Abstract
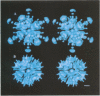
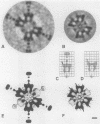
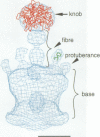
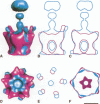
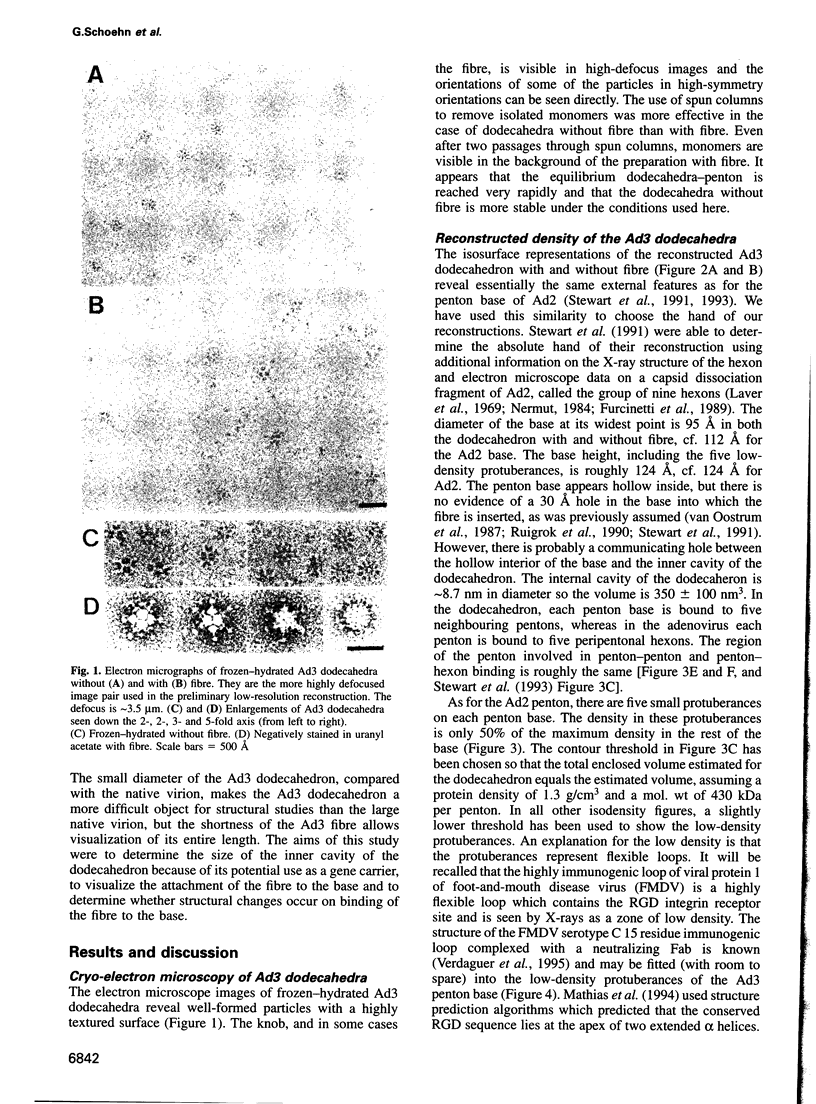
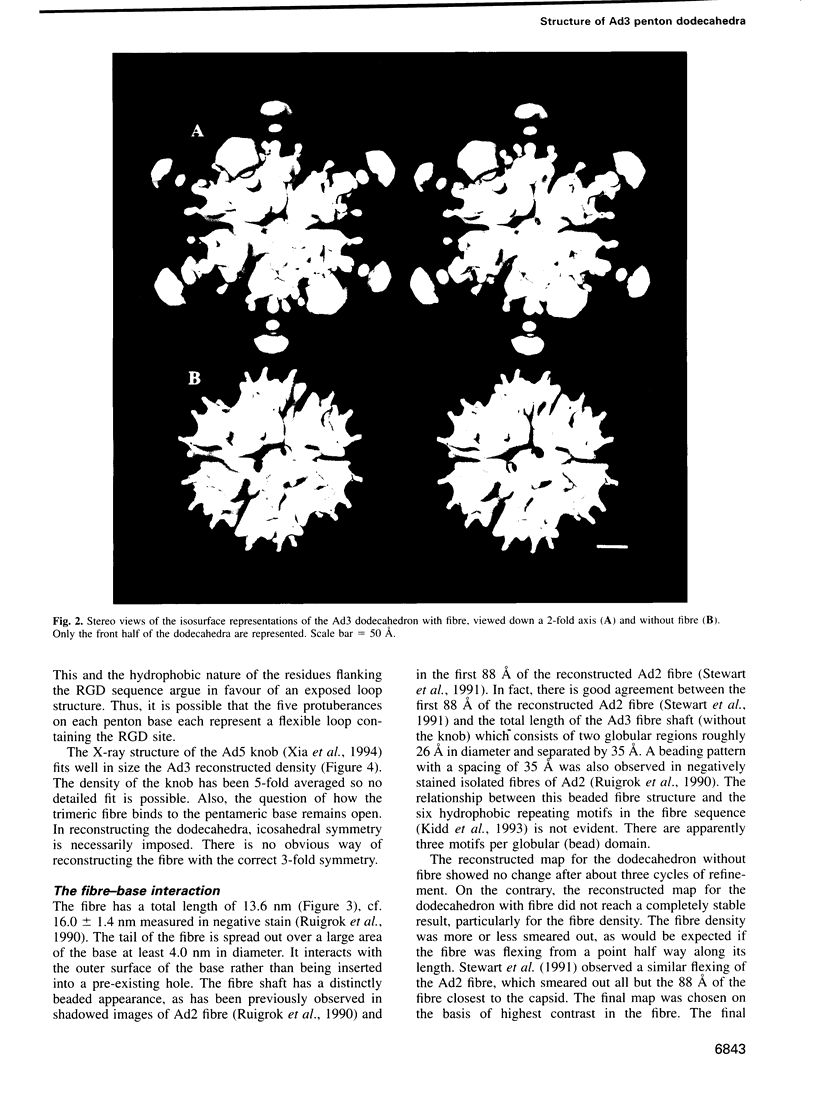
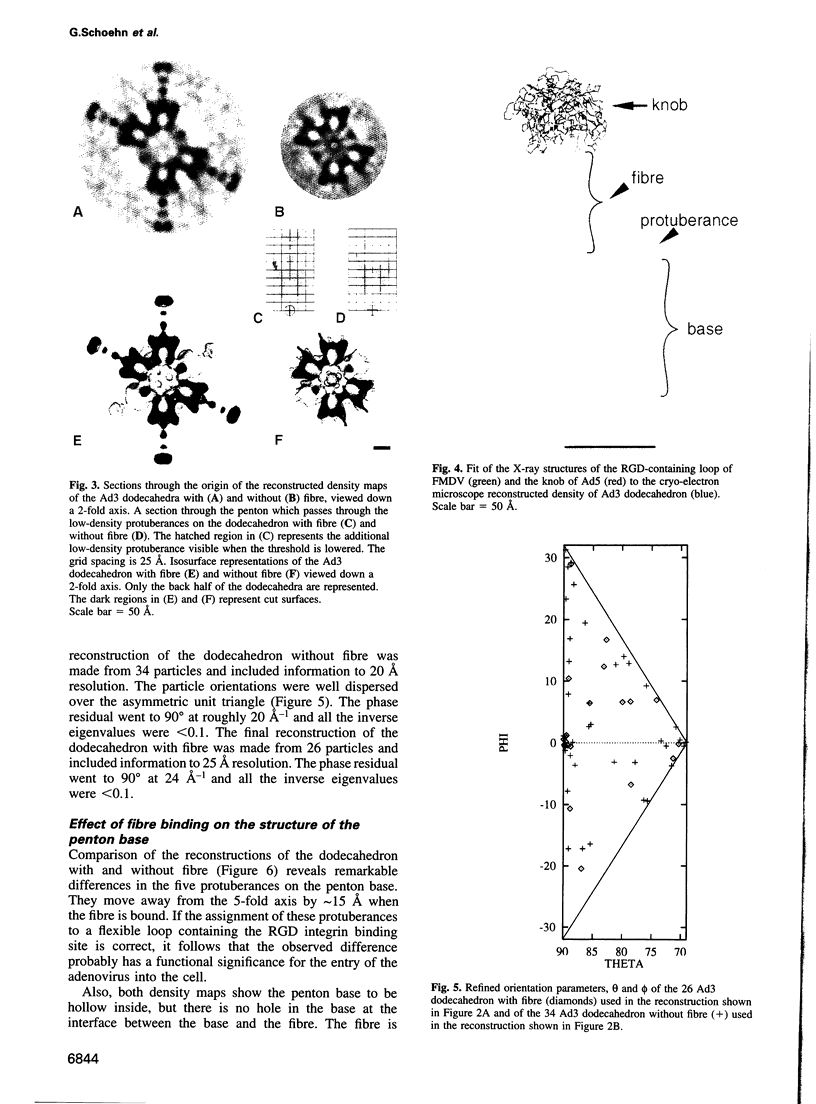
It was recently shown that co-expression of adenovirus type 3 (Ad3) penton base and fibre in the baculovirus system produces dodecahedral particles, as does the expression of the penton base alone. The structure of both of these dodecahedral particles, with and without fibre, has been determined by cryoelectron microscopy and 3-dimensional reconstruction techniques to a resolution of 25 and 20 A, respectively. The general form of the penton base resembles that of the base protein in the recent reconstruction of adenovirus type 2. There is a remarkable difference in the penton base structure with and without the fibre. The five small protuberances on the outer surface of each base move away from the 5-fold axis by approximately 15 A when the fibre is present. These protuberances are of relatively low density and most probably represent a flexible loop possibly containing the RGD site involved in integrin binding. The fibre is apparently bound to the outer surface of the penton base, rather than inserted into it. The fibre is flexible and the shaft contains two distinct globular regions 26 A in diameter. The volume of the inner cavity of the dodecahedron is 350 +/- 100 nm3. This small volume precludes the use of the inner cavity to house genetic information for gene therapy; however, the possibility remains of linking the gene to the dodecahedron surface in the hope that it will be internalized with the dodecahedron.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Cheng R. H. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J Struct Biol. 1996 Jan-Feb;116(1):120–130. doi: 10.1006/jsbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Cuzange A., Chroboczek J., Jacrot B. The penton base of human adenovirus type 3 has the RGD motif. Gene. 1994 Sep 2;146(2):257–259. doi: 10.1016/0378-1119(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Devaux C., Caillet-Boudin M. L., Jacrot B., Boulanger P. