Abstract
Epithelial Na+ channel (ENaC) blockade stimulates stilbene-sensitive conductive Cl− secretion in the mouse cortical collecting duct (CCD). This study's purpose was to determine the co-ion that accompanies benzamil- and DIDS-sensitive Cl− flux. Thus transepithelial voltage, VT, as well as total CO2 (tCO2) and Cl− flux were measured in CCDs from aldosterone-treated mice consuming a NaCl-replete diet. We reasoned that if stilbene inhibitors (DIDS) reduce conductive anion secretion they should reduce the lumen-negative VT. However, during ENaC blockade (benzamil, 3 μM), DIDS (100 μM) application to the perfusate reduced net H+ secretion, which increased the lumen-negative VT. Conversely, ENaC blockade alone stimulated H+ secretion, which reduced the lumen-negative VT. Application of an ENaC inhibitor to the perfusate reduced the lumen-negative VT, increased intercalated cell intracellular pH, and reduced net tCO2 secretion. However, benzamil did not change tCO2 flux during apical H+-ATPase blockade (bafilomycin, 5 nM). The increment in H+ secretion observed with benzamil application contributes to the fall in VT observed with application of this diuretic. As such, ENaC blockade reduces the lumen-negative VT by inhibiting conductive Na+ absorption and by stimulating H+ secretion by type A intercalated cells. In conclusion, 1) in CCDs from aldosterone-treated mice, benzamil application stimulates HCl secretion mediated by the apical H+-ATPase and a yet to be identified conductive Cl− transport pathway; 2) benzamil-induced HCl secretion is reversed with the application of stilbene inhibitors or H+-ATPase inhibitors to the perfusate; and 3) benzamil reduces VT not only by inhibiting conductive Na+ absorption, but also by stimulating H+ secretion.
Keywords: chloride, H+ secretion, pendrin
in cortical collecting ducts (CCDs) from aldosterone-treated mice and rats, robust NaCl absorption is observed. Following epithelial Na+ channel (ENaC) blockade with amiloride analogs, NaCl absorption falls >50% (20, 27), due to inhibition of ENaC-mediated Na+ absorption. However, since ENaC does not transport Cl−, how amiloride reduces Cl− absorption is unclear. In the companion paper (17a), we observed that Cl− secretion increases either with ENaC gene ablation or with chemical inhibitors of ENaC. This ENaC-sensitive Cl− flux is eliminated with stilbene analogs, such as DIDS, which are nonselective Cl− transporter blockers. Benzamil-stimulated Cl− secretion is also reduced with inhibitors of the Na+-K+-2Cl− cotransporter, NKCC1. Since NKCC1 localizes to the basolateral membrane of type A intercalated cells, ENaC blockade may stimulate Cl− secretion by type A intercalated cells.
In addition to reducing renal Cl− absorption, some studies indicate that ENaC blockade in the CCD reduces net H+ secretion (16). Since type A intercalated cells are the primary mediator of electrogenic H+ secretion in the CCD, amiloride likely inhibits the apical H+-ATPase of the type A intercalated cell. In the turtle urinary bladder and in the rabbit CCD, amiloride application lowers H+ secretion (10, 15, 16) by reducing lumen-negative transepithelial voltage, which attenuates the electromotive force for H+ secretion (24, 37). In rats, dogs, and in humans, amiloride administration in vivo increases urinary pH and reduces serum HCO3− concentration, (1, 3, 23), consistent with apical H+-ATPase inhibition. As such, administration of amiloride in vivo generates a distal renal tubular acidosis that is thought to be voltage dependent (1). This amiloride-induced fall in net H+ secretion may modulate Cl− flux.
To further characterize amiloride-sensitive Cl− secretion, the purpose of this study was twofold: 1) to determine the co- or countertransported ion that accompanies benzamil- and DIDS-sensitive Cl− transport, and 2) to determine whether inhibiting this co- or countertransport mechanism attenuates DIDS-sensitive changes in Cl− transport.
METHODS
Animals.
Unless otherwise indicated, experiments were performed in male and female wild-type mice on a 129S6/SvEv Tac background (Taconic Farms). In some experiments, male or female Slc26a4 (−/−) mice on a 129S6/SvEv Tac background, developed by Everett et al. (4), were compared with wild-type mice from the same strain (129S6/SvEv Tac), bred in parallel. Unless otherwise stated, mice were fed a balanced diet (53881300; Zeigler Brothers) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow) supplemented with NaCl (∼0.8 meq NaCl/day, treatment 1) and were given aldosterone by minipump (250 μg·kg body wt−1·day−1, treatment 2) for 5–7 days before euthanasia (11). The Institutional Animal Care and Use Committee at Emory University approved all treatment protocols.
In vitro perfusion of isolated CCD.
For transepithelial flux studies, CCDs were dissected from medullary rays and perfused at flow rates of 2–3 nl/min in the presence of a symmetric, HCO3−-buffered physiological solution (solution 1, Table 1) or in HEPES-buffered solutions (solution 2, Table 1). Tubules were equilibrated at 37°C for 30 min before the collections began. A stock solution of benzamil hydrochloride (3 × 10−3 M) was prepared in water. A stock solution of bafilomycin (10−5 M) was prepared in absolute ethanol. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Table 1.
Solution composition (in mM)
| Solution 1 | Solution 2 | Solution 3 | Solution 4 | Solution 5 | Solution 6 | |
|---|---|---|---|---|---|---|
| NMDG-gluconate | 14 | 14 | 86 | |||
| NMDG-Cl | 82 | |||||
| NaCl | 125 | 132 | 82 | 10 | ||
| NaHCO3 | 24 | |||||
| Na2HPO4 | 1 | 2.5 | ||||
| K-gluconate | 26 | |||||
| LiCl | ||||||
| KCl | 5 | 26 | 26 | 120 | ||
| K2HPO4 | 2.5 | 2 | 2 | 2 | ||
| Choline-HCO3 | 25 | 25 | 25 | |||
| ½Ca-gluconate | 14 | |||||
| CaCl2 | 2 | 1 | 2 | 2 | 2 | |
| MgSO4 | 1.2 | 1 | 1.5 | 1.5 | 1.5 | 1.5 |
| Glucose | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5 |
| HEPES | 10 | 15 |
NMDG, N-methyl-d-glucamine.
Measurement of net transepithelial Cl− flux.
Cl− concentration was measured in the perfusate and collected samples using a continuous-flow fluorimeter and the Cl−-sensitive fluorophore 6 methoxy-N-(3-sulfopropyl) quinolinium (SPQ; Molecular Probes, Eugene, OR), as described previously (6, 32, 33). Transepithelial Cl− flux, JCl, was calculated according to the equation:
where Co and CL are perfusate and collected fluid Cl− concentrations, respectively, Q is the flow rate (in nl/min), and L is tubule length. Net fluid transport was taken to be zero since net fluid flux has not been observed in CCDs when perfused in vitro in the presence of symmetric solutions and in the absence of vasopressin (12, 13). JCl was expressed as picomoles per millimeter per minute.
