We demonstrate that intravenous ketorolac (a nonselective inhibitor of cyclooxygenase that lowers prostanoids) significantly attenuated the blood pressure response to dynamic leg exercise in peripheral arterial disease patients but not in healthy controls. Further understanding this process is important because high blood pressure responses are associated with adverse cardiovascular events in peripheral arterial disease patients.
Keywords: cyclooxygenase, intermittent claudication, exercise physiology
Abstract
Prostanoids are produced during skeletal muscle contraction and subsequently stimulate muscle afferent nerves, thereby contributing to the exercise pressor reflex. Humans with peripheral arterial disease (PAD) have an augmented exercise pressor reflex, but the metabolite(s) responsible for this augmented response is not known. We tested the hypothesis that intravenous injection of ketorolac, which blocks the activity of cyclooxygenase, would attenuate the rise in mean arterial blood pressure (MAP) and heart rate (HR) evoked by plantar flexion exercise. Seven PAD patients underwent 4 min of single-leg dynamic plantar flexion (30 contractions/min) in the supine posture (workload: 0.5–2.0 kg). MAP and HR were measured on a beat-by-beat basis; changes from baseline in response to exercise were determined. Ketorolac did not affect MAP or HR at rest. During the first 20 s of exercise with the most symptomatic leg, ΔMAP was significantly attenuated by ketorolac (2 ± 2 mmHg) compared with control (8 ± 2 mmHg, P = 0.005), but ΔHR was similar (6 ± 2 vs. 5 ± 1 beats/min). Importantly, patients rated the exercise bout as “very light” to “fairly light,” and average pain ratings were 1 of 10. Ketorolac had no effect on perceived exertion or pain ratings. Ketorolac also had no effect on MAP or HR in seven age- and sex-matched healthy subjects who performed a similar but longer plantar flexion protocol (workload: 0.5–7.0 kg). These data suggest that prostanoids contribute to the augmented exercise pressor reflex in patients with PAD.
NEW & NOTEWORTHY
We demonstrate that intravenous ketorolac (a nonselective inhibitor of cyclooxygenase that lowers prostanoids) significantly attenuated the blood pressure response to dynamic leg exercise in peripheral arterial disease patients but not in healthy controls. Further understanding this process is important because high blood pressure responses are associated with adverse cardiovascular events in peripheral arterial disease patients.
peripheral arterial disease (PAD) is a form of atherosclerosis that preferentially affects the lower extremity. Because leg blood flow is impaired in PAD, it is not surprising that dynamic exercise, such as walking, provokes pain in the calf, thigh, or buttocks, termed “intermittent claudication.” Clinical exercise testing (e.g., the Bruce treadmill protocol) also causes a large increase in blood pressure (BP) in PAD patients, and studies (10, 11) have linked this augmented pressor response to cardiovascular morbidity and mortality. In recent years, our laboratory has focused on BP control in patients with PAD. Specifically, we found that 1) the BP response to single-leg dynamic plantar flexion exercise was augmented in PAD patients compared with healthy control subjects; 2) the pressor response occurred at very low workloads and before the onset of pain; 3) the pressor response correlated with the ankle-brachial index (ABI), suggesting that disease severity plays a role; and 4) intravenous infusion of ascorbic acid lowered the pressor response to exercise in PAD (13, 28). To date, the mechanisms for these findings are largely unknown. Understanding BP regulation during exercise in PAD is fundamentally important and also may lead to improved clinical care.
