Abstract
This study examined the role of the brain stem in inhibition of bladder reflexes induced by tibial nerve stimulation (TNS) in α-chloralose-anesthetized decerebrate cats. Repeated cystometrograms (CMGs) were performed by infusing saline or 0.25% acetic acid (AA) to elicit normal or overactive bladder reflexes, respectively. TNS (5 or 30 Hz) at three times the threshold (3T) intensity for inducing toe movement was applied for 30 min between CMGs to induce post-TNS inhibition or applied during the CMGs to induce acute TNS inhibition. Inhibition was evident as an increase in bladder capacity without a change in amplitude of bladder contractions. TNS applied for 30 min between saline CMGs elicited prolonged (>2 h) poststimulation inhibition that significantly (P < 0.05) increased bladder capacity to 30–60% above control; however, TNS did not produce this effect during AA irritation. TNS applied during CMGs at 5 Hz but not 30 Hz significantly (P < 0.01) increased bladder capacity to 127.3 ± 6.1% of saline control or 187.6 ± 5.0% of AA control. During AA irritation, naloxone (an opioid receptor antagonist) administered intravenously (1 mg/kg) or directly to the surface of the rostral brain stem (300–900 μg) eliminated acute TNS inhibition and significantly (P < 0.05) reduced bladder capacity to 62.8 ± 22.6% (intravenously) or 47.6 ± 25.5% (brain stem application). Results of this and previous studies indicate 1) forebrain circuitry rostral to the pons is not essential for TNS inhibition; and 2) opioid receptors in the brain stem have a critical role in TNS inhibition of overactive bladder reflexes but are not involved in inhibition of normal bladder reflexes.
Keywords: neuromodulation, brain stem, opioid, tibial, cat
tibial neuromodulation, a Food and Drug Administration (FDA)-approved therapy for overactive bladder (OAB) symptoms, including urgency, frequency, and incontinence (7, 19, 20, 29), is often used to treat patients whose symptoms are not completely controlled by drugs or who have unacceptable drug side effects (1, 2, 8, 18). However, the mechanisms underlying tibial neuromodulation therapy are uncertain. Understanding the site of action and neurotransmitters involved in the inhibition of bladder reflexes by tibial nerve stimulation (TNS) is important for developing new OAB treatments by combining drug therapies with tibial neuromodulation to achieve a higher efficacy with fewer side effects (14, 33).
Previous studies in cats have demonstrated the inhibitory effect of TNS on bladder reflexes elicited during non-nociceptive saline distention as well as nociceptive acetic acid (AA) irritation (23, 27). The failure of TNS to inhibit reflex bladder contractions during AA irritation in animals with acute spinal cord transection at the T9/T10 level (32) indicates that supraspinal neural circuits are necessary for TNS inhibition. However, it is unknown whether these supraspinal circuits are located in the forebrain or in the brain stem.
A major clinical benefit of TNS is the long-lasting poststimulation inhibitory effect, which allows for the sustained improvement in OAB symptoms by a 30-min stimulation once a month following completion of an initial 12-wk treatment (21). A 30-min TNS in cats has also been shown to produce a sustained poststimulation inhibition lasting for hours (27). This prolonged poststimulation effect, however, has not been demonstrated during AA irritation in cats (23). While AA irritation activates bladder nociceptive C-fiber afferents that trigger a spinal micturition reflex, saline distention primarily activates bladder non-nociceptive Aδ afferents that trigger a supraspinal micturition reflex (5, 9). It is therefore presumed that the ability to elicit a prolonged TNS poststimulation inhibition during non-nociceptive saline distention of the bladder might require supraspinal neural circuits located in the forebrain or brain stem. The goal of this study was to examine the role of the brain stem in TNS inhibition in cats after surgically removing the forebrain by supracollicular decerebration.
MATERIALS AND METHODS
All protocols involving the experimental use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Surgical Procedures
The experiments were conducted using a total of 17 cats (9 male, 8 female, 2.7–4.2 kg) under isoflurane (2–3% in O2) anesthesia during surgery followed by α-chloralose anesthesia (65 mg/kg iv and supplemented as needed; Sigma-Aldrich, St. Louis, MO) during data collection. Systemic blood pressure was monitored throughout the experiment via a catheter inserted into the right carotid artery while the left carotid artery was ligated. Heart rate and O2 saturation were monitored by a pulse oximeter (9847V, NONIN Medical, Plymouth, MN) attached to a front limb. A tracheotomy was performed and an endotracheal tube was inserted to ensure airway patency. A catheter for intravenous infusion of fluid and drugs was inserted into the left cephalic vein. A laparotomy was then performed, and the ureters were isolated, cut, and drained externally. The urethra was then exposed, and a double lumen catheter was inserted through an urethrotomy into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pressure transducer to monitor the pressure within the bladder, and the other was connected to a pump to infuse (1–2 ml/min) the bladder with saline or 0.25% AA. The left tibial nerve was then isolated on the medial side of the hindlimb, and a tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted around the nerve for stimulation. All surgical sites were closed with sutures. Decerebration was then performed through a craniotomy by surgically removing the brain tissue rostral to the superior colliculi. A polyurethane catheter (PE-50) was placed on top of the brain stem underneath the dura for drug delivery. Then, warm liquid agar gel (4 g in 100 ml saline, Fisher Scientific, Waltham, MA) was used to fill the space of the removed brain tissue and close the craniotomy once the agar gel solidified. Immediately following decerebration, the anesthesia was switched from isoflurane to α-chloralose. Then, experiments were conducted in the decerebrated animals after a waiting period of about 30–60 min.