Measurement of transepithelial HCO3− flux, JtCO2.
Total CO2 (HCO3−+H2CO3+CO2) concentration, which is mainly HCO3− in most physiological solutions, was measured in the perfusate and in collected samples using a continuous-flow fluorimeter with the method of Zhou et al. (21, 38). Transepithelial total CO2 flux, JtCO2, was calculated according to the equation JtCO2 = (Co − CL)Q/L, where Co and CL are the ion concentrations measured in the perfusate and the collected fluid, Q is the flow rate (in nl/min), and L is the tubule length.
Measurement of transepithelial voltage.
Transepithelial voltage was measured in the perfusion pipette connected to a high-impedance electrometer through an agar bridge saturated with 0.16 M NaCl and a calomel cell as described previously (29). The reference was an agar bridge from the bath to a calomel cell.
Measurement of intracellular pH.
Intracellular pH was measured in intercalated cells from CCDs perfused in vitro using the esterified form of the pH-sensitive fluorophore BCECF-AM (30, 36). Tubules were mounted on concentric pipettes and then perfused in vitro for 15 min with BCECF-AM (5.4 μmol/l) present in the luminal fluid (solution 1, Table 1). The perfusate was exchanged for a solution that did not contain BCECF and perfused for an additional 15 min before intracellular pH measurements. Emission intensity was measured at an emission wavelength of 535 nm when the excitation wavelength was alternated between 440 and 495 nm using a Polychrome V scanning monochrometer (TILL Photonics, Eugene, OR) with a xenon light source connected to an Olympus inverted microscope and a Hamamatsu ORCA ER digital camera. Imaging Workbench 6 (INDEC BioSystems, Santa Clara, CA) software was used for the unit control and image analysis. In each CCD, four to nine intercalated cells were identified by their characteristic shape and preferential loading with BCECF, and circumscribed as regions of interest (36). Every 15 s, emission intensity was measured continuously at an excitation wavelength of 495 for 20 ms and then at 440 nm for 50 ms under basal conditions and then following the addition and then the withdrawal of benzamil to the perfusate. The perfusate contained Cl−, with or without Na+ (Table 1, solutions 3 and 4). Tubules were bathed in a NaCl-free solution (Table 1, solution 5). A three-point standard curve was constructed for each tubule at the end of each experiment by perfusing the lumen and peritubular bath with a high-K+- containing solution, pH 6.7–7.6 (Table 1, solution 6). The bath calibration solution also contained the K+/H+ ionophore nigericin (14.7 μmol/l).
Statistics.
All data are presented as means ± SE. Each “n” used in the statistical analysis represents data from separate mice. To test for statistical significance between two groups, a paired or an unpaired Student's t-test was used. When results from more than two groups were compared, an ANOVA was used followed by Tukey's protected t-test. The criterion for statistical significance was P < 0.05.
RESULTS
DIDS inhibits H+ secretion mediated by the H+-ATPase, which increases the lumen-negative transepithelial voltage.
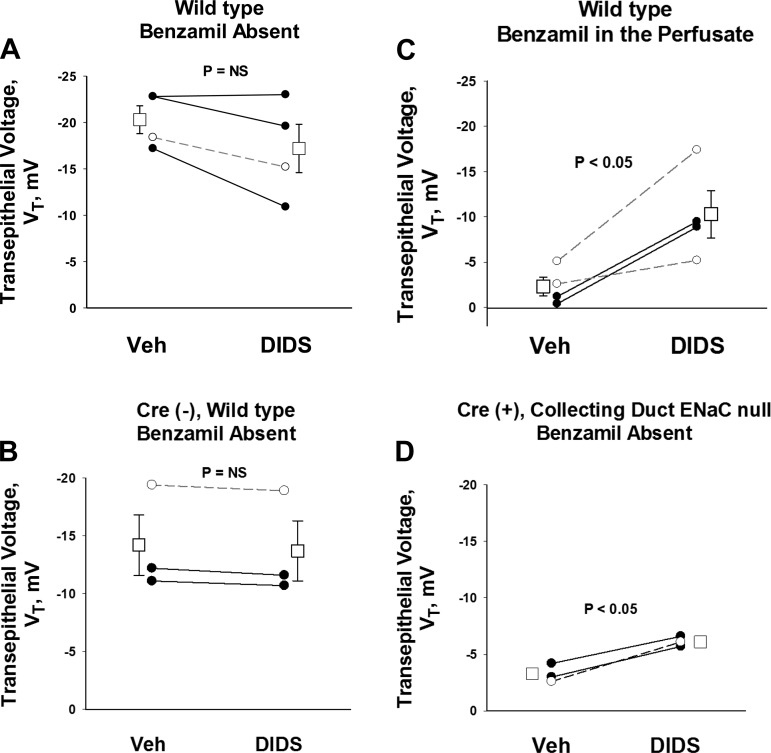
In the companion paper (17a), we observed that ENaC inhibition stimulates conductive Cl− secretion, which is reversed with the application of stilbene inhibitors (DIDS). Further experiments examined the effect of DIDS on transepithelial voltage during ENaC inhibition. We reasoned that stimulating conductive anion secretion should increase the lumen-negative transepithelial voltage, whereas inhibiting conductive anion secretion should reduce the lumen-negative transepithelial voltage. As such, we predicted that when conductive Cl− secretion is inhibited with the nonselective Cl− transport blocker DIDS, the lumen-negative transepithelial voltage will fall, i.e., becomes less lumen negative. To test this prediction, we examined the effect of the DIDS on transepithelial voltage in CCDs from aldosterone-treated mice. Figure 1 shows that in the absence of ENaC blockade, the lumen-negative transepithelial voltage is either unchanged or slightly reduced with DIDS application (Fig. 1, A and B), which is consistent with inhibition of a conductive Cl− secretory pathway. However, when ENaC activity is eliminated either with a chemical inhibitor (benzamil, Fig. 1C) or with ENaC gene ablation (Fig. 1D), DIDS application increased the lumen-negative transepithelial voltage. This DIDS-induced increase in the lumen-negative voltage observed during ENaC blockade occurred in mice given diet alone or diet and aldosterone (Fig. 1).
Fig. 1.
During epithelial sodium channel (ENaC) blockade, DIDS increases the lumen-negative transepithelial voltage, VT. The effect of DIDS on VT was measured in cortical collecting ducts (CCDs) from aldosterone-treated mice in the presence or absence of functional ENaC. The effect of DIDS on VT was examined either with functional ENaC (A and B) or when ENaC function was eliminated with chemical inhibitors of ENaC (benzamil, C) or with ENaC gene ablation (D). In other experiments, we observed that during ENaC blockade, DIDS increased the lumen-negative VT by 2.5 ± 0.5 mV (n = 4) in CCDs from mice receiving diet alone and by 7.6 ± 1.3 mV (n = 7) in mice receiving diet and aldosterone (P < 0.05).
