen with CNI reduction or elimination. Transplant P. 2009;41:4138–46. doi: 10.1016/j.transproceed.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez D, Martinez D, Gutierrez E, et al. Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrologia. 2011;31:27–34. doi: 10.3265/Nefrologia.pre2010.Jul.10512. [DOI] [PubMed] [Google Scholar]
- 26.Hricik DE, Anton HS, Knauss TC, et al. Outcomes of African-American kidney transplant recipients treated with sirolimus, tacrolimus, and corticosteroids. Transplantation. 2002;74:189. doi: 10.1097/00007890-200207270-00008. [DOI] [PubMed] [Google Scholar]
- 27.Podder H, Podbielski J, Hussein I, et al. Sirolimus improves the two-year outcome of renal allografts in African-American patients. Transpl Int. 2005;14:135–42. doi: 10.1007/s001470100315. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Taber DJ, Weimert NA, et al. Potential differences in kidney allograft outcomes between ethnicities when converting to sirolimus base immunosuppression. Transplant P. 2009;41:4131–37. doi: 10.1016/j.transproceed.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 29.Kovarik JM, Tedesco H, Pascual J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced-exposure cyclosporine in de novo kidney transplantation. Ther Drug Monit. 2004;26:499–505. doi: 10.1097/00007691-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Goirand F, Royer B, Hulin A, et al. Level of evidence for therapeutic drug monitoring of everolimus. Therapie. 2011;66:57–61. doi: 10.2515/therapie/2010025. [DOI] [PubMed] [Google Scholar]
- 31.Lorber MI, Ponticelli C, Whelchel J, et al. Therapeutic drug monitoring for everolimus in kidney transplantation using 12-month exposure, efficacy, and safety data. Clin Transplant. 2005;19:145–52. doi: 10.1111/j.1399-0012.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 32.Pascual J. Everolimus in clinical practice—renal transplantation. Nephrol Dial Transpl. 2006;21:S18–23. doi: 10.1093/ndt/gfl300. [DOI] [PubMed] [Google Scholar]