Experimental Protocol
The experimental protocol is summarized in Fig. 1. At the beginning of the experiments, cystometrograms (CMGs) were performed in all cats by slowly infusing saline into the bladder to determine the bladder capacity defined as the bladder volume threshold required to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Multiple CMGs were performed to ensure reproducibility of the saline control capacity. Then, the animals were divided into two groups.
Fig. 1.
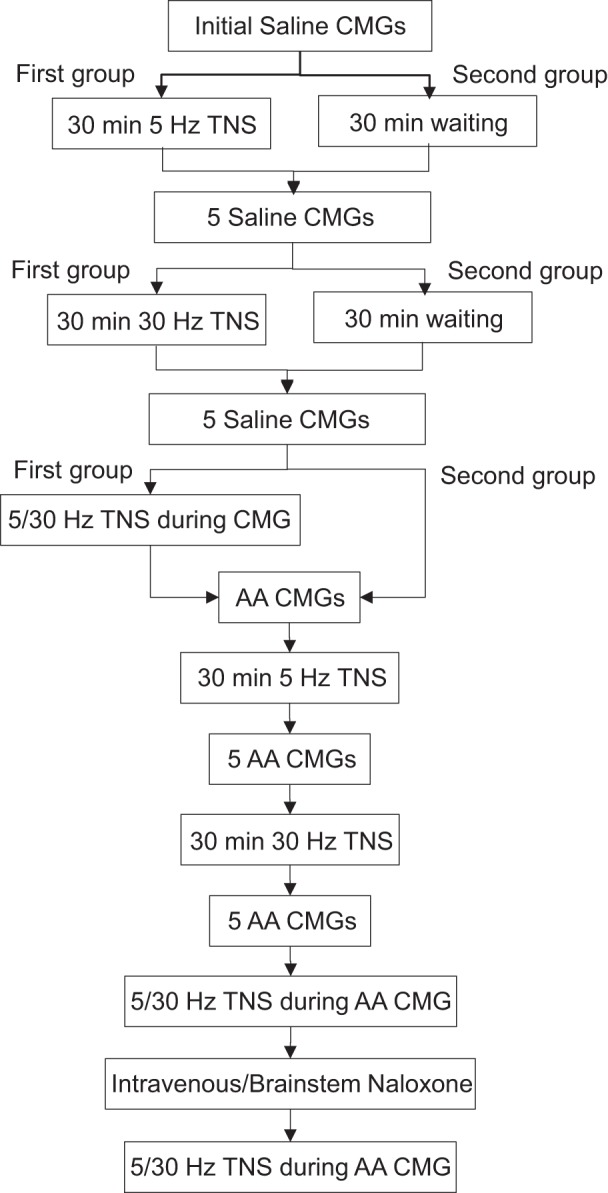
Experimental diagram showing the sequence of different treatments. CMGs, cystometrograms; TNS, tibial nerve stimulation; AA, acetic acid.
TNS during saline CMGs.
In the first group of cats (n = 8), the bladder volume was maintained at the bladder capacity to generate rhythmic bladder contractions, and then TNS (frequency 5 Hz; pulse width 0.2 ms) was applied for 30 min at three times the threshold (3T) intensity for inducing toe movement. After the 30-min TNS, five CMGs were performed within a 1.5- to 2-h period to examine the change in bladder capacity. At the end of the fifth CMG, the bladder volume was again maintained at the bladder capacity and a second 30-min TNS (frequency 30 Hz; pulse width 0.2 ms; intensity 3T) was applied. The poststimulation effect on bladder capacity induced by the second 30-min TNS was evaluated by another five CMGs repeated within 1.5–2 h after the termination of the second stimulation.
After an examination of the poststimulation effect, TNS (5 or 30 Hz, 0.2 ms, 3T) was applied again during repeated CMGs to determine whether TNS could induce an acute inhibitory effect and increase bladder capacity above the level during poststimulation inhibition. TNS parameters were chosen based on our previous studies in cats (24, 27). The bladder was emptied after each CMG, and a 5- to 10-min rest period was inserted between CMGs to allow the distended detrusor to recover.
In the second group of cats (n = 8), the initial procedures similar to those used in the first group were performed, but these animals served as controls and therefore TNS was not applied during either the first or the second 30-min treatment period while the bladder volume was maintained at the capacity. Following each 30-min period, five saline CMGs were performed.
TNS during AA CMGs.
After completion of the first set of experiments in both groups of cats, the saline infusion was switched to 0.25% AA infusion to irritate the bladder and cause bladder overactivity. Multiple CMGs were performed to ensure reproducibility of the AA control capacity. The repeated CMG procedures similar to those used in the first group of cats during saline infusion was again employed to determine the poststimulation effect induced by the 30-min TNS during AA irritation. Finally, in all 17 cats TNS (5 or 30 Hz, 0.2 ms, 3T) was applied during repeated AA CMGs to determine acute TNS inhibition. At the end of the experiments, naloxone hydrochloride dihydrate (an opioid receptor antagonist, Sigma-Aldrich) was administered intravenously (iv) in a dose (1 mg/kg, n = 10 cats) shown in previous experiments (24) to completely block TNS inhibition of bladder overactivity or applied topically to the dorsal surface of the brain stem (300–900 μg in 0.1–0.3 ml saline, n = 7 cats). Five minutes after administration of naloxone, the acute TNS inhibitory effect was tested again during repeated AA CMGs.