Further experiments explored the mechanism behind the unexpected rise in lumen-negative transepithelial voltage observed with stilbene inhibitor application. We hypothesized that during ENaC blockade, DIDS increases the lumen-negative transepithelial voltage by inhibiting secretion of a cation, such as H+, rather than by stimulating secretion of an anion. To test this hypothesis, we examined the effect of DIDS on total CO2 (HCO3−+CO2+H2CO3) flux. Total CO2 flux generally reflects HCO3− flux, JHCO3−, since total CO2 is primarily HCO3− under most physiological conditions (34). Figure 2 shows that during ENaC blockade, DIDS application changed total CO2 flux from net absorption to net total CO2 secretion. The fall in total CO2 absorption observed with DIDS application could be from reduced H+ secretion or increased secretion of OH− equivalents, such as HCO3−. To discriminate between these possibilities, we tested whether the DIDS-induced change in total CO2 flux is eliminated with H+-ATPase inhibitor (bafilomycin) application. We observed that during ENaC blockade, application of DIDS to the perfusate changed total CO2 flux from net absorption to net secretion. However, during blockade of both ENaC and the apical H+-ATPase, DIDS application did not change total CO2 flux [vehicle, 2.2 ± 4.3 vs. 3.6 ± 3.3 pmol·mm−1·min−1 with DIDS, n = 4, P = not significant (NS)]. We conclude that during ENaC blockade, DIDS application inhibits H+ secretion mediated by the apical H+-ATPase.
Fig. 2.
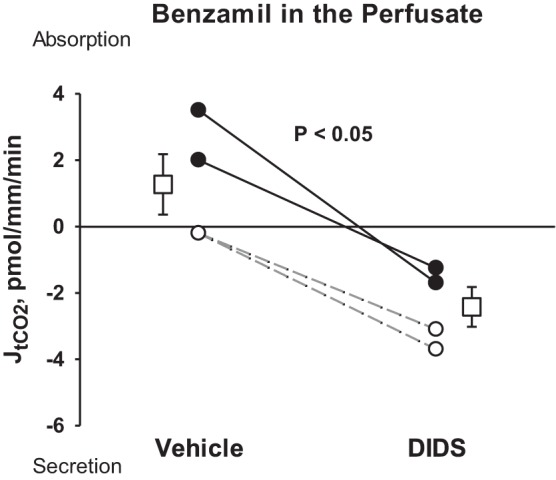
During ENaC blockade, DIDS reduces total CO2 absorption. JtCO2 was measured during ENaC blockade (benzamil) in the presence and absence of a stilbene inhibitor (DIDS). Solid lines display results from experiments where the vehicle was applied in the first period and DIDS applied in the second period. Dashed lines indicate experiments where the order was reversed (i.e., DIDS was applied in the initial period and then removed in the second). Boxes show means ± SE of data under each condition. As shown, DIDS application changed tCO2 flux from net absorption to net secretion.
DIDS reduced total CO2 absorption during ENaC blockade, most likely by inhibiting H+ secretion. Since a fall in electrogenic H+ secretion should increase the lumen-negative transepithelial voltage (26), we asked whether DIDS increases the lumen-negative transepithelial voltage by inhibiting apical H+-ATPase-mediated H+ secretion. If so, H+-ATPase inhibitors should blunt the increment in the lumen-negative transepithelial voltage observed with DIDS application. To test this hypothesis, we examined the effect of DIDS on transepithelial voltage in the presence or absence of an H+-ATPase inhibitor (bafilomycin 5 nM, Fig. 3A). As shown, during ENaC blockade, inhibiting the H+-ATPase blunted the DIDS-induced change in lumen-negative transepithelial voltage (DIDS-sensitive transepithelial voltage).
Fig. 3.
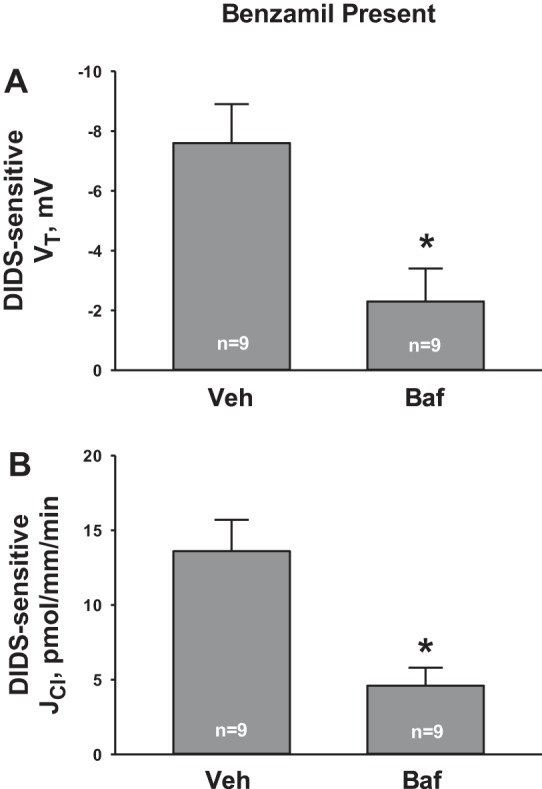
H+-ATPase inhibition attenuates the effect of DIDS on Cl− flux (JCl) and VT. VT and JCl were measured during ENaC blockade in the presence and absence of DIDS. From these data, we calculated the change in JCl and VT that follows DIDS application, or the DIDS-sensitive component of JCl (B) and VT (A). In separate tubules, the experiment was repeated, but in the presence of both apical H+-ATPase (bafilomycin) and ENaC (benzamil) blockade. DIDS-sensitive VT (A) and JCl (B) were compared in the presence and absence of apical H+-ATPase blockade (bafilomycin). *P < 0.05.
During ENaC blockade, DIDS inhibits HCl secretion.
Since DIDS reduces secretion of both H+ and Cl− during ENaC blockade, we asked whether DIDS-sensitive changes in H+ and Cl− flux are coupled. Therefore, we examined the effect of DIDS on Cl− flux during apical H+-ATPase blockade. Whereas DIDS reduced net HCl secretion during ENaC blockade alone, Fig. 3B shows that with blockade of both ENaC and the apical H+-ATPase, the increment in Cl− absorption observed with DIDS application (DIDS-sensitive Cl− absorption) is blunted. We conclude that during ENaC blockade, DIDS-sensitive changes in H+ and Cl− flux depend on the apical H+-ATPase.
ENaC blockade stimulates net H+ secretion.