The exercise pressor reflex is a complex regulatory process in which group III and IV afferents sense mechanical and chemical factors within the contracting skeletal muscle. This information is projected to the spinal cord and brain stem; eventually, sympathetic outflow increases, mean arterial pressure (MAP) increases, and heart rate (HR) increases (16). Prostanoids are produced during skeletal muscle contraction and subsequently stimulate muscle afferent nerves, thereby contributing to the exercise pressor reflex (32–34, 38). Experiments in cats have demonstrated that intravenous indomethacin (a nonselective inhibitor of cyclooxygenase) attenuates group III and IV afferent nerve responses to dynamic exercise (15). A different study (36) in cats showed that intravenous indomethacin attenuated the rise in MAP in response to static hindlimb contraction. Many studies (7, 8, 14, 23, 25) in healthy humans have demonstrated that blocking the prostanoid pathway attenuates reflex cardiovascular responses to exercise, although two studies (9, 12) have contradicted these findings. Currently, it is believed that prostanoids are especially important for sensitizing group III afferents during low-intensity dynamic exercise (i.e., before the onset of muscle metaboreflex activation). Indeed, studies in healthy humans (23) and patients with heart failure (24) have shown that intra-arterial indomethacin attenuates the sympathetic nerve response to rhythmic handgrip. The effect of cyclooxygenase inhibition on reflex cardiovascular responses to exercise in patients with PAD is currently unknown.
In the present study, we tested the hypothesis that intravenous injection of ketorolac tromethamine (a nonselective inhibitor of cyclooxygenase) attenuates the rise in MAP and HR in response to low-intensity, single-leg, dynamic plantar flexion exercise in patients with PAD.
METHODS
Ethical approval.
All experiments were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. All subjects provided written and informed consent.
Design and subjects.
These physiological experiments used a repeated-measures design and had both within-subjects (exercise workload and ketorolac treatment) and between-subjects (PAD and healthy) components. Trials were not randomized because the half-life of ketorolac is several hours; there was also no blinding of the ketorolac administration.
Seven patients with a clinical diagnosis of PAD (Fontaine stages I and II) and symptomatic intermittent claudication volunteered for this investigation (Table 1). The sample size was determined after the first four PAD patients had completed testing. Specifically, we determined that if the mean change in MAP in the first 20 s of exercise (i.e., to isolate the muscle mechanoreflex) before and after ketorolac was 6 mmHg with a SD of 4 mmHg, we would need to enroll seven PAD patients to be able to reject the null hypothesis with 90% power and a type 1 error of 0.05. Exclusion criteria were as follows: 1) age > 75 yr, 2) body mass index > 35 kg/m2, 3) symptomatic coronary artery disease, 4) peripheral neuropathy, 5) heart failure, 6) chronic obstructive pulmonary disease, 7) diabetes, and 8) renal disease (creatinine > 1.5). PAD patients were chronically treated with a variety of medications, including β-blockers (n = 2), statins (n = 5), antiplatelet agents (n = 5 aspirin and n = 2 clopidogrel), angiotensin-converting enzyme inhibitors (n = 4), angiotensin receptor antagonists (n = 1), and diuretics (n = 2). All medications were withheld on the morning of the study except for two patients who took their antiplatelet medication.
Table 1.
Baseline parameters
| PAD Patients |
Healthy Subjects |
|||
|---|---|---|---|---|
| Preketorolac | Postketorolac | Preketorolac | Postketorolac | |
| Men/women | 5/2 | 5/2 | ||
| Age, yr | 65 ± 2 | 65 ± 2 | ||
| Height, cm | 173 ± 4 | 172 ± 4 | ||
| Weight, kg | 78 ± 5 | 76 ± 4 | ||
| Body mass index, kg/m2 | 26.0 ± 1.0 | 25.6 ± 0.8 | ||
| ABI most | 0.54 ± 0.07† | 1.10 ± 0.06 | ||
| ABI least | 0.76 ± 0.10† | 1.09 ± 0.04 | ||
| Systolic blood pressure, mmHg | 129 ± 4† | 135 ± 7 | 116 ± 4 | 121 ± 5 |
| Diastolic blood pressure, mmHg | 77 ± 2 | 78 ± 3 | 74 ± 3 | 75 ± 3 |
| Mean arterial pressure, mmHg | 98 ± 3† | 94 ± 3 | 84 ± 5 | 91 ± 3* |
| Heart rate, beats/min | 58 ± 2 | 56 ± 2 | 57 ± 3 | 54 ± 3 |
Values are means ± SE; n = 7 peripheral arterial disease (PAD) patients and 7 healthy control subjects. ABI most, ankle-brachial index of the most symptomatic leg; ABI least, ankle-brachial index of the least symptomatic leg.
