Abstract
While much of our understanding of stress physiology is derived from biomedical studies, little is known about the downstream molecular consequences of adaptive stress responses in free-living animals. We examined molecular effectors of the stress hormones cortisol and aldosterone in the northern elephant seal, a free-ranging study system in which extreme physiological challenges and cortisol fluctuations are a routine part of life history. We stimulated the neuroendocrine stress axis by administering exogenous adrenocorticotropic hormone (ACTH) and examined the resultant effects by measuring corticosteroid hormones, metabolites, and gene expression before, during, and following administration. ACTH induced an elevation in cortisol, aldosterone, glucose, and fatty acids within 2 h, with complete recovery observed within 24 h of administration. The global transcriptional response of elephant seal muscle tissue to ACTH was evaluated by transcriptomics and involved upregulation of a highly coordinated network of conserved glucocorticoid (GC) target genes predicted to promote metabolic substrate availability without causing deleterious effects seen in laboratory animals. Transcriptional recovery from ACTH was characterized by downregulation of GC target genes and restoration of cell proliferation, metabolism, and tissue maintenance pathways within 24 h. Differentially expressed genes included several adipokines not previously described in muscle, reflecting unique metabolic physiology in fasting-adapted animals. This study represents one of the first transcriptome analyses of cellular responses to hypothalamic-pituitary-adrenal axis stimulation in a free-living marine mammal and suggests that compensatory, tissue-sparing mechanisms may enable marine mammals to maintain cortisol and aldosterone sensitivity while avoiding deleterious long-term consequences of stress.
Keywords: ACTH, corticosteroid, transcriptome, muscle, seal
all animals experience stress at some point in their life histories, which elicits a conserved physiological response that enables organisms to focus their resources on coping with immediate challenges (53). The vertebrate stress response is mediated by activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in secretion of glucocorticoid (GC) hormones. GCs bind to intracellular receptors [glucocorticoid receptors (GR), and mineralocorticoid receptors (MR)] in target tissues such as liver, adipose, and muscle. Once bound with their ligand, these receptors alter the expression of genes that regulate numerous functions including metabolism and growth (51). These transcriptional changes ultimately result in liberation of energy stores and suppression of energetically costly processes such as reproduction and inflammation. Finely tuned negative feedback mechanisms terminate the stress response after the challenge has passed (51). Repeated or sustained stressors, however, can result in chronic activation of the stress axis and may deplete physiological resources and impair an organism's ability to respond appropriately to subsequent stressors (52). This can significantly affect the health of human and animal populations alike and presents significant public health and wildlife conservation challenges.
Much of our current understanding of vertebrate stress physiology derives from biomedical research in humans and laboratory animals in a pathological context (51). Animals in nature, however, may not experience or respond to stressors in the same manner as laboratory species (4). Many organisms are routinely exposed to stressors in their environments, such as predation, food shortages, intraspecific conflicts, and reproductive challenges, among others. If a free-living animal is to survive and reproduce in an unpredictable environment, its reactivity to acute perturbations must be modulated to avoid long-term, deleterious consequences (4). However, little is known about how such responses are regulated at cellular levels in nonlaboratory species, which have only been profiled in a handful of studies (33, 37). This hinders our ability to understand the molecular mechanisms underlying development and pathology of maladaptive, chronic stress in animals, a key research focus in emerging fields such as conservation physiology (10).
Phocid seals such as the northern elephant seal (Mirounga angustirostris) present an ideal free-living vertebrate system for stress studies in an environmental context. As part of their natural life history, elephant seals routinely experience extreme physiological challenges associated with prolonged fasting coupled with energetically demanding life history pressures of breeding, molting, and neonatal development (6, 11, 12, 59). Due to the amenability of this species to research handling and endocrine manipulation (7), much is known about elephant seals' metabolic adaptations (6, 11). Circulating cortisol concentrations increase markedly during fasting in this species (8, 45) but do not result in the typical adverse consequences, such as increased protein catabolism (13, 23) or immunosuppression (46) seen in laboratory animals under similar circumstances. At the same time, fasting seals retain the capacity to elevate adrenocortical hormone levels in response to experimental HPA axis stimulation (9, 17). This suggests that fasting-adapted animals may possess characteristics that mitigate deleterious effects of elevated GCs while maintaining HPA axis sensitivity to acute perturbation.
In response to HPA axis manipulation, downstream GC effectors (such as genes that suppress protein synthesis, glucose uptake, inflammation, and cell survival) can be identified by profiling global gene expression changes in target tissues using transcriptomics. Endogenous GC secretion can be stimulated by administering exogenous adrenocorticotropic hormone (exACTH), mimicking the downstream effects of HPA axis activation in response to stressors (9, 17, 18, 40). This manipulation, when performed under dissociative anesthesia in northern elephant seals, avoids artifacts of capture and handling stress (7). Administration of slow-release exACTH in juvenile and adult elephant seals has been shown to significantly elevate cortisol and aldosterone for 24–48 h. The mineralocorticoid aldosterone is released in response to a variety of stressors, including handling, physical, and thermoregulatory stress in marine mammals and is likely a key component of the stress response in this group (9, 17, 18, 24, 56–58).
We evaluated downstream responses to exACTH administration in juvenile elephant seals during a brief annual haul-out when they are not molting, breeding, or fasting extensively to remove confounds of life history stress (27). We describe the acute endocrine, metabolic, and transcriptional response and subsequent recovery of northern elephant seals following exACTH administration. Gene expression changes were profiled in skeletal muscle, a metabolically active GC target tissue that is easily accessible in marine mammals (29). We previously sequenced and assembled a reference transcriptome obtained from muscle samples collected during the exACTH experiment and conducted preliminary differential expression analysis (28). In this study, we report a detailed analysis of changes in gene expression between 1) baseline and acute response and 2) acute response and recovery conditions and relate them to other physiological measurements (hormones, metabolites) of the response to exACTH. We found that the cellular response of muscle to exACTH administration involved transient upregulation of a highly conserved and coordinated gene network that was suppressed within 24 h. Differentially expressed genes included factors not previously described in muscle tissue in the context of stress, reflecting unique physiological adaptations in free-ranging animals. This study represents one of the first RNA sequencing (RNA-Seq)-based analyses of transcriptome responses to HPA axis manipulation in marine mammals and provides valuable insights into cellular mechanisms utilized by free-living vertebrates to modulate adrenocortical responses.
MATERIALS AND METHODS
Study Animals and Sampling Design
All animal handling procedures were approved by the Sonoma State University Institutional Animal Care and Use Committee and conducted under National Marine Fisheries Service marine mammal permit 14636. Juvenile (0.8 yr old) northern elephant seals (M. angustirostris) were sampled at Año Nuevo State Reserve (San Mateo County, CA) during their 2013 autumn (October-November) haul-out. This life history stage is not confounded by energetically demanding activities such as breeding or molting (27). Seven juvenile animals (5 female, 2 male) that had recently arrived at the rookery and were of similar mass (124.7 ± 8.4 kg) and apparent body condition were selected. Samples from three female animals were used for RNA-Seq (28) and quantitative real-time PCR (QPCR) validation and hormone and metabolite analyses. Samples from an additional two females and two males were used for hormone, metabolite, and QPCR analyses.
Pilot experiments were conducted to determine appropriate sampling times that would capture maximal acute response to endocrine manipulation in this age class of seals. Corticosteroid values were higher 2 h after exACTH administration than at earlier sampling times, consistent with similar experiments in molting juveniles that showed a maximal corticosteroid response within 2–2.5 h (9). We reasoned that 2 h would provide sufficient time to observe changes in gene expression in response to manipulation. Recovery was evaluated by decline of corticosteroids to baseline levels, which occurred within 24 h after exACTH administration and was maintained at 48 h. Field sampling between 2 and 24 h postinjection was not feasible. Therefore, markers were evaluated in three conditions: prior to exACTH administration (“baseline”), 2 h after exACTH administration (“acute response”), and ∼24 h after exACTH (“recovery”).
ACTH Administration and Sample Collection
Study animals were approached while sleeping at the rookery and were immobilized as previously described (27, 28). Briefly, seals were initially sedated with 1 mg/kg intramuscular injection of tiletamine-zolazepam (Telazol; Fort Dodge Laboratories, Fort Dodge, IA). Sedation was maintained with periodic intravenous doses of ketamine and diazepam (Fort Dodge Laboratories). This sedation procedure does not cause an increase in circulating cortisol concentration and thus appears to alleviate any stress response due to animal handling in elephant seals (7). Baseline blood samples were obtained via an 18 G 3.25-inch needle from the extradural vein (3) within 22.1 ± 7.4 min of initial Telazol injection. All blood samples were collected into chilled serum and EDTA-treated plasma tubes and stored on ice until return to the laboratory. After subcutaneous injection with 1 ml lidocaine, samples of the left external abdominal oblique muscle were collected via a 6.0 mm diameter biopsy punch (Miltex, York, PA) and immediately frozen in liquid nitrogen.