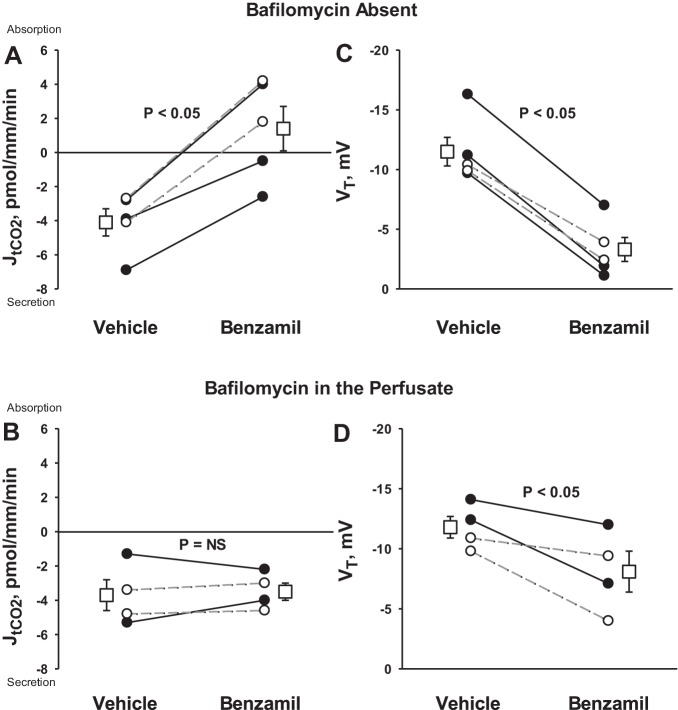
Since DIDS application reduced net H+ secretion during ENaC blockade, we explored the effect of ENaC blockade alone on net H+ secretion. Figure 4 shows that in the absence of inhibitors, CCDs from aldosterone-treated mice secrete total CO2, as reported previously (22). However, total CO2 flux changed from net secretion to net absorption following benzamil application. Therefore, benzamil either stimulates H+ secretion or inhibits OH− secretion, such as through blockade of apical anion exchange.
Fig. 4.
Benzamil increases net H+ secretion. JtCO2 and VT were measured in the presence and absence of benzamil (A and C). This experiment was repeated during apical H+-ATPase blockade achieved with the application of bafilomycin to the perfusate (B and D). Solid lines display results of experiments in which vehicle was applied in the first period and benzamil was applied in the second. Dashed lines indicate experiments where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second). The boxes display means ± SE of all experiments performed.
To test whether ENaC blockade increases H+ secretion, we examined the effect of benzamil on total CO2 flux, during apical H+-ATPase blockade (bafilomycin, Fig. 4B). As shown, blockade of the apical H+-ATPase eliminated the benzamil-sensitive change in total CO2 flux. To test further whether benzamil stimulates H+ secretion or instead inhibits apical anion exchange, intracellular pH was measured in intercalated cells before and after the application of this diuretic to the perfusate (Fig. 5 and Table 2). With Na+ in the luminal fluid, intercalated cell intracellular pH rose with benzamil application, consistent with stimulation of net H+ efflux or inhibition of OH− efflux (Fig. 5A). In contrast, benzamil had no effect on intracellular pH in the absence of luminal Na+ (Fig. 5B), suggesting that benzamil modulates H+ secretion through ENaC-mediated Na+ absorption.
Fig. 5.
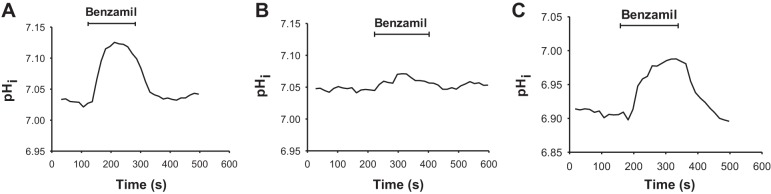
Benzamil increases intercalated cell intracellular pH (pHi) independently of pendrin-mediated apical Cl−/HCO3− exchange. pHi was measured in intercalated cells loaded with BCECF (see methods). The solutions employed are displayed in Table 1. Perfusate K+, Cl−, and HCO3− concentrations were the same in all experiments. In all experiments, NaCl was absent from the bath solution (solution 5). A and B: effect of benzamil applied to the perfusate on intercalated cell pHi in CCDs from wild-type mice, with Na+ present (A, solution 3) or absent (B, solution 4) in the luminal fluid. C: effect of benzamil on intercalated cell pHi in CCDs from pendrin null mice, when perfused in the presence of luminal Na+ (solution 3).
Table 2.
Effect of benzamil on intercalated cell pHi
| Basal pHi | dpHi/dt, pH units/s | ΔpHi | n | |
|---|---|---|---|---|
| Wild-type mice, Na+ present in the luminal fluid | 7.01 ± 0.05 | 0.138 ± 0.005 | 0.094 ± 0.003 | 3 |
| Pendrin null mice, Na+ present in the luminal fluid | 7.04 ± 0.06 | 0.119 ± 0.023 | 0.080 ± 0.021 | 5 |
| Wild-type mice, Na+ absent from the luminal fluid | 6.94 ± 0.06 | 0.054 ± 0.004* | 0.015 ± 0.004* | 4 |
Values are means ± SE.
pHi, intracellular pH.
P < 0.05, ANOVA.
Since benzamil may raise intracellular pH by inhibiting apical Cl−/HCO3− exchange (17, 35) and since most apical anion exchange is pendrin mediated (2, 33), we examined the effect of benzamil on intercalated cell intracellular pH in the absence of pendrin-mediated apical Cl−/HCO3− exchange (pendrin null mice). As shown, benzamil produced a similar increment in intracellular pH in wild-type and pendrin null mice (Fig. 5C and Table 2). We conclude that benzamil increases intracellular pH either by stimulating the apical H+-ATPase or by stimulating a H+ or HCO3− exchanger other than pendrin. However, Figs. 4 and 5 together indicate that benzamil most probably increases intercalated cell intracellular pH and net H+ secretion by stimulating the apical H+-ATPase.
The apical H+-ATPase modulates ENaC-stimulated Cl− secretion.
Since ENaC blockade stimulates secretion of H+ and Cl− through associated pathways, we reasoned that inhibiting the H+-ATPase should reduce Cl− secretion or increase Cl− absorption. To test this hypothesis, we examined the effect of an H+-ATPase inhibitor (bafilomycin, 5 nM) on Cl− absorption and transepithelial voltage. While application of H+-ATPase inhibitors to the perfusate did not change Cl− flux in the absence of ENaC blockade (19), Fig. 6A shows that during ENaC blockade, Cl− absorption increased with the application of bafilomycin to the perfusate, as expected when a pathway mediating Cl− secretion is inhibited.
Fig. 6.