Data Analysis
To analyze the poststimulation effect under either saline or AA infusion conditions, bladder capacities measured during the repeated CMGs were normalized to the initial saline or AA control capacity, respectively, in the same animal (Fig. 2 and Fig. 4). To analyze acute TNS inhibition during repeated saline CMGs, bladder capacities were normalized to the control saline CMG performed immediately before testing TNS (Fig. 3). To analyze acute TNS inhibition during repeated AA CMGs and the effect of naloxone on acute TNS inhibition, bladder capacities were normalized to the control AA CMG performed immediately before testing TNS (Fig. 5). The data were averaged across animals under the same conditions, and the results are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures ANOVA followed by Bonferroni (2-way) or Dunnett (1-way) posttests. Two-way ANOVA was performed between treated and control groups for the five repeated CMGs after each 30-min treatment (Fig. 2 and Fig. 4). One-way ANOVA was performed in different groups of cats (Figs. 3, 5, and 6) with four levels of treatment (control, 5 Hz, 30 Hz, post-control).
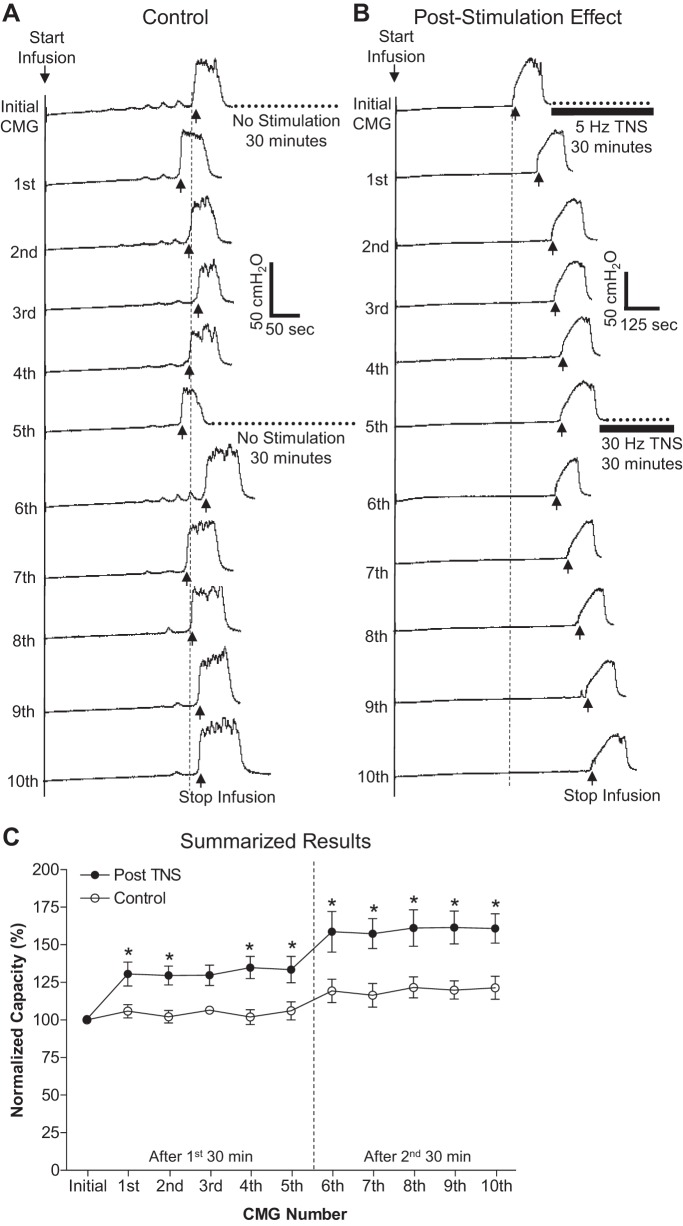
Fig. 2.
Prolonged poststimulation inhibition induced by 30-min TNS during repeated saline CMG tests. A: bladder capacity was not changed during repeated CMGs in the absence of TNS. Note: after the initial and 5th CMG, the bladder was maintained for 30 min in a distended condition Infusion rate: 2 ml/min. B: bladder capacity was significantly increased after the 30-min period of TNS at 5 Hz following the initial CMG, but was not further increased after the second 30-min period of TNS at 30 Hz. Five repeated CMGs (1st-5th, 6th-10th) were performed within 1.5–2 h after each 30-min TNS. The vertical dashed line indicates the control bladder capacity. The horizontal bladder bar indicates the 30-min TNS. Infusion rate: 2 ml/min. Stimulation: pulse width 0.2 ms; intensity 4.2 V (3T). T, intensity threshold for inducing toe movement. C: summarized results. Stimulation: pulse width 0.2 ms, intensity 3–9 V (3T). *Statistically significant (P < 0.05, posttest) difference between the control group (n = 8 cats) and treatment group (n = 8 cats) by 2-way ANOVA for the first 30-min TNS at 5 Hz (between groups: P < 0.0001, F = 50.8, df = 1; interaction: P = 0.94, F = 0.19, df = 4) and for the second 30-min TNS at 30 Hz (between groups: P < 0.0001, F = 53.2, df = 1; interaction: P = 0.99, F = 0.007, df = 4). Vertical dashed line indicates the time of the second 30-min TNS, which did not further significantly (P > 0.05, posttest) increase bladder capacity (2-way ANOVA, between groups: P < 0.0001, F = 21.7, df = 1; interaction: P = 0.99, F = 0.01, df = 4).