After baseline sample collection, each animal received an intramuscular injection of 0.23 ± 0.02 U/kg corticotrophin LA gel (exACTH; Wedgewood Pharmacy, Richmond, VA) on the left side, ∼3 cm anterior to the initial biopsy site. This exACTH preparation is a synthetically produced bioactive peptide containing the first 24 amino acids of ACTH (aa 1–24) in a proprietary viscous gel matrix to provide extended release after intramuscular injection (Wedgewood Pharmacy). Sedation was maintained for 2 h, and a second set of blood and tissue samples (on the right, contralateral side of the animal) were collected 2 h after exACTH administration to capture the acute response. Animals were weighed (MSI tension dynamometer, Seattle, WA), individually marked with rear flipper tags (Dalton Jumbo Roto-tags, Oxon, UK) and black hair dye (Lady Clairol, Stamford, CT) and released to resume normal activity. Study subjects were resighted and immobilized again the following day, 22.3 ± 2.0 h after initial exACTH injection. A set of recovery blood and tissue samples was collected within 16.8 ± 4.4 min of Telazol injection as described above. Muscle tissue was obtained from the right side of each animal at least 3 cm away from the acute biopsy site. We were unable to relocate and collect recovery samples for one of the seven study animals.
Hormone and Metabolite Assays
Blood samples were centrifuged at 5,000 rpm for 15 min at 4°C. Isolated plasma and serum were stored at −80°C. Hormone concentrations were measured by radioimmunoassay or enzyme immunoassay with commercially available kits previously validated for use in elephant seals: cortisol, aldosterone (Siemens, Washington, DC), and insulin (EMD Millipore, St. Charles, MO) (17). All samples were analyzed in duplicate with intra-assay coefficient of variation ≤ 5%.
Glucose and lactate were measured using YSI 2300 STAT plus autoanalyzer (YSI, Yellow Springs, OH). Free fatty acids (FFA; Cayman, Ann Arbor, MI) and blood urea nitrogen (BUN; Stanbio, Boerne, TX) were analyzed with enzymatic fluorometric and colorimetric assays, respectively. All samples were analyzed in duplicate with intra-assay coefficient of variation ≤ 5%.
RNA Isolation
Tissue samples were stored at −80°C until extraction as previously described (28). In brief, 75–165 mg of muscle tissue was minced with a scalpel in a sterile glass dish on ice, transferred to a glass tissue grinder (Kimble-Chase Kontes Duall, Vineland, NJ), and homogenized with 1 ml of TRIzol Reagent (Ambion, Life Technologies, Grand Island, NY). RNA was extracted using chloroform, precipitated with isopropanol, and resuspended in 100 μl RNase-free water according to Ambion protocol. RNA samples were then applied to RNeasy columns and sample cleanup was conducted using RNeasy mini kit with a 30-min on-column DNase I digest (Qiagen, Germantown, MD) according to Qiagen protocol. RNA concentration was quantified on a Qubit fluorometer (Life Technologies, Grand Island, NY).
Transcriptome Analysis
RNA-Seq, reference transcriptome assembly and annotation, and preliminary differential expression analysis were described in a previous study (28). Briefly, paired-end 100 base-pair sequencing was conducted on the Illumina HiSeq 2500 platform. The reference transcriptome was assembled de novo using Trinity assembler (19). Transcripts were mapped to the assembly using bowtie read aligner (32). Transcript abundance was calculated using RSEM (35), and differentially expressed transcripts were identified using EBSeq (34).
Here, we assessed all differentially expressed transcripts and explored potential relationships with other physiological metrics (i.e., hormone and metabolite concentrations). We used the DAVID functional annotation tool (25) to identify KEGG and BioCarta metabolic and signaling pathways enriched in each differentially expressed gene set at P < 0.1. Functional network prediction was conducted using GeneMANIA (60) with human homologs of differentially expressed genes as inputs. The gene network involved in the acute response to exACTH includes corticosteroid receptors GR and MR and 32 genes differentially expressed between baseline and acute response conditions. The gene network involved in recovery from exACTH includes 65 genes differentially expressed between acute response and recovery conditions. Automatically selected weighing method was used for network weighing option, and 10 related genes and 10 related attributes are shown. Edges are based on coexpression data only.
Quantitative RT-PCR
cDNA was synthesized using 1 μg of RNA using QiantiTect reverse transcription kit (Qiagen) with gDNA digest and a 30 min RT incubation step. cDNA samples were diluted 1:20 and 2 μl were used in a 20-μl QPCR reaction using iTaq Universal SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA). QPCR was performed on an ABI 7000 instrument (Applied Biosystems, Grand Island, NY) using the following program: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 60 s at 61°C, and 30 s at 72°C. All reactions were run in triplicate with intra-assay coefficient of variation ≤ 1%.
Primers were designed using Primer3Plus with default QPCR settings, based on M. angustirostris coding sequence obtained by transcriptome sequencing (28). Primer efficiency was calculated from four-point standard curves (1:10, 1:20, 1:40, 1:80 cDNA dilutions) and absence of primer-dimers was verified by melting curve analysis. No-template controls were run for each primer set and showed no amplification. Primers were used at 500 nM with the exception of GAPDH, EF2, and Ccng1, which were used at 100 nM concentrations. Primer sequences are shown in Table 1.
Table 1.
Primers used for QPCR validation of differentially expressed genes
| Gene | Tr ID | Primer Sequence (5′ to 3′) | E | Mean FC | Comparison |
|---|---|---|---|---|---|
| Cedpd | tr398922 | F: ATCGACTTCAGCGCCTACATC | 1.97 | 4.32 ± 1.84 | 2 h / 0 h |
| R: TGTGGTTGCTGTTGAAGAGG | |||||
| Ddit4 | tr404230 | F: TGGTAAGTGTCCCTTCTGTGTG | 1.85 | 2.76 ± 1.36 | 2 h / 0 h |
| R: ACCGCAGAAAACGCTGTAAG | |||||
| Tob2 | tr660220 | F: TGCATGCCATGATGCTTTGG | 1.71 | 1.98 ± 0.77 | 2 h / 0 h |
| R: AGGGCTTTGGTACGTTTTGG | |||||
| Klf15 | tr28582 | F: TGGCAATGCATGTGCTTGAG | 1.72 | 1.53 ± 0.50 | 2 h / 0 h |
| R: TTGTCTGGAAACGGGATGAGG | |||||
| Zfp36 | tr644672 | F: TTCAACCGCATCTCCGTTTC | 1.62 | 1.25 ± 0.29 | 2 h / 0 h |
| R: TGATTGAGTCCCGCAGTTTC | |||||
| Medag | tr95009 | F: TGACCAAGCGTGTATGTGTG | 1.90 | 3.68 ± 2.31 | 24 h / 2 h |
| R: AATGCTTTGGAGACGCGTTC | |||||
| Rbp4 | tr233357 | F: TTCATGCAGCCTTCAACTCG | 1.80 | 3.10 ± 1.26 | 24 h / 2 h |
| R: ACTGAGGTTCCATGTTTGCG | |||||
| Trim62 | tr461529 | F: ATTTGCTGCTGTTGCCCATG | 1.75 | 0.92 ± 0.48 | 24 h / 2 h |
| R: CACGGTGGAAATCGAGTGTAAC | |||||
| Asb15 | tr419155 | F: AGCAGCCGGACAATTTCATG | 1.71 | 0.73 ± 0.20 | 24 h / 2 h |
| R: AAGACGGCGCTGTATTTTGC | |||||
| EF2 | tr388769 | F: TGGCCAAATTTGCTGCCAAG | 1.67 | 0.95 ± 0.40 | 2 h / 0 h |
| R: CTTGCTGAATTTGCCGTTGG | 0.92 ± 0.21 | 24 h / 2 h | |||
| Ccng1 | tr141230 | F: TGGCAGAGATTTGACCTTCTGG | 1.66 | 0.99 ± 0.36 | 2 h / 0 h |
| R: ATGCTTCAATTGCCGTGCAG | 1.07 ± 0.18 | 24 h / 2 h | |||
| Gapdh* | tr223210 | F: GGAAACTGTGGCGTGATGG | 1.78 | NC | 2 h / 0 h |
| R: CCTGCTTCACCACCTTCTTG | 24 h / 2 h |
QPCR, quantitative PCR; Tr ID, transcript ID; E, primer efficiency; Mean FC, mean fold change in gene expression between conditions noted in “Comparison” (n = 6).
Gapdh primer sequences were obtained from Ref. 39. F, forward; R, reverse; NC, no change in expression.
Candidate reference genes were selected based on stable expression in the RNA-Seq dataset. Expression stability in all QPCR samples was evaluated using Bestkeeper (48) and Normfinder (1) tools. GAPDH (SD = 0.28, CV = 1.63%, r = 0.804, P < 0.001; primers from Ref. 39) was selected as the most stable gene (stability value = 0.006) in the dataset for use as reference. Fold-changes in gene expression in acute response relative to baseline samples, and recovery relative to acute response samples were calculated by the Pfaffl (2001) method (47). QPCR was conducted with three matched samples from each of six animals.
Statistical Analysis
Data analyses were conducted using JMP 11 (SAS, Cary, NC). Changes in hormone and metabolite concentrations in response to exACTH were analyzed using linear mixed models with time as an ordinal fixed effect and animal ID as a random effect. Response variables were log-transformed as necessary to meet distribution and variance assumptions. Post hoc comparisons among repeated samples were conducted using Tukey's honest significant difference test. The relationship between log2 fold-change in gene expression values obtained by RNA-Seq and those obtained by QPCR was evaluated by Pearson correlation analysis.