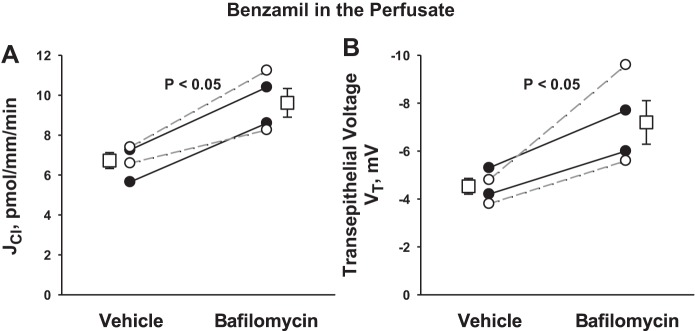
During ENaC blockade, apical H+-ATPase inhibition increases Cl− absorption. JCl (A) and VT (B) were measured during ENaC blockade in CCDs from aldosterone-treated mice in the presence and absence of bafilomycin (5 nM) in the luminal fluid. Solid lines display results of experiments in which vehicle was applied in the first period and bafilomycin applied in the second. Dashed lines indicate experiments where the order was reversed (i.e., bafilomycin was applied in the initial period and then removed in the second). The boxes display means ± SE of all experiments performed.
Since DIDS application increased the lumen-negative transepithelial voltage during ENaC blockade, we reasoned that DIDS increases the lumen-negative voltage, not by changing anion flux, but by reducing the secretion of a cation, i.e., H+. Further experiments therefore tested the effect of inhibiting H+ secretion on transepithelial voltage. Figure 6B shows that during ENaC blockade, apical H+-ATPase inhibition (bafilomycin) increased the lumen-negative voltage, as expected when conductive cation secretion falls. We conclude that during ENaC blockade, inhibiting electrogenic H+ secretion increases the lumen-negative transepithelial voltage and reduces conductive Cl− secretion.
H+-ATPase inhibition reduces benzamil-sensitive transepithelial voltage.
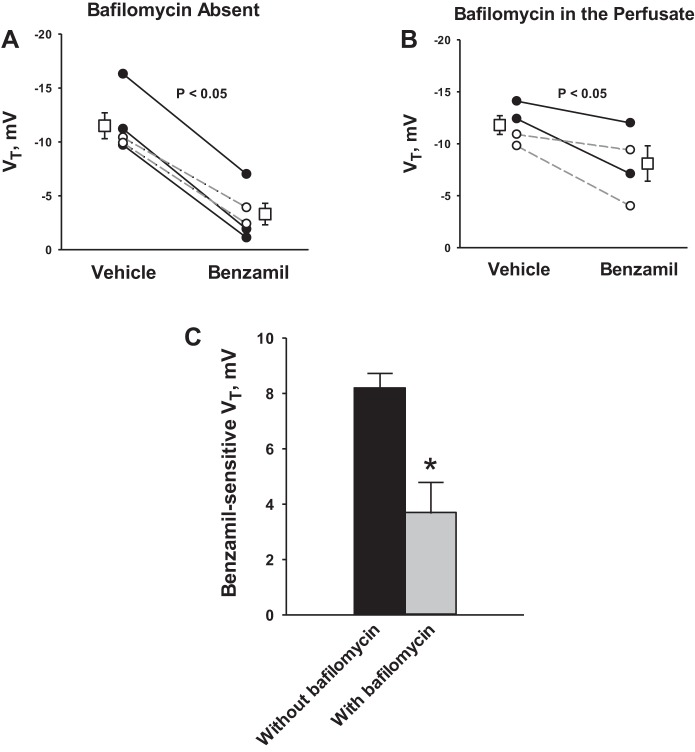
Since ENaC blockade stimulates the apical H+-ATPase, ENaC inhibitors may reduce the lumen-negative transepithelial voltage not only by reducing Na+ absorption but also by stimulating H+ secretion. As such, we examined the contribution of apical H+ secretion to the benzamil-sensitive change in transepithelial voltage. Figure 7 shows that benzamil reduces transepithelial voltage either in the presence (Fig. 7B) or the absence (Fig. 7A) of apical H+-ATPase blockade. However, the magnitude of benzamil-sensitive transepithelial voltage fell by ∼60% with apical H+-ATPase blockade. We conclude that benzamil reduces the lumen-negative transepithelial voltage not only by reducing the absorption of Na+ but also by stimulating the secretion of H+.
Fig. 7.
Apical H+-ATPase inhibition reduces benzamil-sensitive VT. VT was measured before and after ENaC blockade in CCDs from aldosterone-treated mice in the presence (B) and in the absence (A) of bafilomycin (5 nM) in the luminal fluid. Solid lines display results of experiments in which vehicle was applied in the first period and benzamil was applied in the second. Dashed lines indicate experiments where the order was reversed (i.e., bafilomycin was applied in the initial period and then removed in the second). The boxes display the means ± SE of all experiments performed. C: benzamil-sensitive component of VT measured in each group. *P < 0.05.
Benzamil-sensitive transepithelial voltage falls with removal of CO2/HCO3−.
Since H+-ATPase blockade reduced the benzamil-sensitive component of transepithelial voltage, further experiments asked whether H+-ATPase blockade reduces the benzamil-sensitive component of Cl− absorption. To answer this question, H+ secretion was reduced not only by applying bafilomycin to the perfusate but also by eliminating CO2 as a source of H+ for luminal acidification. To do so, HCO3−/CO2 was eliminated from the perfusate and bath (26, 31). We detected a benzamil-sensitive component of Cl− absorption in both the presence and in the absence of H+-ATPase blockade (Fig. 8A). However, the magnitude of benzamil-sensitive Cl− absorption (Fig. 8A) fell whether H+ secretion was reduced with chemical inhibitors (bafilomycin) or with elimination of CO2 as a H+ source. We conclude that benzamil-sensitive Cl− flux is dependent on the apical H+-ATPase.
Fig. 8.
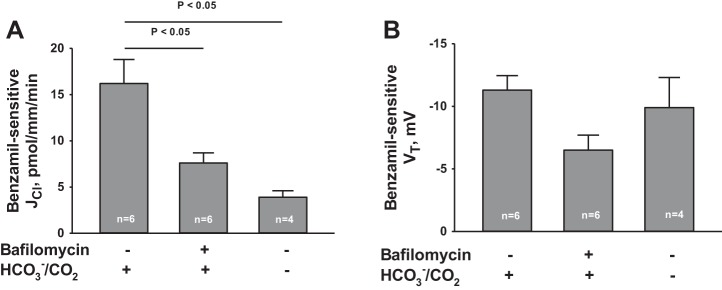
H+-ATPase inhibition attenuates benzamil-induced Cl− secretion. The effect of benzamil on JCl and VT was examined in the presence and absence of apical H+-blockade achieved with bafilomycin (5 nM) added to the luminal fluid or with HCO3−/CO2 removed from the bath and perfusate. Benzamil-induced Cl− secretion (i.e., the benzamil-sensitive component of JCl) and benzamil-sensitive VT were calculated from data obtained under each of these treatment conditions. *P < 0.05, ANOVA.