Difference between preketorolac and postketorolac in healthy subjects; †difference between groups at the specific time point (P < 0.05).
Seven age- and sex-matched healthy control subjects (ABI > 0.9) who were not taking any prescription medications also participated in this study (Table 1).
Study protocol.
All experiments were conducted during the morning hours after an overnight fast. Room temperature was consistently between 22 and 25°C. Upon subject arrival at the laboratory, height and weight were obtained, a brief physical examination was conducted, and ABI was measured for each leg. Subjects were supine and instrumented with a three-lead ECG (Cardiocap/5, GE Healthcare), a finger cuff to monitor beat-by-beat BP (Finometer, FMS), a pneumobelt to monitor breathing movements, and one intravenous catheter in the antecubital space for blood samples and drug administration. After a 15-min quiet rest period, baseline BPs were obtained via automated oscillometry of the brachial artery (Philips SureSigns Vs3), and a blood sample was obtained for the measurement of thromboxane B2 (stable marker of thromboxane A2) and 6-keto-PGF1α (stable marker of prostacyclin, Penn State Core Endocrine Laboratory, Hershey, PA). The calf of the supine volunteer was supported by cushions to assure a full range of motion (i.e., active plantar flexion), and a custom sandal was affixed to the foot, consistent with previous studies in our laboratory (13, 28).
After a 3-min baseline period, PAD patients underwent a 4-min bout of plantar flexion exercise with their most symptomatic leg (30 contractions/min following the pace of a metronome). We chose this paradigm to most closely mimic walking. Weight was progressively added (i.e., 0.5 kg during the first minute, 1.0 kg during the second minute, 1.5 kg during the third minute, and 2.0 kg during the fourth minute). After a 20-min rest period, the same procedures were repeated for the less symptomatic leg. Control subjects performed single-leg plantar flexion up to 7.0 kg (14 min total with the first 4 min identical to that performed in PAD patients). We chose this extended exercise bout in healthy subjects because our prior publication (28) showed the 2.0-kg paradigm to have minimal effects on HR and BP in healthy control subjects. At the end of each minute, subjects verbally rated their perceived exertion and leg pain (6).
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug clinically used for short-term management of moderate to severe pain. It is a nonselective inhibitor of cyclooxygenase, and a previous physiological study (26) has shown that intravenous administration of 30–45 mg reduces prostanoids throughout the body. In the present study, ketorolac tromethamine (30 mg in 30 ml, Hospira, Lake Forest, IL) was injected over 1 min. The 30-mg dose was chosen because it is effective at reducing postoperative pain (1). We suspected that stimulation of muscle afferents would occur at lower prostanoid levels compared with pain (18, 39). A 30-min waiting period occurred after injection to ensure the drug was sufficiently active in the blood (26), and a blood sample was then obtained for the measurement of thromboxane B2 (stable marker of thromboxane A2) and 6-keto-PGF1α (stable marker of prostacyclin, Penn State Core Endocrine Laboratory). Plantar flexion trials were then repeated in the presence of cyclooxygenase blockade.
In an attempt to minimize an order effect, six of seven PAD patients repeated the control trial (no ketorolac injection) on a separate day. However, these trials did not include a 30-ml saline infusion.
Data collection and statistical analysis.