RESULTS
Endocrine and Metabolic Response to exACTH
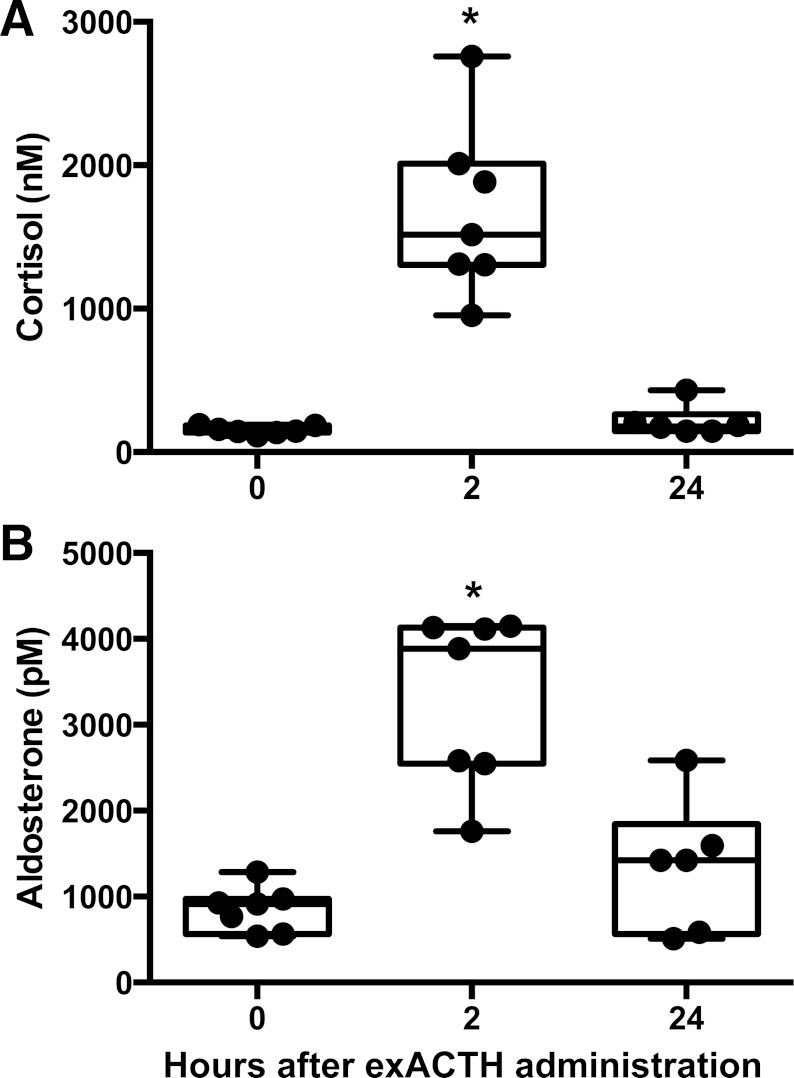
We evaluated the response of juvenile elephant seals to exACTH by comparing hormone (cortisol, aldosterone) and metabolite (glucose, lactate, FFA, BUN) measurements between three experimental conditions: 1) baseline, prior to exACTH administration, 2) acute response, 2 h after exACTH administration, and 3) recovery from exACTH, 24 h after administration. Baseline values of hormones in study animals were within the range reported in this species (17, 27) and did not appear to vary by sex. ExACTH administration induced a robust spike in cortisol and aldosterone within 2 h. Cortisol increased 11-fold (F2,11 = 125.67, P < 0.0001; Fig. 1A), while aldosterone increased 3.9-fold (F2,11 = 27.55, P < 0.001; Fig. 1B). Both hormones recovered to baseline values within 24 h. Insulin levels were not affected by exACTH (F2,11 = 1.75, P = 0.22).
Fig. 1.
Exogenous adrenocorticotropic hormone (ExACTH) administration induced an acute corticosteroid response in elephant seals. Cortisol (A) and aldosterone (B) were measured in juvenile seals prior to (0 h) and 2 (2 h) and 24 h (24 h) after exACTH administration. *Values that are different from baseline (0 h) at P < 0.05.
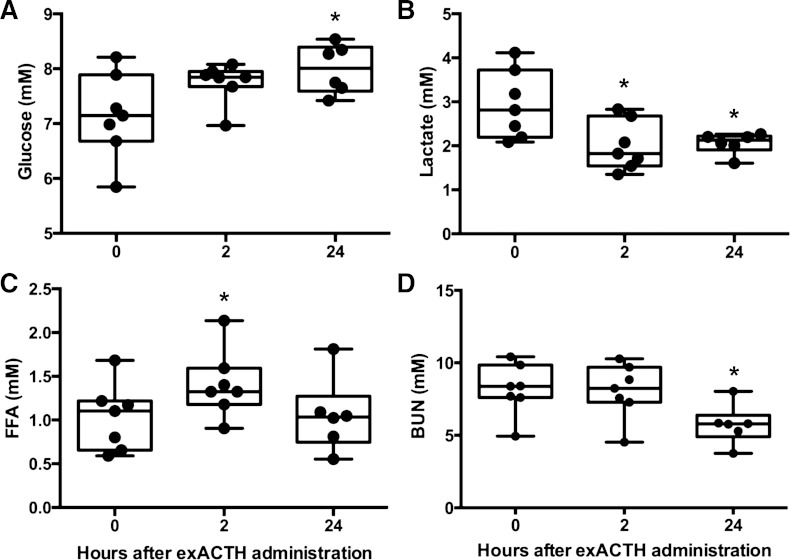
Baseline metabolites were consistent with others reported in this species (22). Glucose was not altered at 2 h (P > 0.05) but was slightly elevated, by 12%, from baseline values 24 h after exACTH administration (F2,11 = 6.76, P < 0.05; Fig. 2A). Lactate decreased 32% within 2 h and remained suppressed at 24 h (F2,11 = 8.09, P < 0.05; Fig. 2B). FFA increased 37% within 2 h of exACTH administration but returned to baseline within 24 h (F2,11 = 4.76, P < 0.05; Fig. 2C). BUN was not altered at 2 h (P > 0.05) but decreased 30% from baseline values after 24 h (F2,10 = 45.02, P < 0.0001; Fig. 2D). Therefore, the peripheral response of juvenile elephant seals to exACTH administration involved an increase of circulating cortisol, aldosterone, glucose, and FFA with a concomitant decrease of lactate and BUN.
Fig. 2.
ExACTH administration induced changes in carbohydrate, lipid, and protein metabolism. Glucose (A), lactate (B), free fatty acids (FFA, C), and blood urea nitrogen (BUN, D) were measured prior to (0 h) and 2 (2 h) and 24 h (24 h) after exACTH administration. *Values that are different from baseline (0 h) at P < 0.05.
Acute Transcriptome Response to exACTH
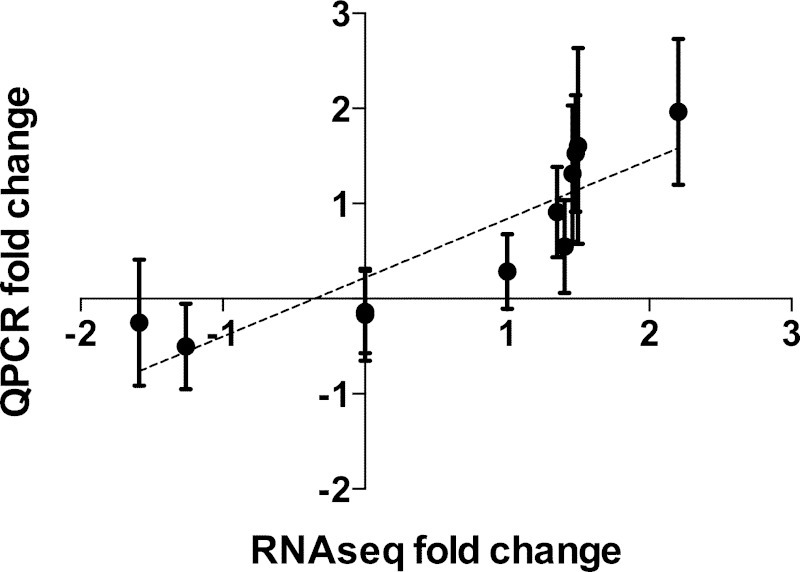
Global gene expression changes in elephant seal muscle sampled during the exACTH administration were previously quantified by RNA-Seq (28). In this study, we conducted extensive annotation and analysis of all differentially expressed transcripts. Expression levels were compared between muscle samples collected at baseline and acute response conditions in three female juvenile seals. We identified 48 transcripts that were differentially expressed at P < 0.05 and false discovery rate (FDR) < 0.05 and expressed by at least 1 FPKM (fragments per kilobase of transcript per million mapped reads, Supplementary File 1).1 Thirty-seven transcripts were upregulated by at least 1.1-fold, of which 14 were upregulated by twofold or greater. These included several isoforms of the same genes (Cebpd, two isoforms; Tob2, three isoforms; Pdk4, three isoforms; Rab3gap1, two isoforms). Only 11 transcripts were suppressed during the acute response, of which four were downregulated by at least twofold. Two downregulated transcripts were isoforms of the same gene (Zpf36L1). Three significantly upregulated transcripts were not annotated due to lack of gene models in any mammalian system. Expression levels of five upregulated (Cebpd, Ddit4, Tob2, Klf15, Zfp36) and two unaltered (EF2, Ccng1) transcripts were verified by QPCR in the same and three additional samples. Fold-change values quantified by the two methods were significantly correlated (Pearson r = 0.88, P < 0.001; Fig. 4, Table 1).