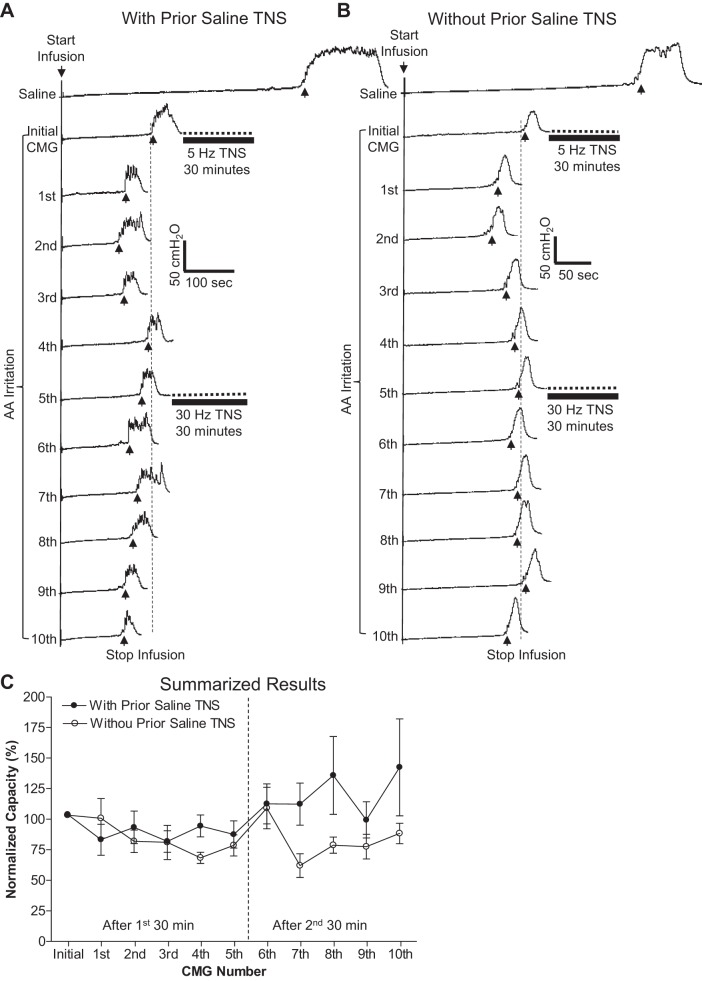
Fig. 4.
In decerebrate cats, the poststimulation inhibitory effect induced by TNS was absent during repeated CMGs with AA infusion. A: bladder capacity was not increased during repeated AA CMGs by the 30-min TNS when it had already been applied during prior saline CMGs. B: bladder capacity was not changed either during repeated AA CMGs by the 30-min TNS even though it had never been applied during prior saline CMGs. Records in A and B are from different cats. Five repeated CMGs (1st-5th, 6th-10th) were performed within 1.5–2 h after each 30-min TNS. The vertical dashed line indicates the control bladder capacity. The horizontal bladder bar indicates the 30-min TNS. Infusion rate: 2 ml/min in A and B. Stimulation: pulse width 0.2 ms; intensity (3T) 3.6 V in A and 0.66 V in B. C: summarized results. Stimulation: pulse width 0.2 ms, intensity 0.66–9 V (3T). Vertical dashed line indicates the time of the second 30 min TNS; n = 8 cats/group.
Fig. 3.
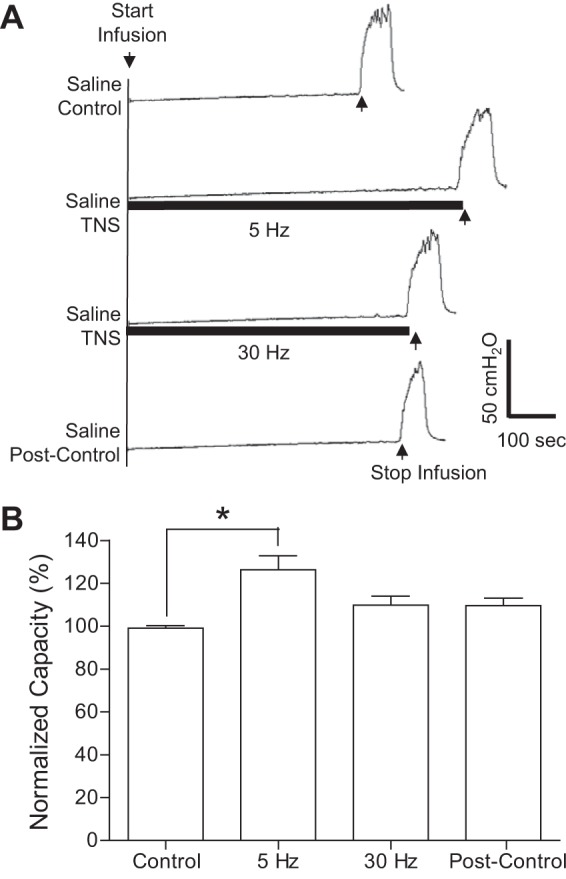
Acute inhibition of normal bladder reflex induced by TNS applied during saline CMGs. A: CMGs during saline infusion with/without TNS. Black bars under pressure trace indicate the duration of TNS (5 or 30 Hz, 0.2 ms, 3T = 3.0 V). Infusion rate = 1 ml/min. B: summarized results from 8 cats. The bladder capacity is normalized to the control CMG without TNS. *Significantly different (P < 0.01, posttest) by 1-way ANOVA (P < 0.0001, F = 12.55, treatment df = 3, individual df = 7). TNS: 5 or 30 Hz, 0.2 ms, 3T = 3–9 V. Infusion rate = 1–2 ml/min.
Fig. 5.