Figure 8B shows, however, that under the same treatment conditions benzamil-induced Cl− flux did not change in tandem with benzamil-sensitive transepithelial voltage. These data argue against the possibility that apical H+-ATPase inhibition reduces benzamil-sensitive Cl− absorption exclusively by changing Cl− transport across tight junctions.
H+-ATPase inhibition blocks NKCC1-dependent Cl− secretion.
We have shown previously that the basolateral membrane Na-K-2Cl transporter NKCC1 mediates Cl− uptake across the basolateral plasma membrane of type A intercalated cells in the rat outer medullary collecting duct (OMCD) (32) and mouse CCD (20), which is then secreted across the apical membrane into the luminal fluid (Fig. 9). When NKCC1 is inhibited with bumetanide, Cl− secretion in the CCD is reduced (20, 32). Because type A intercalated cells mediate the secretion of H+ in tandem with Cl− secretion, we asked whether apical H+-ATPase blockade abolishes the effect of NKCC1 inhibitors (bumetanide) on transepithelial Cl− transport (Fig. 10). To answer this question, we examined the effect of bumetanide application on Cl− absorption in the presence and absence of H+-ATPase blockade. As shown, during ENaC blockade alone, bumetanide application to the bath increased Cl− absorption from 2.7 ± 4.1 to 9.6 ± 3.2 pmol·mm−1·min−1 (P < 0.05), consistent with our previous observations in mouse CCD (20). However, during blockade of both ENaC and the apical H+-ATPase, Cl− absorption was unchanged with application of this NKCC1 inhibitor (Fig. 10). We conclude that across type A intercalated cells, NKCC1 mediates Cl− uptake across the basolateral membrane. Cl− is then secreted across the type A intercalated cell apical plasma membrane through a yet to be identified apical conductive Cl− transport pathway in tandem with H+ secretion mediated by the apical plasma membrane H+-ATPase.
Fig. 9.
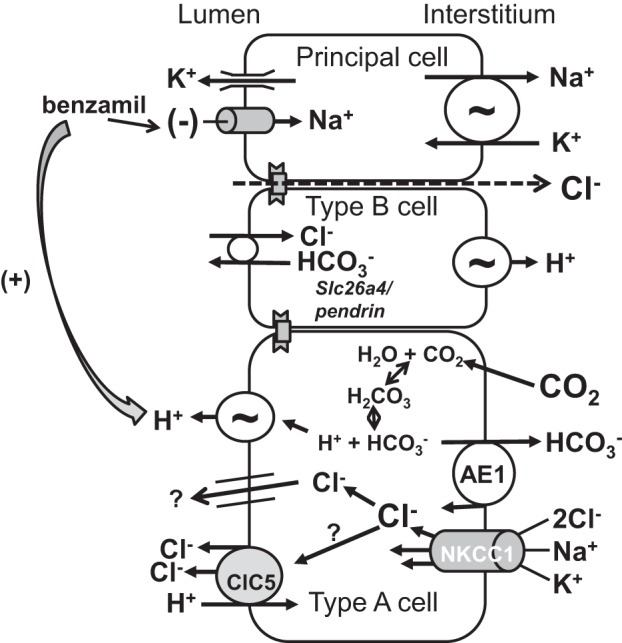
Proposed mechanism of benzamil-induced Cl− secretion. The Na-K-2Cl cotransporter, NKCC1, mediates Cl− uptake across the basolateral membrane in type A intercalated cells. Cl− is then secreted across the apical plasma membrane through a yet to be identified pathway. H+ is secreted by type A cells through the apical H+-ATPase, which is associated with Cl− secretion mediated by this pathway. However, there is evidence that NKCC1 localizes to the basolateral regions of not only type A intercalated cells (14, 20), but also to the basolateral regions of principal cells (14).
Fig. 10.
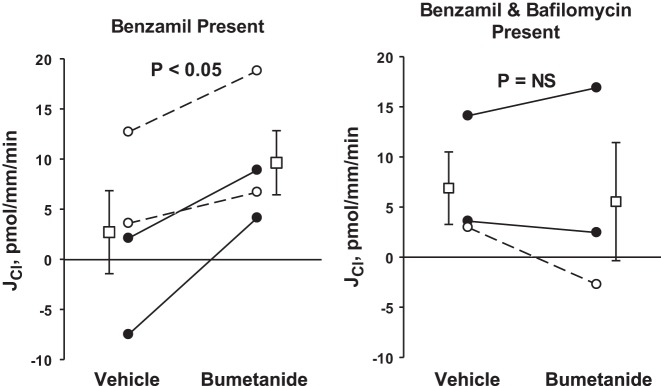
H+-ATPase inhibition eliminates bumetanide-induced changes in Cl− flux. Mice were fed the NaCl-replete gelled diet described in methods and were given aldosterone by minipump (200 μg·kg body wt−1·day−1) for 5–7 days before euthanasia. The effect of bumetanide (100 μM) on JCl was examined during ENaC blockade (3 μM benzamil in the luminal fluid) in the presence (right) and absence (left) of apical H+ blockade (bafilomycin, 5 nM, the luminal fluid). As shown, H+-ATPase blockade prevented the increase in Cl− absorption observed with bumetanide application. Solid lines display results of experiments in which bumetanide vehicle was applied in the first period and bumetanide (plus vehicle) was applied in the second. Dashed lines indicate experiments where the order was reversed (i.e., bumetanide was applied in the initial period and then removed in the second). The boxes display means ± SE of all experiments performed. *P < 0.05.
Model predictions of the bafilomycin-sensitive HCl secretion.
In the companion study (Tables 2 and 3 in Ref. 17a), we used a CCD model in which ClC-5 (1H+/2Cl−) is present in the type A intercalated cell luminal membrane, to simulate the effect of bafilomycin on Cl− and HCO3− transport. In the absence of ClC-5, the model predicts that H+-ATPase inhibition will increase the lumen-negative transepithelial voltage, which should slightly increase Cl− absorption. With ClC-5 present, however, the model predicts a greater increase in Cl− absorption with bafilomycin application and predicts that bafilomycin will reduce intracellular pH within type A intercalated cells, thereby activating ClC-5-mediated Cl− absorption and H+ secretion. The model also predicts that in the CCD, H+-ATPase inhibition will promote Cl− absorption independently of ENaC inhibition. In the absence of benzamil, the model predicts that bafilomycin will increase Cl− absorption by 9.0 pmol·mm−1·min−1, due to a 10.7 pmol·mm−1·min−1 increase in ClC-5-mediated Cl− flux. However, during ENaC blockade, where Cl− flux is secretory, bafilomycin should reduce this Cl− secretion by 11.5 pmol·mm−1·min−1. In both cases, bafilomycin depolarizes the luminal membrane of the intercalated cell, thus providing an electrical force favoring movement of Cl− from lumen to cell.