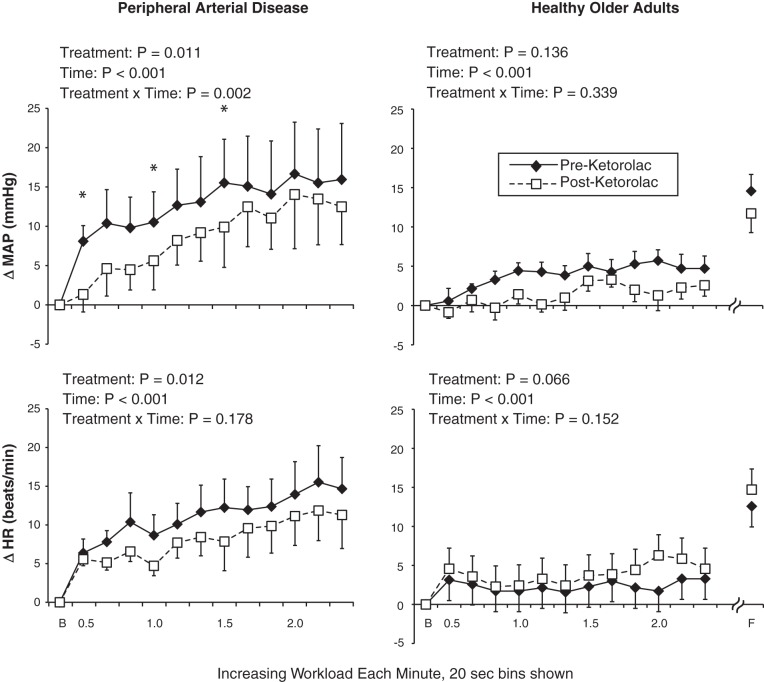
Data were collected continuously at 200 Hz and analyzed offline (PowerLab, AD Instruments). Statistics were performed using SPSS 21.0, and graphics were produced with Microsoft Excel and Adobe Illustrator CS5. Exercise data were averaged into 20-s bins (i.e., 3 bins/workload). Absolute changes (Δ) in MAP and HR from the preceding baseline were used (as shown in Fig. 1). Because PAD patients and healthy subjects performed slightly different protocols, the groups were analyzed separately (i.e., to evaluate the effect of ketorolac). The overall study used a repeated-measures design, and, therefore, 2 treatment (preketorolac and postketorolac) × 5 time (baseline, 0.5 kg. 1.0 kg, 1.5 kg, and 2.0 kg) repeated-measures ANOVAs were conducted. The first 20 s of each workload were included in the ANOVA based on our previous findings in PAD patients (28). In healthy control subjects, physiological responses at the 7-kg workload were compared with a paired-samples t-test (i.e., this time point was not used in the ANOVA). When a significant interaction was found, the Holm adjustment to the Bonferroni correction was used. Nonparametric tests were used to evaluate ratings of perceived exertion and leg pain. Physiological data are presented as means ± SE and perceptual data as medians with ranges in parentheses. P values of <0.05 were considered statistically significant.
Fig. 1.
Hemodynamic responses to exercise in patients with peripheral arterial disease (PAD; left) and healthy control subjects (right). MAP, mean arterial pressure; HR, heart rate; B, baseline; F, fatigue. Values are means ± SE. *Significant difference between treatments at the specific time point (P < 0.05).
RESULTS
As shown in Table 1, PAD patients and healthy control subjects were well matched for age, height, weight, body mass index, and resting HR. Systolic BP (P = 0.037) and MAP (P = 0.041) were higher at baseline in PAD patients, but diastolic BP was comparable between groups (P = 0.353). Injection of ketorolac had no effect on resting systolic BP, diastolic BP, MAP, or HR in PAD patients, whereas ketorolac caused a significant rise in MAP in healthy control subjects (P = 0.048). As shown in Table 2, ketorolac significantly reduced thromboxane B2 (P = 0.047) and 6-keto-PGF1α (P = 0.024) in healthy control subjects. It is worth noting that PAD patients and healthy control subjects had different baseline levels of thromboxane B2 and 6-keto-PGF1α, likely because PAD patients were all treated with chronic antiplatelet agents, which block cyclooxygenase (n = 5 aspirin and n = 2 clopidogrel).
Table 2.
Blood markers pre- and postketorolac
| PAD Patients |
Healthy Subjects |
|||
|---|---|---|---|---|
| Preketorolac | Postketorolac | Preketorolac | Postketorolac | |
| Thromboxane B2, pg/ml | 120 ± 28† | 84 ± 4 | 714 ± 32 | 232 ± 3* |
| 6-Keto-PGF1α, pg/ml | 88 ± 8† | 87 ± 7 | 137 ± 88 | 22 ± 6* |
Values are means ± SE; n = 7 PAD patients and 7 healthy control subjects.