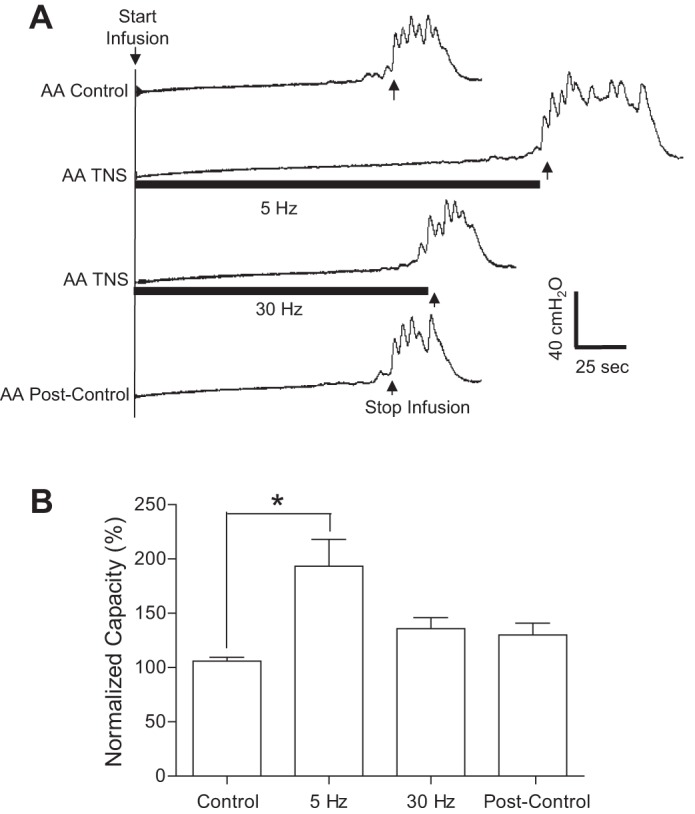
Acute inhibition of overactive bladder reflex induced by TNS applied during AA CMGs. A: CMGs during AA infusion with/without TNS. Black bars under pressure trace indicate the duration of TNS (5 or 30 Hz, 0.2 ms, 3T = 3.6 V). Infusion rate = 2 ml/min. B: summarized results from 17 cats. The bladder capacity is normalized to the control CMG without TNS. *Significantly different (P < 0.01, posttest) by 1-way ANOVA (P < 0.0001, F = 9.1, treatment df = 3, individual df = 16). TNS: 5 or 30 Hz, 0.2 ms, 3T = 0.66–9 V. Infusion rate = 1–2 ml/min.
Fig. 6.
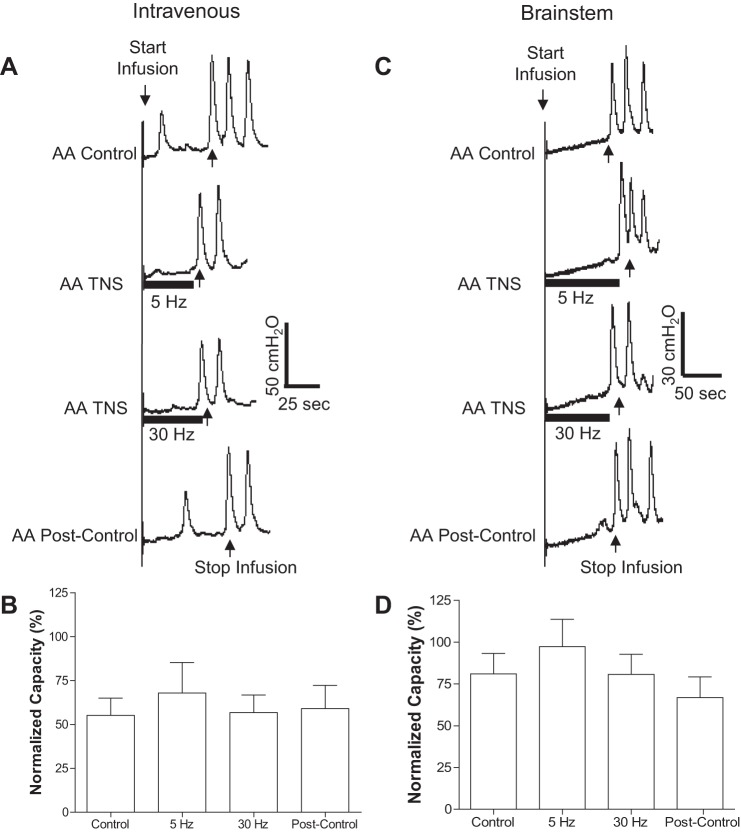
Inhibition of overactive bladder reflex by TNS during AA infusion was removed by naloxone administered either intravenously (A and B) or by topical application to the surface of the brain stem (C and D). A and C: CMG traces with or without TNS. B and D: summarized results for intravenous (n = 10 cats) or brain stem (n = 7 cats) administration of naloxone. TNS: 5 Hz or 30 Hz, 0.2 ms pulse width, intensity 3T = 0.66–9 V.
RESULTS
TNS Poststimulation Inhibition During Saline CMGs
In the unstimulated control group of cats, bladder capacity remained relatively constant during repeated saline CMGs performed over a 3- to 4-h period, which included two 30-min periods of bladder distension without application of TNS (Fig. 2A). Rhythmic bladder contractions occurred during the 30-min period in seven of the eight control cats.