However, the model predicts a different effect of ENaC inhibition on H+ secretion from that observed experimentally. The model predicts that benzamil application will reduce the driving force for electrogenic H+ secretion by the apical membrane H+-ATPase, due to the fall in lumen-negative voltage expected with the application of this diuretic. The predicted result is reduced H+ secretion and reduced intracellular pH within type A intercalated cells (Table 3 in Ref. 17a). Experimental data displayed in Figs. 4 and 5 (the present study) show that benzamil instead alkalinizes intercalated cells and increases H+ secretion. Our experimental data show that the primary mechanism by which benzamil increases H+ secretion and increases intercalated cell intracellular pH is by stimulating the apical membrane H+-ATPase in the type A intercalated cell. Benzamil stimulates H+ secretion through the apical H+-ATPase, despite the fall in lumen-negative transepithelial voltage, which reduces the driving force for electrogenic H+ secretion. As such, this diuretic does not alter H+ secretion through changes in transepithelial voltage. Therefore, our observation in the companion paper (17a) that DIDS-sensitive Cl− transport occurs only in the presence of benzamil is consistent with model predictions that compared total Cl− flux in the presence or absence of ClC-5. However, our observations regarding the impact of benzamil on H+ flux show a lack of concordance between experiment and model.
DISCUSSION
We and others have shown that inhibiting ENaC reduces Cl− absorption in CCDs from mice given aldosterone and a NaCl-replete diet (19, 20, 28). The mechanism of benzamil-sensitive Cl− absorption is thought to occur through paracellular Cl− transport across tight junctions driven by the lumen-negative transepithelial voltage generated by ENaC (8). However, the present and previous (20) studies have shown that benzamil-sensitive changes in transepithelial voltage and Cl− flux do not always change in tandem.
The apical H+-ATPase generates a lumen-positive transepithelial voltage in the late distal convoluted tubule (DCT) (5) and in the OMCD (32). In the inner stripe of the OMCD, apical H+ and Cl− secretion are dependent on HCO3−/CO2 as a H+ source since the lumen-positive voltage and H+ secretion fall markedly when HCO3−/CO2 is eliminated (31, 32). Whereas HCl secretion was observed in the absence of ENaC inhibitors in the inner stripe of the OMCD from rats given an aldosterone analog (31, 32), the present study observed HCl secretion in CCDs from aldosterone-treated mice only during ENaC blockade. The HCl secretion observed in the in the inner stripe of the rat OMCD in the absence of ENaC blockade (31) may reflect the lack of net Na+ absorption, and presumably the lack of significant ENaC-mediated Na+ absorption, in this segment when perfused in vitro (25, 31).
The present study demonstrates that in the CCD, inhibiting ENaC stimulates HCl secretion that is sensitive to H+-ATPase inhibitors. Our observations are consistent with those of Stoner, Burg, and Orloff (26), who showed that adding the ENaC inhibitor amiloride to the luminal fluid generates a lumen-positive transepithelial voltage in rabbit CCDs perfused in vitro. Since this amiloride-induced lumen-positive transepithelial voltage is eliminated with either carbonic anhydrase inhibition or with removal of CO2, these authors concluded that amiloride stimulates H+ secretion. Later studies by McKinney and Burg (15, 16) showed, however, that in rabbit CCDs perfused in vitro, amiloride application to the perfusate increases net HCO3− secretion, or reduces net H+ secretion. In the turtle urinary bladder, Husted and Steinmetz (10) also observed that amiloride reduces H+ secretion. Because H+ secretion falls when the transepithelial voltage is less lumen negative (24), they concluded that amiloride reduces H+ secretion, in part, by hyperpolarizing the luminal membrane. However, a large increase in the lumen-negative transepithelial voltage produces only a small increment in net H+ secretion in the turtle bladder. As such, while the reason for the different results of the present study and those of Steinmetz is unclear, what is clear is that electrochemical driving force is not the major regulator of net H+ secretion along the distal nephron. The present study shows that ENaC inhibition does not stimulate H+ secretion through changes in driving force since ENaC inhibition increases H+ secretion while reducing the lumen-negative transepithelial voltage, which decreases the driving force for H+ secretion. ENaC inhibition may instead alter H+-ATPase activity through changes in downstream luminal fluid composition or through a paracrine mechanism.
It is well established that amiloride application in vivo reduces net H+ excretion in the mouse, rat, and human, thereby generating a metabolic acidosis (1, 9, 23, 37). What is not clear is why amiloride analogs increase H+ secretion in CCDs from aldosterone-treated mice perfused in vitro, but reduce H+ secretion in CCDs from rabbits, at least under basal conditions (15) or following NaHCO3 ingestion (16). Amiloride may affect transport differently in the mouse and rabbit. Alternatively, the increase in H+ secretion observed during ENaC blockade may be more physiologically significant during high-aldosterone states. Chronic dietary NaCl restriction increases aldosterone release, which stimulates renal Cl− transporters, such as pendrin, thereby increasing Cl− absorption. If a NaCl-rich meal is then consumed, serum aldosterone concentration falls, which reduces apical plasma membrane pendrin abundance probably over a period of hours to days. Without a means to rapidly reduce Na+ and Cl− absorption, a sudden increase in NaCl intake would markedly increase total body NaCl balance. The HCl secretion observed following ENaC blockade might represent a mechanism for rapidly reducing Cl− absorption. High NaCl intake increases distal Na+ delivery, which rapidly downregulates ENaC through Na+ self-inhibition (7). This ENaC inhibition should stimulate HCl secretion, thereby minimizing net NaCl absorption.
In the present study, benzamil did not change Cl− and H+ flux in a 1:1 relationship since the change in Cl− flux observed with benzamil application is about twofold higher than the change in CO2 flux observed with the application of this diuretic. Changes in total CO2 flux may, however, underestimate H+ secretion since a disequilibrium pH is observed in the CCD (12). Alternatively, this conductive pathway or conductive complex might secrete 2 Cl− for each secreted H+ (Fig. 9). For example, if 2 H+ are secreted through the apical H+-ATPase in tandem with the secretion of 2 Cl− and the absorption of 1 H+ through an electrogenic Cl−/H+ exchanger, net secretion of 1 H+ and 2 Cl− would be observed, although other mechanisms are also possible. For example, we cannot exclude the possibility that cations other than H+ are cosecreted with benzamil-stimulated Cl− secretion. However, K+ is not likely a cosecreted cation since amiloride reduces K+ secretion.
The benzamil-sensitive change in transepithelial voltage is commonly used as a measure of ENaC-mediated Na+ absorption. However, the present study shows that benzamil reduces transepithelial voltage not only by reducing ENaC-mediated Na+ absorption but also by stimulating H+ secretion. As such, benzamil-sensitive transepithelial voltage reflects, in part, apical H+-ATPase-mediated H+ secretion.