Fig. 4.
Log2 fold-change expression values measured by RNA-Seq (n = 3) and those measured by quantitative PCR (QPCR, n = 6) for 11 genes of interest (Cebpd, Ddit4, Tpb2, Klf15, Zfp36, Medag, Rbp4, Trim62, Asb15, EF2, Ccng1) were highly correlated (Pearson r = 0.88, P < 0.001).
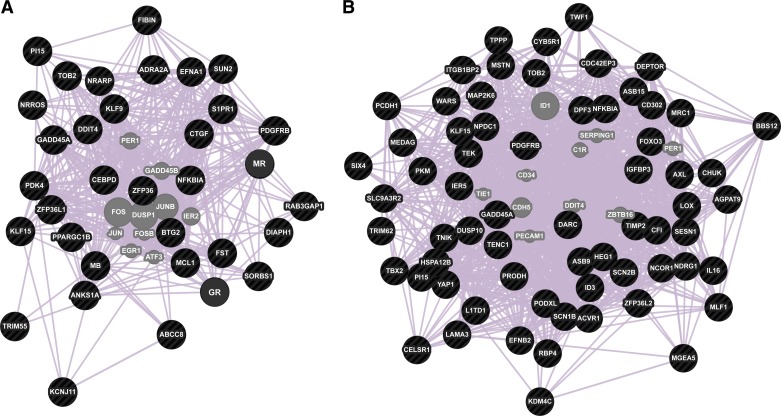
We mapped mouse homologs of differentially expressed seal genes to signaling and metabolic pathways using DAVID functional annotation tool (25). Few pathways were significantly enriched at P < 0.1 relative to the entire mouse proteome due to small size of the query dataset. The only KEGG pathway overrepresented in the differentially expressed gene set was Type II diabetes mellitus, while the sole enriched Biocarta pathway was ATM signaling pathway (Table 2). To predict functional interactions between cortisol, aldosterone, and differentially expressed genes identified in our dataset, we conducted network analysis using the hormone receptors GR and MR and 32 differentially expressed genes as input using GeneMANIA network prediction tool (60). Figure 3A depicts connections between human homologs of these 34 genes based on existing coexpression data, with a maximum of 10 related genes and attributes displayed. Most genes (nodes) altered during the acute response to elevated corticosteroids are likely to be functionally related based on coexpression data (edges), with each node connected to a minimum of three edges in the network (Fig. 3A).
Table 2.
KEGG and Biocarta pathways overrepresented during the acute response to exACTH and recovery from exACTH
| Pathway | Gene | P Value |
|---|---|---|
| Acute response to exACTH | ||
| ATM signaling† | Gadd45α | 0.075 |
| IκBα | ||
| Type II diabetes mellitus* | Abcc8 | 0.098 |
| Kcnj11 | ||
| Recovery from exACTH | ||
| NF-κB activation by nontypeable H. influenzae† | IKKα | 0.022 |
| Signal transduction through IL1R† | MKK6 | 0.025 |
| TNF/stress related signaling† | IκBα | 0.025 |
| Keratinocyte differentiation† | 0.058 | |
| Toll-like receptor signaling* | 0.073 | |
| MAPK signaling* | IKKα | 0.030 |
| Dusp10 | ||
| Gadd45α | ||
| MKK6 | ||
| PDGFRβ | ||
| p53 signaling* | Gadd45α | 0.038 |
| Igfbp3 | ||
| Sesn1 | ||
| Small cell lung cancer* | IKKα | 0.056 |
| Lama3 | ||
| IκBα | ||
| Prostate cancer* | IKKα | 0.062 |
| IκBα | ||
| PDGFRβ | ||
| dsRNA-induced gene expression† | IKKα | 0.079 |
| IκBα | ||
KEGG;
Biocarta.
Fig. 3.
Acute transcriptional responses to exACTH administration were highly coordinated. Gene network prediction based on coexpression studies is shown for transcripts differentially expressed in elephant seal muscle during acute response to exACTH (A) and recovery from exACTH (B). Input genes are shown in black; 10 transcription factors that are predicted to associate with the network are shown in gray.
Transcripts differentially expressed during the acute response to exACTH included known transcriptional regulators of GR signaling, such as Ddit4, Klf15, Pdk4, and Sorbs1 (29, 30). We identified 15 differentially expressed seal orthologs of primary GR targets in mouse myotubes (Table 3). An additional 10 differentially expressed genes are indirectly associated with GR; these are correlated with GR activity but have not been shown to contain specific promoter elements (Table 3). Using literature search, we broadly categorized differentially expressed transcripts as participants in 1) metabolism, 2) cell proliferation/death, 3) immune signaling, or 4) generalized tissue functions. Six differentially expressed genes did not fit these categories and are not discussed here (Supplementary File 1).
Table 3.
Genes differentially expressed during acute response to exACTH
| Fold Change | Transcript ID | Gene Symbol | Gene Name |
|---|---|---|---|
| Metabolism | |||
| 4.61 | tr398922 | Cebpd† | CCAAT/enhancer-binding protein delta |
| 2.75 | tr404230 | Ddit4* | DNA damage-inducible transcript 4 |
| 2.65 | tr28582 | Klf15* | Krueppel-like factor 15 |
| 2.09 | tr417255 | Fst† | follistatin |
| 1.66 | tr248344 | Adra2* | alpha-2A/B/C adrenergic receptor |
| 1.66 | tr152271 | Abcc8 | ATP-binding cassette, subfamily C, member 8 |
| 1.46 | tr218113 | Pdk4* | pyruvate dehydrogenase kinase, isozyme 4 |
| 1.43 | tr514865 | Kcnj11 | ATP-sensitive inward rectifier potassium channel 11 |
| 1.18 | tr73996 | Sorbs1* | sorbin and SH3 domain-containing protein 1 |
| −1.87 | tr342376 | PGC1β† | PPAR gamma, coactivator 1 beta |
| Cell proliferation/death | |||
| 2.55 | tr660220 | Tob2* | transducer of ERBB2, 2 |
| 1.64 | tr138042 | Gadd45α | growth arrest and DNA damage-inducible protein |
| 1.60 | tr172628 | S1pr1* | sphingosine 1-phosphate receptor 1 |
| 1.49 | tr247792 | Anks1a* | ankyrin repeat and SAM domain-containing protein 1A |
| 1.42 | tr20240 | Mcl1† | induced myeloid leukemia cell differentiation protein |
| 1.36 | tr585312 | Klf9* | Krueppel-like factor 9 |
| −1.45 | tr478784 | Zfp36l1 | ZFP36 ring finger protein-like 1 |
| −1.75 | tr245847 | Btg2† | BTG family member 2 |
| −3.40 | tr359172 | Nrarp | NOTCH-regulated ankyrin repeat protein |
| Immune signaling | |||
| 2.00 | tr644672 | Zfp36* | tristetraprolin |
| 1.78 | tr428989 | IκBα† | NF-kappa-B inhibitor alpha |
| −2.87 | tr222774 | Nrros | negative regulator of reactive oxygen species |
| Tissue function | |||
| 3.51 | tr216922 | Fibin† | fin bud initiation factor homolog |
| 2.09 | tr91732 | CTGF* | connective tissue growth factor |
| 1.80 | tr58004 | Pi15* | peptidase inhibitor 15 |
| 1.34 | tr110381 | Diaph1† | protein diaphanous homolog 1 |
| 1.34 | tr332339 | MuRF2 | tripartite motif containing 55 |
| 1.32 | tr129132 | Sun2 | SUN domain-containing protein 2 |
| 1.22 | tr331492 | Rab3 gap1* | rab3 GTPase-activating protein catalytic subunit |
| −1.32 | tr148788 | Mb† | myoglobin |
| −1.34 | tr441283 | PDGFRβ* | platelet-derived growth factor receptor beta |
| −1.93 | tr154980 | Efna1* | ephrin-A1 isoform 1 |
EBSeq, P < 0.05, false discovery rate (FDR) < 0.05.
Primary glucocorticoid receptor targets;
glucocorticoid-associated genes.
Metabolism.
GCs increase circulating glucose to meet increased energy demands by inhibiting insulin signaling and tissue glucose uptake and stimulating protein catabolism to provide amino acid substrates for gluconeogenesis (29). Accordingly, six genes that inhibit components of insulin signaling and glucose utilization (Abcc8, Kcnj11, Adra2, Sorbs1, Pdk4) were significantly upregulated in elephant seal muscle during the acute response, while a positive regulator of oxidative metabolism (PGC-1β) was downregulated (Table 3). The three most highly upregulated genes during acute response were key mediators of GC-driven proteolysis (Cebpd, Klf15, Ddit4). We did not detect changes in expression of FoxO factors or secondary targets such as atrogenes at this sampling point. Follistatin, a positive regulator of muscle growth and mTOR signaling, was upregulated by 2.09-fold during the acute response to exACTH (Table 3). Therefore, exACTH induced alterations in metabolic factors that may be involved in inhibiting insulin signaling and initiating a proteolytic signaling cascade in elephant seal muscle.