In the group of cats to be used for stimulation, bladder capacity was relatively constant during control CMGs. At the beginning of the 30-min period of bladder distension before TNS, rhythmic bladder contractions occurred in six of the eight cats but were completely inhibited by the 5-Hz TNS at 3T. Following the first 30-min period of 5-Hz TNS, bladder capacity during subsequent CMGs was significantly increased for 2 h (Fig. 2B). Before the second 30-min TNS (30 Hz), bladder distension again elicited rhythmic bladder contractions in seven of the eight cats. These contractions were completely inhibited by TNS in two cats, partially inhibited in four cats, and unaffected in one cat. TNS (30 Hz) produced only a slight additional increase in bladder capacity during the poststimulation CMGs (Fig. 2B). The amplitude of the bladder contractions that occurred at the end of each CMG was not altered by 5- or 30-Hz TNS (Fig. 2B).
The poststimulation inhibitory effect of 30-min TNS is summarized in Fig. 2C. In the control unstimulated cats (n = 8), bladder capacity did not significantly (P > 0.05) change during 10 repeated saline CMGs performed over a 3- to 4-h period. On the other hand, in the stimulated cats (n = 8) bladder capacity was significantly (P < 0.05) increased after the first 30-min TNS (5 Hz) to 131.6 ± 2.1% on average of initial saline control (10.9 ± 4.4 ml). The second 30-min TNS (30 Hz) further increased the bladder capacity to 159.9 ± 1.6% on average of initial saline control, but this second increase was not significant compared with the bladder capacities after the first TNS.
Acute Inhibitory Effect Induced by TNS During Saline CMGs
After induction of poststimulation inhibition with two 30-min periods of stimulation, TNS applied continuously at 5 Hz during the CMGs (Fig. 3) elicited a further significant (P < 0.01) increase in bladder capacity to 127.3 ± 6.1% of the saline control (termed acute inhibition) but did not change the amplitude of the bladder contraction at the end of the CMG. However, a subsequent 30-Hz TNS failed to significantly increase the bladder capacity (Fig. 3).
Absence of TNS Poststimulation Inhibition During AA CMGs
Following repeated saline CMGs, intravesical infusion of AA irritated the bladder and significantly (P < 0.001) reduced bladder capacity (Fig. 4, A and B, second traces from the top) to 30.0 ± 15.7% of the control capacity measured during the final saline CMGs (Fig. 4, A and B, top traces). There was no significant difference in AA-induced reduction in bladder capacity for cats that had previously been exposed to TNS under saline conditions (32.5 ± 5.7% of final saline control, n = 8 cats) and those that had served as saline unstimulated controls (27.8 ± 5.6% of final saline control, n = 9 cats). During repeated AA CMGs, 30-min TNS (5 or 30 Hz) failed to induce poststimulation inhibition and a significant increase in bladder capacity in the eight cats that had been exposed to 30-min TNS during saline CMGs and during which their saline bladder capacity had been significantly increased (Fig. 4, A and C). TNS for 30 min at 5 or 30 Hz also failed to increase bladder capacity in the eight cats that had never been exposed to TNS during the prior saline CMGs (Fig. 4, B and C). Rhythmic bladder contractions occurred in the first 30-min period in 13 of the 16 cats, and TNS (5 Hz) completely inhibited the contractions in 7 cats, partially inhibited the contractions in 4 cats, and had no effect in 2 cats. During the second 30-min period, rhythmic bladder contractions occurred in 15 of the 16 cats, while TNS (30 Hz) completely inhibited the contractions in 4 cats, partially inhibited the contractions in 8 cats, and had no effect in 3 cats.
Acute Inhibitory Effect Induced by TNS During AA CMGs
After failing to detect poststimulation inhibition following two 30-min periods of stimulation during AA CMGs, TNS was applied continuously at 5 or 30 Hz during the CMGs (Fig. 5) to study acute inhibition. TNS at 5 Hz significantly (P < 0.01) increased bladder capacity to 187.6 ± 5.0% of the AA control (n = 17 cats), but 30-Hz TNS was ineffective (Fig. 5).
Naloxone Effect on AA-Induced Bladder Overactivity and on Acute TNS Inhibition
Previous studies have shown that intravenous administration of a large dose (1 mg/kg) of naloxone (an opioid receptor antagonist) during saline CMGs in the central nervous system (CNS) of intact cats stimulates bladder reflexes and reduces bladder capacity (24). This dose of naloxone also completely blocks acute TNS inhibition of AA-induced bladder overactivity (24). In the present experiments naloxone administered either intravenously (1 mg/kg, n = 10 cats) or by topical application to the surface of the brain stem (doses ranging from 300 to 900 μg applied in 0.1–0.3 ml saline, n = 7 cats) during AA CMGs eliminated acute TNS (5 Hz) inhibition (Fig. 6) and significantly (P < 0.05) reduced bladder capacity to 62.8 ± 22.6% (intravenous injection) and 47.6 ± 25.5% (application to the brain stem) of AA control measured immediately before naloxone treatment. The reduction in bladder capacity was not significantly different in the two groups of cats. TNS inhibition was blocked by topical application of 300 μg of naloxone in six of seven experiments and by 600 μg in another experiment. Bladder capacity was reduced by 300 μg in four of seven experiments and by 600 μg in two and 900 μg in one experiment.