Difference between preketorolac and postketorolac in healthy subjects; †difference between groups at the specific time point (P < 0.05).
In PAD patients, the pressor response to 4 min of single-leg dynamic plantar flexion with the most symptomatic leg was attenuated by ketorolac (Fig. 1, top left). Indeed, at 0.5 kg (P = 0.005), 1.0 kg (P = 0.036), and 1.5 kg (P = 0.011) of exercise, ΔMAP was significantly reduced after ketorolac. In PAD patients, 2 × 5 ANOVA for HR did not reveal a treatment × time interaction (Fig. 1, bottom left), but there was a main effect for treatment such that ΔHR was lower after ketorolac. Ratings of perceived exertion and leg pain data were low and not significantly affected by ketorolac (Table 3).
Table 3.
Perceptual responses to plantar flexion exercise
| PAD Patients |
Healthy Subjects |
|||
|---|---|---|---|---|
| Preketorolac | Postketorolac | Preketorolac | Postketorolac | |
| Rating of perceived exertion | ||||
| 0.5 kg | 7 (6–9) | 6 (6–9) | 6 (6–7) | 6 (6–7) |
| 1.0 kg | 9 (6–11) | 8 (6–10) | 7 (6–9) | 7 (6–7) |
| 1.5 kg | 10 (8–13) | 9 (8–13)* | 8 (6–11) | 7 (6–9) |
| 2.0 kg | 11 (9–14) | 10 (9–14)* | 9 (7–12) | 9 (7–10) |
| 7.0 kg | 15 (13–17) | 15 (14–17) | ||
| Pain | ||||
| 0.5 kg | 0 (none) | 0 (0–2) | 0 (none) | 0 (none) |
| 1.0 kg | 0 (0–3) | 0 (0–3) | 0 (none) | 0 (none) |
| 1.5 kg | 0 (0–4) | 0 (0–5) | 0 (none) | 0 (none) |
| 2.0 kg | 0 (0–5) | 0 (0–5) | 0 (none) | 0 (none) |
| 7.0 kg | – | – | 0 (none) | 0 (0–3) |
Data are shown as medians with ranges in parentheses; n = 7 PAD patients and 7 healthy control subjects.
Significant difference between groups at the specific time point. There were no effects of ketorolac treatment. For ratings of perceived exertion, 6 = no exertion, 9 = very light, 13 = somewhat hard, and 17 = very hard. For measures of leg pain, 0 = no pain, 2 = mild, 4 = moderate, 6 = severe, 8 = very severe, and 10 = worst possible.
When the less symptomatic leg was analyzed in PAD patients, ketorolac significantly reduced ΔMAP at 2.0 kg (before: 17 ± 5 mmHg vs. after: 7 ± 2 mmHg, P = 0.036). At 0.5 kg (before: 5 ± 2 mmHg vs. after: 2 ± 2 mmHg), 1.0 kg (before: 9 ± 2 mmHg vs. after: 4 ± 1 mmHg), and 1.5 kg (before: 15 ± 6 mmHg vs. after: 8 ± 2 mmHg), comparisons did not reach statistical significance (P > 0.2 for all).
In an attempt to minimize an order effect, six of seven PAD patients repeated the control trial (no ketorolac injection) on a separate day. ΔMAP in the first 20 s of exercise with the most symptomatic leg was 7 ± 2 mmHg during this repeat trial compared with 8 ± 2 mmHg (as shown in Fig. 1). Thus, the effect of ketorolac on MAP (ΔMAP = 1 ± 2 mmHg in these six subjects) was much larger than any time or learning effect of this paradigm.
In healthy subjects, ketorolac had no effect on MAP or HR responses during the first 4 min of single-leg dynamic plantar flexion (Fig. 1, top right and bottom right). Because we expected the physiological response to be minimal in these healthy subjects based on our previous publication (28), we purposely had the exercise to a maximum of 7 kg or fatigue (to raise MAP and HR to comparable levels observed in PAD patients). At peak exercise, ketorolac still had no significant effect on either MAP (P = 0.777) or HR (P = 0.654) in healthy control subjects.