Cell proliferation and death.
Excessive use of GCs is linked to musculoskeletal pathologies resulting from increased apoptosis and reduced cell proliferation (15). Four proliferation repressors (Tob2, Anks1a, Gadd45α, Klf9) were upregulated during the acute response to exACTH in elephant seal muscle. However, three additional proliferation repressors (Btg2, Nrarp, Zfp36l1) were downregulated, with Nrarp being the most strongly suppressed gene during the acute response (Table 3). Additionally, two apoptosis inhibitors (Mcl1, S1pr1) were upregulated, suggesting that elephant seal muscle tissue may be protected from deleterious effects of GCs by inhibiting apoptotic signaling but not cell proliferation (Table 3).
Immune signaling.
GCs play a well-described role in immunosuppression and are commonly used to treat inflammation (53). Two inflammation inhibitors [Zfp36 (tristetraprolin), IκBα] were upregulated during acute response to exACTH, while another (Nrros) was the second-most highly downregulated gene at 2 h (Table 3). Therefore, the effect of exACTH administration on immune signaling in muscle was mainly repressive.
Tissue function.
Seven genes involved in generalized cell and tissue functions were upregulated during the acute response to exACTH (Table 3). These included tissue development (fibin) and remodeling (CTGF, Pi15) factors, cytoskeletal proteins (Diaph1, Sun2), a sarcomere assembly regulator [MuRF2 (Trim55)], and an autophagy mediator (Rab3gap1). Downregulated genes included myoglobin, angiogenesis-promoting growth factor receptor (PDGFRβ), and neuronal guidance ephrin ligand (Efna1). This suggests that the acute response of muscle to exACTH administration includes maintenance of some tissue functions but suppression of genes involved in other, energy-intensive processes.
Transcriptional Changes during Recovery from exACTH
To identify transcriptional events that may underlie tissue recovery from exACTH injection, we compared gene expression profiles between acute response and recovery conditions. We found 122 differentially expressed genes (P < 0.05, FDR < 0.05) that were expressed by at least 1 FPKM during recovery (Supplementary File 2). Of these, 50 were upregulated and 72 were downregulated by at least 1.1-fold. One upregulated and seven downregulated transcripts were not annotated. Expression levels of two upregulated (Medag, Rbp4), two downregulated (Trim62, Asb15), and two unaltered (EF2, Ccng1) transcripts were verified by QPCR and were highly correlated with RNA-Seq data (Pearson r = 0.88, P < 0.001; Fig. 4, Table 1).
Five KEGG pathways were overrepresented during recovery at P < 0.1, which included MAPK, p53, Toll-like receptor (TLR), and two cancer signaling pathways (Table 2). Enriched Biocarta pathways included NF-κB activation, signal transduction through IL-1R, TNF/stress related signaling, keratinocyte differentiation, and double-stranded RNA-induced gene expression. Differentially expressed genes were grouped into functional categories based on literature search as described above (Table 4). Network prediction, conducted using 65 differentially expressed genes, showed that network topology during recovery was more complex than that seen during acute response (Fig. 3B). Each node was connected by a minimum of seven edges to other nodes in the network, suggesting that transcriptional changes accompanying recovery from exACTH are highly coordinated.
Table 4.
Genes differentially expressed during recovery from exACTH
| Fold Change | Transcript ID | Gene Symbol | Gene Name |
|---|---|---|---|
| Metabolism | |||
| 2.83 | tr95009 | Medag | mesenteric estrogen-dependent adipogenesis protein |
| 2.79 | tr233357 | Rbp4 | retinol-binding protein 4 |
| 2.21 | tr308263 | Prodh | proline dehydrogenase 1, mitochondrial precursor |
| 2.03 | tr87386 | Igfbp3 | insulin-like growth factor-binding protein 3 |
| 1.45 | tr234547 | Tenc1 | tensin-like C1 domain-containing phosphatase |
| −1.29 | tr84952 | Ncor1 | nuclear receptor corepressor 1 |
| −1.35 | tr665318 | Pkm | pyruvate kinase, muscle isozyme |
| −1.44 | tr44234 | Cyb5r1 | NADH-cytochrome b5 reductase 1 |
| −1.44 | tr680927 | FoxO3 | forkhead box protein O3 |
| −1.67 | tr394732 | Gpat3 | glycerol-3-phosphate acyltransferase 3 |
| −1.92 | tr434317 | Asb9 | ankyrin repeat and SOCS box protein 9 |
| −1.93 | tr82435 | Deptor | DEP domain containing MTOR-interacting protein |
| −2.11 | tr331847 | Bbs12 | Bardet-Biedl syndrome 12 protein |
| −2.22 | tr28582 | Klf15 | Krueppel-like factor 15 |
| −2.36 | tr492052 | Sesn1 | sestrin-1 |
| −2.39 | tr59849 | Asb15 | ankyrin repeat and SOCS box containing 15 |
| −2.95 | tr69985 | Mstn | growth/differentiation factor 8/myostatin |
| −3.00 | tr461529 | Trim62 | tripartite motif-containing protein 62 |
| Cell proliferation/differentiation/death | |||
| 3.35 | tr89806 | Id3 | DNA-binding protein inhibitor ID-3 |
| 2.58 | tr37168 | Podxl | podocalyxin |
| 2.38 | tr398685 | Ier5 | immediate early response gene 5 |
| 2.04 | tr235316 | Tbx2 | T-box transcription factor TBX2 |
| 1.97 | tr452841 | Dusp10 | dual specificity protein phosphatase 10 |
| 1.90 | tr518088 | Ndrg1 | N-myc downstream regulated 1 |
| 1.80 | tr45785 | Npdc1 | neural proliferation differentiation and control protein 1 |
| 1.70 | tr125345 | Axl | AXL receptor tyrosine kinase |
| 1.66 | tr298730 | Zfp36l2 | zinc finger protein 36, C3H1 type-like 2 |
| 1.46 | tr256010 | Yap1 | yorkie homolog isoform 1 |
| −1.30 | tr3706 | Alk2 | activin receptor type-1 |
| −1.40 | tr138042 | Gadd45α | growth arrest and DNA damage-inducible protein |
| −1.46 | tr207432 | Zfp277 | zinc finger protein 277 |
| −1.52 | tr1470 | Melusin | integrin beta-1-binding protein 2 |
| −1.63 | tr85092 | Jmjd2c | JmjC domain-containing histone dimethyl. protein 2C |
| −2.00 | tr387679 | Six4 | homeobox protein SIX4 |
| −2.29 | tr361527 | L1td1 | LINE-1 type transposase domain-containing protein 1 |
| −2.34 | tr72612 | Mlf1 | myeloid leukemia factor 1 |
| −2.38 | tr660220 | Tob2 | transducer of ERBB2, 2 |
| −3.48 | tr512514 | Dpf3 | D4, zinc and double PHD fingers, family 3 |
| −3.57 | tr19867 | MKK6 | mitogen-activated protein kinase kinase 6 |
| Immune response | |||
| 2.42 | tr471772 | Ackr1 | Duffy antigen/chemokine receptor |
| 2.27 | tr326094 | IL16 | pro-interleukin-16 |
| 2.05 | tr91018 | Mrc1 | macrophage mannose receptor 1 |
| 1.45 | tr191856 | CFI | complement factor I |
| 1.28 | tr88481 | CD302 | CD302 antigen |
| −1.74 | tr428989 | IκBα | NF-kappa-B inhibitor alpha |
| −6.86 | tr414006 | IKKα | conserved helix-loop-helix ubiquitous kinase (Chuk) |
| Tissue function | |||
| 2.67 | tr578885 | Lox | lysyl oxidase |
| 2.38 | tr209872 | Nherf2 | Na+/H+ exchange regulatory cofactor NHE-RF2 |
| 2.35 | tr89641 | Efnb2 | ephrin B-2 |
| 2.00 | tr75251 | Lama3 | laminin subunit alpha-3 |
| 1.96 | tr38072 | Hspa12b | heat shock 70 kDa protein 12B |
| 1.77 | tr222951 | Scn1b | sodium channel subunit beta-1 |
| 1.76 | tr292276 | Heg1 | heart development protein with EGF-like domains 1 |
| 1.71 | tr370984 | Pcdh1 | protocadherin-1 |
| 1.56 | tr441283 | PDGFRβ | platelet-derived growth factor receptor beta |
| 1.53 | tr360174 | Twf1 | twinfilin-1 |
| 1.52 | tr401835 | Tek | angiopoietin-1 receptor |
| 1.50 | tr211855 | Timp2 | metalloproteinase inhibitor 2 |
| 1.45 | tr440941 | Mgea5 | meningioma expressed antigen 5 (hyaluronidase) |
| 1.28 | tr559289 | Wars | tryptophan-tRNA ligase, cytoplasmic |
| −1.97 | tr71877 | Tnik | traf2 and NCK-interacting protein kinase |
| −2.05 | tr61611 | Cdc42ep3 | cdc42 effector protein 3 |
| −2.15 | tr58004 | Pi15 | peptidase inhibitor 15 |
| −2.39 | tr400735 | Tppp | tubulin polymerization promoting protein |
| −3.12 | tr364915 | Scn2b | sodium channel, voltage-gated, type II, beta subunit |
| −4.66 | tr223880 | Celsr1 | cadherin EGF LAG seven-pass G-type receptor 1 |
EBSeq, P < 0.05, FDR < 0.05.