DISCUSSION
Previous studies in cats with an intact CNS revealed that the effects of TNS on normal reflex bladder activity triggered by intravesical infusion of saline are different from the effects on bladder overactivity initiated by intravesical infusion of dilute AA (24). In the present study, these differences persisted after removal of the forebrain. In decerebrate cats, TNS applied for 30 min at 5 Hz induced a prolonged (>2 h) poststimulation inhibition of normal reflex bladder activity (evident as an increase in bladder capacity) (Fig. 2), but did not elicit poststimulation inhibition of bladder overactivity (Fig. 4). TNS (5 Hz) applied for a shorter period during CMGs in both the normal and overactive bladder models elicited a rapidly reversible increase in bladder capacity (termed acute inhibition) (Figs. 3 and 5). Naloxone, an opioid receptor antagonist, administered intravenously or applied locally to the surface of the brain stem blocked the acute inhibition of bladder overactivity (Fig. 6). In cats with an intact CNS (24), intravenous naloxone had a similar effect on TNS inhibition of bladder overactivity but did not alter the inhibition of normal bladder activity. These results indicate that inhibition of the overactive bladder by TNS is mediated by activation of opioid receptors and is not dependent on neural circuitry in the forebrain. Based on these findings, it is reasonable to conclude that TNS activates at least three types of inhibitory mechanisms in the CNS: 1) poststimulation inhibition, 2) naloxone-sensitive acute inhibition, and 3) naloxone-resistant acute inhibition.
The three types of TNS inhibition could occur at multiple sites in the CNS because bladder function is controlled by reflex pathways organized in the brain stem and spinal cord. A spinal-brain stem-spinal reflex in CNS intact animals which is activated by non-nociceptive Aδ bladder afferent fibers passes through supraspinal relay stations in the periaqueductal gray (PAG) and the pontine micturition center (PMC) (6). This reflex is present in decerebrate animals but is blocked by transection of the spinal cord. However, when the spinal transection is rostral to the sacral segments, thereby sparing sacral pathways, irritation of the bladder by intravesical infusion of dilute AA unmasks reflex contractions mediated by spinal reflex circuitry activated by C-fiber bladder afferents (32). Thus in the present experiments in decerebrate cats that have an intact spinal-brain stem-spinal reflex, the TNS inhibition during saline CMGs could occur supraspinally possibly at the level of the PAG-PMC circuitry or in the spinal cord via suppression of the ascending or descending limbs of the supraspinal reflex. However, during AA CMGs TNS inhibition probably involves activation of neuronal circuits in the brain stem, because in acute spinal transected cats TNS cannot inhibit the C-fiber-mediated spinal reflex bladder activity induced by AA irritation of the bladder (32). This proposal is further supported by the fact that application of naloxone to the brain stem blocked the TNS inhibition of AA-induced bladder overactivity (Fig. 6).
The present results demonstrating an inhibitory function of supraspinal opioid receptors are consistent with results of studies in decerebrate cats and dogs showing that injections of fentanyl, a μ-opioid receptor agonist, into the PMC increases bladder capacity during saline CMGs, an effect reversed by injection of naloxone into the PMC (17). This experiment establishes the presence of opioid-inhibitory receptors in the PMC. In the absence of fentanyl, injection of naloxone into the PMC (17) or intracerebroventricularly (10) decreases bladder capacity, indicating that the micturition switching circuitry in the PAG-PMC is tonically inhibited by an opioid receptor mechanism. During AA CMGs in the present experiments, naloxone intravenously or applied locally to the brain stem also reduced bladder capacity, indicating that the tonic supraspinal opioid inhibition was active under the conditions of our experiments. Therefore, it is probable that the acute TNS inhibition of bladder overactivity is mediated by activation of supraspinal opioid receptors that suppress excitatory transmission in the PAG-PMC circuitry, although the more remote possibility that a supraspinal opioid mechanism activates a descending inhibitory input to the sacral spinal cord cannot be ruled out.
TNS has a prominent effect on bladder capacity with a minimal effect on the amplitude of isovolumetric bladder contractions that occur at the end of bladder filling. This suggests that TNS can increase the storage function of the bladder but may not influence voiding. This will be directly evaluated in future experiments by testing TNS during voiding CMGs. The selective action of TNS on bladder capacity in anesthetized cats is similar to the clinical observations in OAB patients that different types of neuromodulation improve urine storage without reducing voiding efficiency (3, 11, 16, 30). This selectivity suggests that neuromodulation targets the afferent limb of the micturition reflex or the supraspinal switching mechanism in the PAG-PMC circuitry but does not alter the efferent (motor) limb of the circuit. Local injections of neurotransmitters or their antagonists into the PMC of decerebrate cats can elicit similar selective changes in bladder capacity without altering the amplitude of reflex bladder contractions (12, 17), providing further support for the proposal that the PAG-PMC circuitry is a likely site of action of TNS.
The sites of action and neurotransmitter mechanisms underlying naloxone-resistant acute TNS inhibition and the TNS poststimulation inhibition during saline CMGs are uncertain. However, it is clear that these two types of inhibition persist after removal of the forebrain and therefore must also occur in the brain stem or involve a modulation of the spinal-brain stem-spinal pathway at the spinal level.
Minor differences between the efficacy of TNS inhibition in CNS intact and decerebrate cats raise the possibility that the forebrain has an influence on the response to TNS. For example, in CNS intact cats 30- and 5-Hz stimulation are almost equally effective in increasing bladder capacity during AA CMGs (23). However, in decerebrate cats only 5-Hz stimulation significantly increased bladder capacity (Fig. 5). Thus forebrain circuitry may contribute to the inhibitory effects of higher frequency TNS.
The effects of naloxone are also different in CNS intact and decerebrate cats. As mentioned above, naloxone significantly decreases bladder capacity during AA CMGs in decerebrate cats but in previous studies did not significantly reduce bladder capacity during AA CMGs in CNS intact cats (15, 24). This raises the possibility that a tonic opioid-inhibitory mechanism might be suppressed by nociceptive afferent input from the bladder in CNS intact cats and that this suppression is dependent on forebrain circuitry. This putative reduction in opioid inhibition by AA might also contribute to the bladder overactivity in CNS intact cats.