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fiber. Virology. 1987 Nov;161(1):121–128. doi: 10.1016/0042-6822(87)90177-2. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Butcher S. J., Cheng R. H., Baker T. S. Three-dimensional reconstruction of icosahedral particles--the uncommon line. J Struct Biol. 1996 Jan-Feb;116(1):48–55. doi: 10.1006/jsbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Furcinitti P. S., van Oostrum J., Burnett R. M. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989 Dec 1;8(12):3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M., Wrigley N. G., Russell W. C., Martin S. R., McLachlan A. D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983;2(8):1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L. J., Xia D., Wilke M. E., Deisenhofer J., Gerard R. D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994 Aug;68(8):5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewat E. A., Booth T. F., Loudon P. T., Roy P. Three-dimensional reconstruction of baculovirus expressed bluetongue virus core-like particles by cryo-electron microscopy. Virology. 1992 Jul;189(1):10–20. doi: 10.1016/0042-6822(92)90676-g. [DOI] [PubMed] [Google Scholar]
- Hewat E. A., Booth T. F., Roy P. Structure of bluetongue virus particles by cryoelectron microscopy. J Struct Biol. 1992 Jul-Aug;109(1):61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Chroboczek J., Cusack S., Ruigrok R. W. Adenovirus type 40 virions contain two distinct fibers. Virology. 1993 Jan;192(1):73–84. doi: 10.1006/viro.1993.1009. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Wrigley N. G., Pereira H. G. Removal of pentons from particles of adenovirus type 2. Virology. 1969 Nov;39(3):599–604. doi: 10.1016/0042-6822(69)90111-1. [DOI] [PubMed] [Google Scholar]
- Louis N., Fender P., Barge A., Kitts P., Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994 Jun;68(6):4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias P., Wickham T., Moore M., Nemerow G. Multiple adenovirus serotypes use alpha v integrins for infection. J Virol. 