Metabolism.
Expression changes in metabolic factors during recovery from exACTH suggest that finely tuned mechanisms may restore metabolic homeostasis (Table 4). Downregulated metabolic genes included muscle growth inhibitors (Klf15, FoxO3, myostatin), an anabolic factor (Asb15), an atrogene (Trim62), and ubiquitin ligase (Asb9). We found that three inhibitors of insulin signaling (Rbp4, Igfbp3, Tenc1) were upregulated during recovery, while an insulin resistance factor (Bbs12), two mTOR inhibitors (Deptor, Sesn1), a pyruvate kinase (Pkm), and an oxidative metabolism repressor (Ncor1) were downregulated (Table 4). Two adipokines (Medag, Gpat3) and two mitochondrial enzymes (Prodh, Cyb5r1) were also differentially expressed during recovery. Therefore, metabolic recovery from exACTH included suppression of proteolytic factors and derepression of some genes involved in anabolism and substrate oxidation.
Cell proliferation/differentiation/death.
Cell cycle-related genes altered during recovery included both inducers and inhibitors of proliferation (Table 4). Factors that stimulate myocyte proliferation and suppress differentiation (Id3, Tbx2, Yap1) were strongly upregulated; Id3 was the most highly upregulated transcript during recovery. Other upregulated genes are known to stimulate proliferation and survival in other cell types (Zfp36l2, podocalyxin, L1td1, Axl). Three proliferation repressors (Ier5, Ndrg1, Npdc1) were upregulated concomitant with downregulation of three others (Mlf1, Tobb2, Gadd45α). Other downregulated genes include those involved in differentiation and cell fate specification (Zfp277, Jmjd2c, Dpf3), myocyte differentiation (Six4, melusin), and osteogenesis (Alk2; Table 4). We also found evidence for suppression of p38 MAPK signaling, as a p38 activator (MKK6) was downregulated while an opposing phosphatase (Dusp10) was upregulated. Therefore, muscle tissue may recover following an exACTH challenge by stimulating proliferation and survival and suppressing differentiation of various progenitor cell types.
Immune signaling.
Few immune signaling factors were differentially expressed during recovery from exACTH, which included a cytokine (IL-16), immune signaling receptors (Ackr1, Mrc1, CD302), and a complement protein regulator (Cfi; Table 4). Paradoxically, both an inhibitor (IκBα) and an activator (IKKα) of NF-κB signaling were downregulated during recovery; the latter was the second-most highly downregulated gene in the transcriptome. Recovery from exACTH may include complex fine-tuning of NF-κB activity and recovery of some immune signaling components.
Tissue function.
We found 20 general tissue and cell maintenance-related genes differentially expressed during recovery, of which 14 were upregulated and six were downregulated (Table 4). These included extracellular matrix remodeling factors (Lox, Timp2, Mgea5, Lama3, Pi15), cell adhesion proteins (Pcdh1, Celsr), cytoskeletal proteins (Twf1, Tnik, Cdc42ep3, Tppp), solute channels (Scn1b, Scn2b, Nherf2), and angiogenesis-related genes (Tek, Efnb2, Hspa12b, Heg1, Wars, PDGFRβ). Stimulation of vital tissue maintenance and repair processes may enable recovery of muscle tissue from exACTH.
DISCUSSION
We used transcriptome analysis to identify changes in gene expression during an acute response to exACTH administration in free-ranging elephant seals. ExACTH significantly elevated cortisol, aldosterone, and metabolites and upregulated a highly specific, conserved, and coordinated set of genes in muscle tissue. These did not, however, include GR targets associated with maladaptive GC effects seen in laboratory systems. Recovery from exACTH occurred within 24 h of administration as measured by recovery of corticosteroid hormones to baseline levels, downregulation of GR target genes, and derepression of positive regulators of tissue metabolism, growth, and remodeling. Metabolic factors differentially expressed during recovery include adipokines not previously described in muscle, reflecting species-specific adaptations to lipid-based fasting metabolism. We found that muscle response to HPA axis stimulation in free-living marine mammals is similar to that seen in laboratory animals but is context-dependent and highly selective to avoid deleterious effects of elevation of GC hormones that may occur as a natural part of their life histories.
Endocrine and Metabolic Response to exACTH
Administration of exACTH in this study group substantially increased circulating cortisol concentrations within 2 h, from 154 ± 28 nM to 1,687 ± 592 nM; these values overlapped with the maximal cortisol response elicited by capture and physical restraint (without sedation) in weaned pups [1,101 ± 232 nM (7)] that may mimic psychological and physiological responses to natural events (e.g., predation or conspecific aggression). ExACTH also caused significant elevation of aldosterone, a mineralocorticoid largely known for its osmoregulatory function in the context of the renin-angiotensin-aldosterone system. A number of studies, however, have now shown that aldosterone is elevated in response to stress in both pinnipeds (9, 17, 18) and cetaceans (24), suggesting that it may play a role in stress responses in these animals. The corticosteroid response to exACTH was terminated within 24 h of administration.
The response to exACTH administration and subsequent corticosteroid release had a significant effect on gene expression and substrates involved in carbohydrate, lipid, and protein metabolism. The apparent stimulatory effects of acute response on gluconeogenesis [increased glucose and reduced lactate, the preferred gluconeogenic substrate in this species (10, 57)] and lipolysis (increased FFA) in elephant seals are consistent with a typical stress response, despite the already high rates of both pathways in this species. However, we found no effect of exACTH administration on BUN levels after 2 h, consistent with very low rates of protein catabolism in elephant seals that enable effective sparing of lean tissue during fasting (13, 23). BUN concentrations decreased 24 h after exACTH administration, which may suggest a catabolism-limiting negative feedback mechanism in fasting-adapted animals.
Acute Transcriptome Response to exACTH
Metabolic changes elicited by exACTH administration result from glucocorticoid (through GR and MR), mineralocorticoid (through MR), and ACTH (through melanocortin 2 receptors, MC2R) effects on gene expression in target tissues (16, 51). Therefore, the gene expression changes observed in our study are likely the result of tissue responses to all three hormones. However, since the roles of aldosterone and ACTH in muscle physiology are poorly understood, we mainly focus our discussion on known GR effects on muscle gene expression. Muscle is a major GC target that is susceptible to catabolism promoted by elevated cortisol in humans and laboratory animals (29). We hypothesized that muscle tissue, critical for continued organismal function throughout fasting, would be protected from GC-induced degradation and that protective mechanisms would be apparent at the transcriptome level.
We found that 48 and 122 transcripts were differentially expressed during acute response to and recovery from exACTH, respectively. We included transcripts altered by less than twofold in our analysis because conservative fold-change thresholds often disregard changes that may be biologically meaningful (41, 50). For instance, while Sorbs1 was upregulated by only 1.18-fold during acute response, it is a well-described muscle GR target that is involved in tissue stress responses (29).
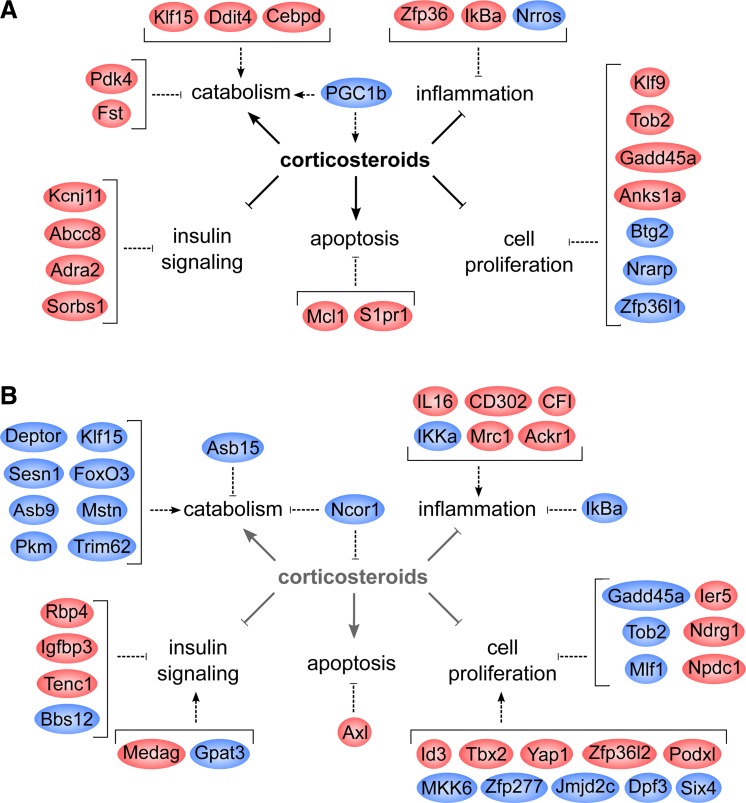
Acute response to exACTH was characterized by upregulation of a number of primary GR target genes previously identified in mouse myocytes (30). These factors are known to promote catabolism and inhibit insulin signaling, tissue glucose uptake, and oxidative metabolism, contributing to the increase in circulating glucose in response to exACTH. The proteolytic effect of GCs is mediated by master catabolism regulators (FoxO3, Klf15) that activate secondary transcriptional networks of E3 ubiquitin ligases (atrogenes), which target myofibrillar protein for degradation (54). The three most highly upregulated genes during acute response to exACTH were master regulators of proteolysis (Cebpd, Klf15, Ddit4). Ddit4 antagonizes protein synthesis by repressing anabolic mTOR signaling, while Cebpd and Klf15 directly activate atrogenes (54). The gene expression data indicating increased proteolysis conflict with static metabolite measures of BUN concentration that failed to detect changes in protein mobilization. This may suggest that the timing of sampling captured changes in gene expression before physiological measures of protein mobilization, such as BUN concentrations, were apparent. As no other key proteolytic factors (e.g., FoxO) or downstream atrogenes (e.g., myostatin) were also upregulated, the 2 h sampling point likely captured an early transcriptional response to exACTH. In the absence of upregulation of atrogenes, protein catabolism may not be initiated, and therefore changes in circulating BUN would not yet be detectable. It would be informative to determine when secondary effectors of proteolysis are activated during the response to exACTH. Surprisingly, we found that the atrogene inhibitor follistatin was significantly upregulated in seal muscle during the acute response to exACTH. Follistatin is usually repressed by GR in other systems (61), and further investigation is necessary to determine if it plays a role in protein sparing during corticosteroid elevation in seals.