Information about TNS inhibition of reflex bladder activity may also provide insights into the mechanisms of action of other types of neuromodulation such as foot stimulation because the latter and TNS exhibit similar properties, including naloxone sensitivity (15, 24, 25) and prolonged poststimulation inhibition (4, 27). Inhibition by TNS or foot stimulation also has similar frequency characteristics during AA CMGs, producing an increase in bladder capacity over a wide range of frequencies (5–30 Hz) (23, 26). On the other hand, inhibitory modulation of reflex bladder activity elicited by pudendal nerve stimulation (PNS) in the cat is markedly different than TNS and foot neuromodulation. PNS inhibition of bladder overactivity is resistant to naloxone (13), does not produce poststimulation inhibition (22), and is mediated in part by activation of GABAergic receptors in the spinal cord (31). PNS inhibition also has a different frequency-response curve, eliciting an increase in bladder capacity only at low frequencies (5–10 Hz) and not at higher frequencies (20–40 Hz) (28).
In our previous study (27), 30- and 5-Hz TNS were applied in random order during the first and second 30-min stimulation periods and produced an average of a nearly 50% increase in bladder capacity during the first period of stimulation and no further increase during the second period of stimulation. In the present study, 5-Hz TNS applied during the first 30-min period produced an average increase of ∼30% increase in bladder capacity, while 30-Hz TNS applied during the second 30-min period produced an additional 20% increase in capacity that maintained capacity at ∼50% above the saline control capacity before TNS (see Fig. 2C). However, the additional increase in capacity elicited by 30 Hz was not statistically significant. Based on these results, it seems reasonable to conclude that 5 Hz produces a near maximal poststimulation response and that decerebration does not change this response. However, to answer this question with certainty, additional experiments with the 30-Hz TNS applied in the first 30-min period are needed.
In summary this study revealed that neural circuits in the brain stem play an important role in TNS inhibition of bladder reflexes and that opioid receptors in the brain stem are involved in TNS inhibition of bladder overactivity. Understanding the sites of action and the neurotransmitter receptors involved in TNS inhibition may ultimately identify the mechanisms underlying tibial neuromodulation, an FDA-approved therapy for OAB.
GRANTS
This study is supported by the National Institutes of Diabetes and Digestive and Kidney Diseases under Grants DK-094905, DK-090006, DK-102427, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. provided conception and design of research; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. performed experiments; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. analyzed data; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. prepared figures; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. drafted manuscript; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; M.C.F., R.C.S., B.S., Z.X., J.W., A.L., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63, Suppl 1: 32–41, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatment of urinary incontinence. Pharmacol Rev 56: 581–631, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bartley J, Gilleran J, Peters KM. Neuromodulation for overactive bladder. Nat Rev Urol 10: 513–521, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Larson JA, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. J Urol 187: 338–343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol 200: 87–108, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govier FE, Litwiller S, Nitti V, Kreder KJ, Rosenblatt P. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol 165: 1193–1198, 2001. [PubMed] [Google Scholar]
- 8.Gulur DM, Drake MJ. Management of overactive bladder. Nat Rev Urol 7: 572–582, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisamitsu T, de Groat WC. The inhibitory effect of opiod peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res 298: 51–65. [DOI] [PubMed] [Google Scholar]
- 11.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 5: 657–666, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res 546: 310–320, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mally AD, Zhang F, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. J Urol 188: 2426–2432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuta Y, Mally AD, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Contribution of opioid and metabotropic glutamate receptor mechanisms to inhibition of bladder overactivity by tibial nerve stimulation. Am J Physiol Regul Integr Comp Physiol 305: R126–R133, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakib N, Siegel S. Neuromodulation versus neurotoxin for the treatment of refractory detrusor overactivity: for neuromodulation. Nat Clin Pract Urol 5: 118–119, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Noto H, Roppolo JR, de Groat WC, Hishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int 47, Suppl 1: 19–22, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Oefelein MG. Safety and tolerability profiles of anticholinergic agents used for the treatment of overactive bladder. Drug Saf 34: 733–754, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Peters KM, Carrico DJ, Wooldridge LS, Miller CJ, MacDiarmid SA. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 189: 2194–2201, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Schwen Z, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am J Physiol Renal Physiol 305: F663–F671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai C, Chen M, Shen B, Wang J, Roppolo JR, de Groat WC. Irritation induced bladder overactivity is suppressed by tibial nerve stimulation in cats. J Urol 186: 326–330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai C, Larson JA, Ogagan D, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai C, Ogagan PD, Chen G, Larson JA, Shen B, Wang J, Roppolo JR, de Groat WC. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. J Urol 188: 1012–1016, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai C, Shen B, Chen M, Wang J, Liu H, Roppolo JR, de Groat WC. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int 107: 303–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai C, Shen B, Wang J, Liu H, Subbaroyan J, Roppolo JR, de Groat WC. Inhibition of bladder overactivity by stimulation of feline pudendal nerve using transdermal amplitude-modulated signal (TAMS). BJU Int 109: 782–787, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JP, Debruyne FM, Kiemeney LA, Bemelmans BL. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn 22: 227–232, 2003. [DOI] [PubMed] [Google Scholar]
- 30.van Kerrebroeck PEV, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z, Reese J, Schwen Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 306: F781–F789, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am J Physiol Renal Physiol 307: F673–F679, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F, Mally AD, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol 302: F1576–F1582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]