DISCUSSION
The purpose of the present study was to evaluate the effect of cyclooxygenase inhibition on reflex HR and MAP responses to dynamic plantar flexion exercise in human subjects. Consistent with our hypothesis, in PAD patients, ΔMAP was significantly attenuated by ketorolac and ΔHR also tended to be lower across time and workload. In healthy control subjects, ketorolac did not have a significant effect on ΔMAP or ΔHR. The present data suggest that inhibition of exercise-induced prostanoid synthesis significantly influences the exercise pressor reflex in patients with PAD. The physiological relevance and potential clinical implications of these findings are discussed below.
The cardinal symptom of PAD, intermittent claudication, is provoked by dynamic exercise (i.e., walking), and many previous studies have quantified cardiovascular responses to walking in these patients. The current consensus is that treadmill walking and supine plantar flexion elicit augmented HR and MAP response in patients with PAD compared with control subjects (2, 3, 11, 20, 31). Importantly, those patients with higher exercise BP have a higher risk of adverse cardiovascular events (10, 11, 17, 40). Despite this heightened cardiovascular risk during exercise in PAD, there have been very few studies evaluating the mechanisms of exercise BP regulation in PAD and/or therapies to mitigate the pressor response. Because acute limb ischemia raises thromboxane B2 (i.e., a stable product of arachidonic acid metabolism) (22) and because animal models of PAD have suggested that the group III component of the exercise pressor reflex (predominantly muscle mechanoreceptors) is augmented in this disease (18, 39), we chose to evaluate the cyclooxygenase-prostanoid pathway during mild, lower limb exercise in the present study.
The majority of studies in healthy humans have shown that inhibiting exercise-induced prostanoid synthesis attenuates reflex cardiovascular responses (7, 8, 14, 23, 25). These studies all used handgrip exercise, and they all administered either indomethacin or ketoprofen. It is thought that ketorolac and indomethacin have equivalent physiological effects (27). To our knowledge, we are the first to evaluate the cyclooxygenase-prostanoid pathway during exercise in humans with PAD, albeit with a different, more clinically relevant exercise modality. In other words, we believe physiological responses to simulated dynamic walking in PAD are more relevant than the response to handgrip. It should be pointed out that ΔMAP and ΔHR in healthy control subjects were not affected by ketorolac. We suspect the lack of effect in healthy subjects is due to the exercise modality (i.e., dynamic compared with isometric in previous studies). Moreover, ketorolac did not have an effect on rating of perceived exertion or leg pain in either group.
The present data in humans are in general agreement with recent studies that used a rodent model of simulated PAD. In particular, 72 h of femoral artery ligation elicited a higher BP response to static contraction compared with control conditions (i.e., freely perfused femoral artery), and this augmented pressor response was attenuated by daltroban, a thromboxane receptor antagonist (18). In another study, Yamauchi and colleagues (41) showed that 72 h of femoral artery ligation increased endoperoxidade 4 receptor (a receptor for PGE2) protein in the dorsal root ganglia; blockade of these receptors lowered the exercise pressor reflex. More recently, it has been shown that ketorolac administered into the spinal cord attenuated the pressor response to static contraction in rats (37). A study by Li and colleagues (19) has also shown that nerve growth factor levels increase after hindlimb ischemia, and this raises the expression of several muscle afferent receptors, thereby potentiating the exercise pressor reflex in rodents with PAD. Considering the overlap between inflammation, oxidative stress, and local hypoxia on vascular control, it is likely that exercise BP regulation in PAD is influenced by numerous factors (21).
Limitations.
Because we infused a nonselective inhibitor of cyclooxygenase, it is likely that vasodilating prostaglandins were also inhibited, and this may contribute to the rise in MAP observed at rest in healthy subjects (35). Ketorolac could also raise arterial stiffness and central BP, which has been shown in previous studies (4, 5).