1994 Oct;68(10):6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. M., White J. L., Grütter M. G., Burnett R. M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986 May 30;232(4754):1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Barge A., Albiges-Rizo C., Dayan S. Structure of adenovirus fibre. II. Morphology of single fibres. J Mol Biol. 1990 Oct 20;215(4):589–596. doi: 10.1016/S0022-2836(05)80170-6. [DOI] [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Adenovirus 3 fiber polypeptide gene: implications for the structure of the fiber protein. J Virol. 1985 Feb;53(2):672–678. doi: 10.1128/jvi.53.2.672-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. L., Burnett R. M., Cyrklaff M., Fuller S. D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991 Oct 4;67(1):145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- Stewart P. L., Fuller S. D., Burnett R. M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993 Jul;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouten P. F., Sander C., Ruigrok R. W., Cusack S. New triple-helical model for the shaft of the adenovirus fibre. J Mol Biol. 1992 Aug 20;226(4):1073–1084. doi: 10.1016/0022-2836(92)91053-r. [DOI] [PubMed] [Google Scholar]
- Verdaguer N., Mateu M. G., Andreu D., Giralt E., Domingo E., Fita I. Structure of the major antigenic loop of foot-and-mouth disease virus complexed with a neutralizing antibody: direct involvement of the Arg-Gly-Asp motif in the interaction. EMBO J. 1995 Apr 18;14(8):1690–1696. doi: 10.1002/j.1460-2075.1995.tb07158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Xia D., Henry L. J., Gerard R. D., Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994 Dec 15;2(12):1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li Q., Ertl H. C., Wilson J. M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995 Apr;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Rosenfeld M. A., Seth P., Crystal R. G. Adenovirus-mediated augmentation of cell transfection with unmodified plasmid vectors. J Biol Chem. 1993 Feb 5;268(4):2300–2303. [PubMed] [Google Scholar]
- van Oostrum J., Smith P. R., Mohraz M., Burnett R. M. The structure of the adenovirus capsid. III. Hexon packing determined from electron micrographs of capsid fragments. J Mol Biol. 1987 Nov 5;198(1):73–89. doi: 10.1016/0022-2836(87)90459-1. [DOI] [PubMed] [Google Scholar]