Additional muscle-sparing mechanisms may include resistance to GC-mediated apoptosis and maintenance of tissue homeostasis. While GC exposure has been shown to induce apoptosis in laboratory systems (15), we found that two apoptosis inhibitors were upregulated in elephant seal muscle during the acute response to exACTH. Some proliferation inhibitors were upregulated, while others (Nrarp, Zfp36l1) were downregulated, reflecting the heterogeneity of cell types that comprise muscle tissue. Nrarp and Zfp36l1 are repressors of Notch signaling, which drives satellite cell proliferation during muscle repair and confers resistance to GC-mediated apoptosis (42). Their downregulation may reflect a negative feedback mechanism for preserving muscle tissue during corticosteroid elevation.
Consistent with the anti-inflammatory effect of GCs seen in other systems, we found that three immunosuppressants (Zfp36, IκBα, Nrros) were differentially expressed in seal muscle during acute response to exACTH. Zfp36 promotes mRNA degradation of proinflammatory cytokines (55), while IκBα inhibits inflammatory signaling by NF-κB (21). Nrros (Lrrc33, Table 3) is a potent immunosuppressant that inhibits TLR-mediated inflammatory signaling and limits ROS generation by phagocytes during inflammation (44). Its role in muscle tissue is currently unknown.
Tissue homeostasis during acute exACTH response may be maintained by upregulation of factors necessary to maintain basal tissue function (cytoskeletal and extracellular matrix remodeling, development, growth and survival) and downregulation of those involved in energetically costly processes (angiogenesis, innervation, myoglobin production). The second most highly upregulated gene was fibin (31), a GC-responsive factor that may be involved in muscle development. Therefore, the transcriptional response of elephant seal muscle to acute HPA axis perturbation is highly specific and involves upregulation of gene targets that may promote limited catabolism and basal tissue function while suppressing energy-intensive insulin signaling, cell proliferation, inflammation, and tissue development processes (Fig. 5A).
Fig. 5.
Transcriptional changes in response to exACTH administration are mediated by corticosteroids and affect processes such as catabolism, cell proliferation, apoptosis, and inflammation (solid arrows). Selected genes differentially expressed (red, upregulated; blue, downregulated) during acute response to exACTH (A) and recovery from exACTH (B) and their putative effects on these processes (dashed arrows) are shown.
Transcriptional Changes during Recovery from exACTH
Changes in the transcriptional landscape between acute response and recovery states suggest that restoration of tissue metabolism and growth occur within 24 h of exACTH administration. This includes downregulation of GR targets induced earlier (Klf15, Gadd45α, Tob2, Pi15), secondary catabolic factors (FoxO3, myostatin), and mTOR inhibitors, and upregulation of genes involved in glucose uptake and utilization. Downregulation of both primary and secondary proteolytic network genes during the ∼22 h of recovery from exACTH administration may be sufficient to drive the observed decrease in BUN levels, potentially protecting protein stores. Sampling muscle tissue more frequently or earlier than 24 h after exACTH administration may reveal additional negative feedback mechanisms that potentially suppress catabolism and reduce circulating BUN following HPA axis stimulation. Paradoxically, suppression of insulin, an anabolic hormone, was maintained during recovery as evidenced by several upregulated insulin signaling inhibitors. Circulating insulin levels in study animals were low and unaffected by exACTH, consistent with the hypoinsulinemia and peripheral insulin resistance described in this species (22). It appears that despite low baseline insulin secretion, suppression of insulin sensitivity in target tissues is critical during adrenocortical responses in a fasting mammal. Mechanisms by which fasting animals maintain protein stores despite suppressed insulin signaling are currently unknown. Four adipokines (Rbp4, Medag, Gpat3, Bbs12) were differentially expressed in elephant seal muscle during recovery from exACTH administration. Their role in muscle metabolism, with the exception of Rbp4, is not well defined but may parallel nutrient uptake roles seen in adipose (5, 38, 62) and reflect reliance on lipid oxidation in fasting species, especially marine mammals. Further studies of muscle-expressed adipokines may yield insight on mechanisms used to conserve energy stores during fasting and acute adrenocortical responses. We also found two mitochondrial enzymes (Prodh, Cyb5r1) differentially expressed during recovery from exACTH. Prodh catalyzes proline catabolism in response to nutrient stress and may play a role in protection from oxidative damage (43), while Cyb5r1 is involved in fatty acid elongation and cholesterol biosynthesis. Negative feedback on GR signaling within muscle tissue was evidenced by downregulation of a nuclear receptor coactivator [PGC-1β (20)] at 2 h and downregulation of a negative regulator of GR activity [Ncor1 (49)] at 24 h.
Recovery from HPA axis perturbation may involve stimulation of cell proliferation and inhibition of differentiation, potentially to replenish the myocyte progenitor pool negatively affected by elevated GCs. Differentially expressed genes included chromatin remodeling factors (Zfp277, Jmjd2c, Dpf3), which may play a role in epigenetic regulation of cell stress responses and should be further explored. We found evidence that p38 signaling, which may regulate muscle development, proliferation, metabolism, and inflammation (14), was suppressed at 24 h. Recovery of some components of the immune response was evidenced by upregulation of factors associated with immune signaling, although an activator of NF-κB was the most highly downregulated gene at this time. The role of NF-κB in muscle responses to elevated corticosteroids is likely complex and warrants further investigation. Tissue maintenance genes altered during recovery from exACTH included extracellular matrix remodeling and cytoskeletal factors, solute channels, and angiogenesis regulators. Therefore, elephant seals may restore muscle homeostasis after an HPA axis challenge with finely tuned and highly coordinated alterations in metabolic, proliferative, immune, and tissue repair pathways (Fig. 5B).
Conclusions
To our knowledge, this is one of the first descriptions of a global transcriptome response to HPA axis stimulation in a free-living marine mammal profiled by RNA-Seq. We found that genes differentially expressed in response to exACTH and elevated corticosteroids include highly conserved muscle GR targets in addition to factors not previously described in this context in laboratory species. These may represent novel biomarkers of acute adrenocortical responses in free-ranging marine mammals, which may be used to evaluate stress states in species subject to frequent environmental disturbance (26). We suggest that animals regularly exposed to corticosteroid fluctuations may use buffering mechanisms to protect vital tissues from their adverse effects while retaining the capacity to mount adaptive stress responses, which are likely tightly regulated to avoid long-term consequences deleterious to an animal's fitness. Examination of exACTH-induced gene expression in other tissues such as adipose, the main energy depot in marine mammals and one that may be more directly affected by aldosterone (36) and ACTH (2), will provide valuable insights on coordination of energy usage during organismal stress responses. In addition, comparison of molecular responses to brief vs. sustained exposure to elevated corticosteroids may elucidate mechanisms involved in the pathological progression from acute to chronic stress, critical to conservation physiology. In the meantime, this study represents a critical step toward a gene-level understanding of marine mammal responses to HPA axis stimulation in the context of natural environments.