In our study, ketorolac significantly reduced thromboxane B2 and 6-keto-PGF1α at baseline (before exercise) in healthy older adults but not in patients with PAD. The standard of care for PAD patients is treatment with antiplatelet agents, which lowers prostanoids (29, 30). It would not be clinically appropriate to have PAD patients withhold these medications for 7–14 days for the purpose of our study. Similarly, finding newly diagnosed PAD patients who have not been treated with aspirin and/or clopidogrel would be very difficult because all of our clinical partners (who help with recruitment) optimize medical treatment of these patients before, during, and after they participate in our study. We suspect that exercise-induced increases in prostanoids are the most physiologically relevant in PAD, but to detect these changes, blood samples would have been required from the venous effluent (e.g., the femoral vein) and/or the working skeletal muscle (e.g., with muscle microdialysis). Presently, we have no experimental evidence that prostanoids increased in the skeletal muscle during this modest bout of exercise. Our data also cannot identify how ketorolac lowered the exercise pressor response (e.g., muscle, afferent pathway, brain stem, efferent pathway, vascular smooth muscle, etc.), but it had an effect nonetheless.
Considering that baseline levels of prostanoids were lower in PAD compared with controls (Table 2), yet ketorolac significantly reduced the exercise pressor reflex (Fig. 1), we suggest that PAD patients have a heightened afferent sensitivity to exercise-induced prostanoids. To prove this concept will require new approaches in a larger sample of PAD patients. At the current time, we believe that measuring muscle sympathetic nerve activity (in the nonexercising leg) as well as popliteal blood flow (in the exercising leg) will help distinguish the underlying mechanisms. Performing postexercise circulatory occlusion could also provide insights into group III versus group IV afferent contribution. Based on the route of administration, we cannot exclude an effect of ketorolac on the central nervous system. An intra-arterial infusion (femoral artery catheter) could theoretically circumvent this problem but would involve a greater risk to the subject; systemic spillover could also occur because the leg has more blood volume than the forearm, which has been used in previous studies (23, 24). Moving forward, the key question is whether the heightened exercise pressor response in PAD patients serves a practical purpose (e.g., to improve muscle blood flow and oxygenation) or whether it is just a damaging reflex to the end organs over time (the heart, brain, and kidney).
Conclusions.
Ketorolac significantly attenuated the pressor response to dynamic exercise in PAD patients but not in healthy control subjects. These data suggest that prostanoids contribute to the augmented exercise pressor reflex in patients with PAD.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-096570 and UL1-TR-000127 (both to L. I. Sinoway).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.M., C.A.B., and L.I.S. conception and design of research; M.D.M., R.C.D., A.J.R., C.A.B., and A.E.C. performed experiments; M.D.M. and A.J.R. analyzed data; M.D.M., R.C.D., A.J.R., C.A.B., A.E.C., M.P.K., and L.I.S. interpreted results of experiments; M.D.M. prepared figures; M.D.M. drafted manuscript; M.D.M., R.C.D., A.J.R., C.A.B., A.E.C., M.P.K., and L.I.S. edited and revised manuscript; M.D.M., R.C.D., A.J.R., C.A.B., A.E.C., M.P.K., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Herr for engineering support, Matt Heffernan for technical assistance, Dr. Amy Reed and Dr. Faisal Aziz for help with subject recruitment, Anne Muller for preparing the graphics for this study, and Kris Gray and Jen Stoner for administrative support.