Eliminating the lumen-negative voltage of the CCD with benzamil increases the driving force for conductive Cl− secretion. Conversely, inhibiting conductive Cl− secretion with stilbene inhibitors should reduce the lumen-negative transepithelial voltage. However, we observed instead that application of stilbene inhibitors to the luminal perfusate increased the lumen-negative transepithelial voltage most likely by inhibiting apical H+-ATPase-mediated H+ secretion (18). DIDS likely inhibits conductive Cl− secretion as an indirect consequence of H+-ATPase blockade.
In conclusion, in CCDs from aldosterone-treated mice the majority of Cl− absorption occurs through a pathway that is modulated by ENaC. Inhibiting ENaC increases conductive, DIDS-sensitive Cl− secretion, which is associated with apical H+-ATPase-mediated H+ secretion. While benzamil may modulate transepithelial Cl− absorption by changing Cl− flux across tight junctions through changes in transepithelial voltage, this study demonstrates that other transport mechanisms are possible.
GRANTS
This study was supported by DK 46493 (to S. M. Wall), DK 29857 (to A. M. Weinstein), T32 DK07656 (to Y. Lazo-Fernandez), and by ASN Career Development Grant 145596 (to V. Pech).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.N., V.P., Y.L.-F., and A.M.W. performed experiments; M.N., V.P., Y.L.-F., A.M.W., and S.M.W. analyzed data; M.N., V.P., Y.L.-F., A.M.W., and S.M.W. interpreted results of experiments; M.N., V.P., A.M.W., and S.M.W. edited and revised manuscript; M.N., V.P., Y.L.-F., A.M.W., and S.M.W. approved final version of manuscript; V.P., A.M.W., and S.M.W. provided conception and design of research; V.P., A.M.W., and S.M.W. prepared figures; S.M.W. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Steve Sansom for helpful suggestions.
REFERENCES
- 1.Allen GG, Barratt LJ. An in vivo study of voltage-dependent renal tubular acidosis induced by amiloride. Kidney Int 35: 1107–1110, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M. Deletion of the anion exchanger Slc26a4 (pendrin) decreases the apical Cl−/HCO3− exchanger activity and imipairs bicarbonate secretion in the kidney collecting duct. Am J Physiol Cell Physiol 299: C33–C41, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda JAL, Subbarayudu K, Dytko G, Mola R, Kurtzman NA. Voltage-dependent distal acidification defect induced by amiloride. J Lab Clin Med 95: 407–416, 1980. [PubMed] [Google Scholar]
- 4.Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10: 153–161, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez R, Bosqueiro JR, Cassola AC, Malnic G. Role of Cl− in electrogenic H+ secretion by cortical distal tubule. J Membr Biol 157: 193–201, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Garcia NH, Plato CF, Garvin JL. Fluorescent determination of chloride in nanoliter samples. Kidney Int 55: 321–325, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–395, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA 107: 18010–18015, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulter HN, Ilnicki LP, Light JH, Sebastian A. On the mechanism of diminished urinary carbon dioxide tension caused by amiloride. Kidney Int 21: 8–13, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Husted RF, Steinmetz PR. The effects of amiloride and ouabain on urinary acidification by turtle bladder. J Pharmacol Exp Ther 210: 264–268, 1979. [PubMed] [Google Scholar]
- 11.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Schreck C, Coleman RA, Wade JB, Henandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinney TD, Burg MB. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 234: F141–F145, 1978. [DOI] [PubMed] [Google Scholar]
- 16.McKinney TD, Burg MB. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest 61: 1421–1427, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milton AE, Weiner ID. Regulation of B-type intercalated cell apical anion exchange activity by CO2/HCO3−. Am J Physiol Renal Physiol 274: F1086–F1094, 1998. [DOI] [PubMed] [Google Scholar]
- 17a.Nanami M, Lazo-Fernandez Y, Pech V, Verlander JW, Agazatian D, Weinstein AM, Bao H, Eaton DC, Wall SM. ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. I. Stilbene-sensitive Cl− secretion. Am J Physiol Renal Physiol. First published April 29, 2015; doi: 10.1152/ajprenal.00471.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obrdlik P, Diekert K, Watzke N, Keipert C, Pehl U, Brosch C, Boehm N, Bick I, Ruitenberg M, Volknandt W, Kelety B. Electrophysiological characterization of ATPases in native synaptic vesicles and synaptic plasma membranes. Biochem J 427: 151–159, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Pech V, Thumova M, Dikalov S, Hummler E, Rossier BC, Harrison DG, Wall SM. Nitric oxide reduces Cl− absorption in the mouse cortical collecting duct through an ENaC-dependent mechanism. Am J Physiol Renal Physiol 304: F1390–F1397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pech V, Thumova M, Kim YH, Agazatian D, Hummler E, Rossier BC, Weinstein AM, Nanami M, Wall SM. ENaC inhibition stimulates Cl− secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am J Physiol Renal Physiol 303: F45–F55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells in mouse cortical collecting duct. J Am Soc Nephrol 19: 84–91, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlueter W, Keilani T, Hizon M, Kaplan B, Batlle DC. On the mechanism of impaired distal acidification in hyperkalemic renal tubular acidosis: evaluation with amiloride and bumetanide. J Am Soc Nephrol 3: 953–964, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Steinmetz PR. Characteristics of hydrogen ion transport in urinary bladder of water turtle. J Am Soc Nephrol 11: 1160–1169, 2000. [PubMed] [Google Scholar]
- 25.Stokes JB, Ingram MJ, Williams AD, Ingram D. Heterogeneity of rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int 20: 340–347, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F519–F528, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Wall SM. NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Wall SM. Ouabain reduces net acid secretion and increases pHi by inhibiting NH4+ uptake on rat tIMCD Na+-K+-ATPase. Am J Physiol Renal Physiol 273: F857–F868, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Wall SM, Fischer MP. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to transepithelial transport of H+, NH4+, K+ and Na+ in rat outer medullary collecting duct. J Am Soc Nephrol 13: 827–835, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to Cl− secretion in rat outer medullary collecting duct. Am J Physiol Renal Physiol 280: F913–F921, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Wall SM, Sands JM, Flessner MF, Nonoguchi H, Spring KR, Knepper MA. Net acid transport by isolated perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 258: F75–F84, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 85: 274–281, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F957–F964, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein AM. A mathematical model of distal nephron acidification: diuretic effects. Am J Physiol Renal Physiol 295: F1353–F1364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Bouyer P, Boron WF. Role of tyrosine kinase in the CO2-induced stimulation of HCO3− reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol 291: F358–F367, 2006. [DOI] [PubMed] [Google Scholar]