GRANTS
This work was conducted under National Marine Fisheries Service permit 14636, supported by National Institutes of Health Grant HL-091767 to R. M. Ortiz and D. E. Crocker and Office of Naval Research Grant N000141110434 to D. E. Crocker, and approved by the Sonoma State University Institutional Animal Care and Use Committee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.I.K., C.D.C., and D.E.C. conception and design of research; J.I.K. and C.D.C. performed experiments; J.I.K. and L.P. analyzed data; J.I.K., C.D.C., and D.E.C. interpreted results of experiments; J.I.K. and C.D.C. prepared figures; J.I.K. and C.D.C. drafted manuscript; J.I.K., C.D.C., L.P., R.M.O., and D.E.C. edited and revised manuscript; J.I.K., C.D.C., L.P., R.M.O., and D.E.C. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank J. Sharick, D. Somo, D. Esminger, H. Peck, and R. Berger for assistance with field procedures and sample collection.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Betz MJ, Hatiboglu N, Mauracher B, Hadaschik D, Sauter A, Demmelmair H, Koletzko B, Beuschlein F, Slawik M. Mc2 receptor knockdown modulates differentiation and lipid composition in adipocytes. Horm Metab Res 44: 670–675, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Blix AS. The venous system of seals, with new ideas on the significance of the extradural intravertebral vein. J Exp Biol 214: 3507–3510, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Boonstra R. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–23, 2013. [Google Scholar]
- 5.Cao J, Perez S, Goodwin B, Lin Q, Peng H, Qadri A, Zhou Y, Clark RW, Perreault M, Tobin JF, Gimeno RE. Mice deleted for GPAT3 have reduced GPAT activity in white adipose tissue and altered energy and cholesterol homeostasis in diet-induced obesity. Am J Physiol Endocrinol Metab 306: E1176–E1187, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Champagne CD, Crocker DE, Fowler MA, Houser DS. Fasting physiology of the pinnipeds: the challenges of fasting while maintaining high energy expenditure and nutrient delivery for lactation. In: Comparative Physiology of Fasting, Starvation, and Food Limitation, edited by McCue MD. Berlin: Springer-Verlag, 2012, p. 309–336. [Google Scholar]
- 7.Champagne CD, Houser DS, Costa DP, Crocker DE. The effects of handling and anesthetic agents on the stress response and carbohydrate metabolism in northern elephant seals. PLoS One 7: e38442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne CD, Houser DS, Crocker DE. Glucose metabolism during lactation in a fasting animal, the northern elephant seal. Am J Physiol Regul Integr Comp Physiol 291: R1129–R1137, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Champagne CD, Tift MS, Houser DS, Crocker DE. Adrenal sensitivity to stress is maintained despite variation in baseline glucocorticoids in moulting seals. Conserv Physiol 3: cov004, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: cot001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocker DE, Champagne CD, Fowler MA, Houser DS. Adiposity and fat metabolism in lactating and fasting northern elephant seals. Adv Nutr 5: 57–64, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocker DE, Ortiz RM, Houser DS, Webb PM, Costa DP. Hormone and metabolite changes associated with extended breeding fasts in male northern elephant seals (Mirounga angustirostris). Comp Biochem Physiol A 161: 388–394, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Crocker DE, Webb PM, Costa DP, Le Boeuf BJ. Protein catabolism and renal function in lactating northern elephant seals. Physiol Zool 71: 485–491, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 429: 403–417, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Dirks-Naylor AJ, Griffiths CL. Glucocorticoid-induced apoptosis and cellular mechanisms of myopathy. J Steroid Biochem Mol Biol 117: 1–7, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Dores RM, Liang L. Analyzing the activation of the melanocortin-2 receptor of tetrapods. Gen Comp Endocrinol 203: 3–9. [DOI] [PubMed] [Google Scholar]
- 17.Ensminger DC, Somo DA, Houser DS, Crocker DE. Metabolic responses to adrenocorticotropic hormone (ACTH) vary with life-history stage in adult male northern elephant seals. Gen Comp Endocrinol 204: 150–157, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Gulland FMD, Haulena M. Adrenal function in wild and rehabilitated Pacific harbor seals (Phoca vitulina richardii) and in seals with phocine herpesvirus-associated adrenal necrosis. Marine Mamm Sci 15: 810–827, 1999. [Google Scholar]
- 19.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, Macmanes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, Leduc RD, Friedman N, Regev A. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8: 1494–1512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine Rev 27: 728–735, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 12: 86, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houser DS, Champagne CD, Crocker DE. A non-traditional model of the metabolic syndrome: the adaptive significance of insulin resistance in fasting-adapted seals. Front Endocrinol 4: 164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houser DS, Costa DP. Protein catabolism in suckling and fasting northern elephant seal pups (Mirounga angustirostris). J Comp Physiol B 171: 635–642, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Houser DS, Yeates LC, Crocker DE. Cold stress induces an adrenocortical response in bottlenose dolphins (Tursiops truncatus). J Zoo Wildl Med 42: 565–571, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Hunt KE, Moore MJ, Rolland RM, Kellar NM, Hall AJ, Kershaw J, Raverty SA, Davis CE, Yeates LC, Fauquier DA, Rowles TK, Kraus SD. Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv Physiol 1: cot006, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelso EJ, Champagne CD, Tift MS, Houser DS, Crocker DE. Sex differences in fuel use and metabolism during development in fasting juvenile northern elephant seals. J Exp Biol 215: 2637–2645, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Khudyakov JI, Preeyanon L, Champagne CD, Ortiz RM, Crocker DE. Transcriptome analysis of northern elephant seal (Mirounga angustirostris) muscle tissue provides a novel molecular resource and physiological insights. BMC Genomics 16: 64, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo T, Harris CA, Wang JC. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380: 79–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci USA 109: 11160–11165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakner J, Seyer C, Hermsdorf T, Schoneberg T. Characterization of the expression, promoter activity and molecular architecture of fibin. BMC Biochem 12: 26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavergne SG, McGowan PO, Krebs CJ, Boonstra R. Impact of high predation risk on genome-wide hippocampal gene expression in snowshoe hares. Oecologia 176: 613–624, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, Haag JD, Gould MN, Stewart RM, Kendziorski C. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 29: 1035–1043, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo P, Dematteo A, Wang Z, Zhu L, Wang A, Kim HS, Pozzi A, Stafford JM, Luther JM. Aldosterone deficiency prevents high-fat-feeding-induced hyperglycaemia and adipocyte dysfunction in mice. Diabetologia 56: 901–910, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia A, Warr GW, Chapman RW. A transcriptomic analysis of the stress induced by capture-release health assessment studies in wild dolphins (Tursiops truncatus). Mol Ecol 17: 2581–2589, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Marion V, Mockel A, De Melo C, Obringer C, Claussmann A, Simon A, Messaddeq N, Durand M, Dupuis L, Loeffler JP, King P, Mutter-Schmidt C, Petrovsky N, Stoetzel C, Dollfus H. BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell Metab 16: 363–377, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Martinez B, Sonanez-Organis JG, Vazquez-Medina JP, Viscarra JA, MacKenzie DS, Crocker DE, Ortiz RM. Prolonged food deprivation increases mRNA expression of deiodinase 1 and 2, and thyroid hormone receptor beta-1 in a fasting-adapted mammal. J Exp Biol 216: 4647–4654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashburn KL, Atkinson S. Evaluation of adrenal function in serum and feces of Steller sea lions (Eumetopias jubatus): influences of molt, gender, sample storage, and age on glucocorticoid metabolism. Gen Comp Endocrinol 136: 371–381, 2004. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy DJ, Smyth GK. Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics 25: 765–771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mourikis P, Tajbakhsh S. Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev Biol 14: 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan SK, Zhu W, Liang X, Zhang L, Demers AJ, Zimmerman MC, Simpson MA, Becker DF. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic Biol Med 53: 1181–1191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noubade R, Wong K, Ota N, Rutz S, Eidenschenk C, Valdez PA, Ding J, Peng I, Sebrell A, Caplazi P, DeVoss J, Soriano RH, Sai T, Lu R, Modrusan Z, Hackney J, Ouyang W. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 509: 235–239, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 280: R790–R795, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Peck HE, Costa DP, Crocker DE. Body reserves influence allocation to immune responses in capital breeding female northern elephant seals. Funct Ecol. In press. [Google Scholar]
- 47.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol 33: 1711–1722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Romero LM, Dickens MJ, Cyr NE. The Reactive Scope Model - a new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–389, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev 21: 55–89, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, Nakae J, Tagata Y, Nishitani S, Takehana K, Sano M, Fukuda K, Suematsu M, Morimoto C, Tanaka H. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab 13: 170–182, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol 26: 9126–9135, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.St. Aubin DJ, Geraci JR. Adrenocortical function in pinniped hyponatremia. Marine Mamm Sci 2: 243–250, 1986. [Google Scholar]
- 57.St. Aubin DJ, Geraci JR. Stress and marine mammals. In: CRC Handbook of Marine Mammal Medicine, edited by Dierauf LA, Gulland FMD. New York: CRC, 2001, p. 253–269. [Google Scholar]
- 58.Thomson CA, Geraci JR. Cortisol, aldosterone, and leucocytes in the stress response of bottlenose dolphins, tursiops truncatus. Can J Fish Aquat Sci 43: 1010–1016, 1986. [Google Scholar]
- 59.Vazquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol 213: 2524–2530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38: W214–W220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, Harrison CA, McMullen JR, Chamberlain JS, Gregorevic P. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J Cell Biol 197: 997–1008, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Chen X, Sairam MR. Novel genes of visceral adiposity: identification of mouse and human mesenteric estrogen-dependent adipose (MEDA)-4 gene and its adipogenic function. Endocrinology 153: 2665–2676, 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.