REFERENCES
- 1.Avellaneda C, Gomez A, Martos F, Rubio M, Sarmiento J, de la Cuesta FS. The effect of a single intravenous dose of metamizol 2 g, ketorolac 30 mg and propacetamol 1 g on haemodynamic parameters and postoperative pain after heart surgery. Eur J Anaesthesiol 17: 85–90, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barnes JN, Casey DP, Hines CN, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition augments central blood pressure and aortic wave reflection in aging humans. Am J Physiol Heart Circ Physiol 302: H2629–H2634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes JN, Charkoudian N, Matzek LJ, Johnson CP, Joyner MJ, Curry TB. Acute cyclooxygenase inhibition does not alter muscle sympathetic nerve activity or forearm vasodilator responsiveness in lean and obese adults. Physiol Rep 2: e12079, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 7.Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman CS, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol 293: H1861–H1868, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J, Moradkhan R, Mascarenhas V, Momen A, Sinoway L. Cyclooxygenase inhibition attenuates sympathetic responses to muscle stretch in humans. Am J Physiol Heart Circ Physiol 294: H2693–H2700, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davy KP, Herbert WG, Williams JH. Effect of indomethacin on the pressor responses to sustained isometric contraction in humans. J Appl Physiol 75: 273–278, 1993. [DOI] [PubMed] [Google Scholar]
- 10.de L II, Welten GM, Verhagen HJ, van Domburg RT, Stolker RJ, Poldermans D. Exercise blood pressure response and perioperative complications after major vascular surgery. Coron Artery Dis 22: 228–232, 2011. [DOI] [PubMed] [Google Scholar]
- 11.de Liefde I, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol 102: 921–926, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Doerzbacher KJ, Ray CA. Muscle sympathetic nerve responses to physiological changes in prostaglandin production in humans. J Appl Physiol 90: 624–629, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, Cui J, Reed AB, Sinoway LI. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 1: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana GA, Pantaleo T, Bongianni F, Cresci F, Lavorini F, Guerra CT, Panuccio P. Prostaglandin synthesis blockade by ketoprofen attenuates respiratory and cardiovascular responses to static handgrip. J Appl Physiol 78: 449–457, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory, and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc, 1996, sect. 12, chapt. 10, p. 381–447. [Google Scholar]
- 17.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke 32: 2036–2041, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Leal AK, McCord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H2140–H2146, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Xing J, Lu J. Nerve growth factor, muscle afferent receptors and autonomic responsiveness with femoral artery occlusion. J Mod Physiol Res 1: 1–18, 2014. [PMC free article] [PubMed] [Google Scholar]
- 20.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. [DOI] [PubMed] [Google Scholar]
- 21.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mathieson MA, Dunham BM, Huval WV, Lelcuk S, Stemp LI, Valeri CR, Shepro D, Hechtman HB. Ischemia of the limb stimulates thromboxane production and myocardial depression. Surg Gynecol Obstet 157: 500–504, 1983. [PubMed] [Google Scholar]
- 23.Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol 287: H1944–H1949, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Cyclooxygenase products sensitize muscle mechanoreceptors in humans with heart failure. Am J Physiol Heart Circ Physiol 294: H1956–H1962, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Momen A, Cui J, McQuillan P, Sinoway LI. Local prostaglandin blockade attenuates muscle mechanoreflex mediated renal vasoconstriction during muscle stretch in humans. Am J Physiol Heart Circ Physiol 294: H2184–H2190, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monahan KD, Ray CA. Cyclooxygenase inhibition and baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol 288: H737–H743, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Morley-Forster P, Newton PT, Cook MJ. Ketorolac and indomethacin are equally efficacious for the relief of minor postoperative pain. Can J Anaesth 40: 1126–1130, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller MD, Reed AB, Leuenberger UA, Sinoway LI. Physiology in medicine: peripheral arterial disease. J Appl Physiol 115: 1219–1226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; . TASC II Working Group, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 33, Suppl 1: S1–S75, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Ritti-Dias RM, Meneses AL, Parker DE, Montgomery PS, Khurana A, Gardner AW. Cardiovascular responses to walking in patients with peripheral artery disease. Med Sci Sports Exerc 43: 2017–2023, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol 259: H745–H750, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol 66: 2721–2724, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol 68: 861–867, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59: 645–654, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Stone AJ, Copp SW, Kaufman MP. Role of prostaglandins in spinal transmission of the exercise pressor reflex in decerebrated rats. Neuroscience 277: 26–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol 71: 1837–1842, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 121: 2109–2116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]