Abstract
Human milk contains biologically important amounts of transforming growth factor-β2 isoform (TGF-β2), which is presumed to protect against inflammatory gut mucosal injury in the neonate. In preclinical models, enterally administered TGF-β2 can protect against experimental necrotizing enterocolitis, an inflammatory bowel necrosis of premature infants. In this study, we investigated whether TGF-β bioactivity in human preterm milk could be enhanced for therapeutic purposes by adding recombinant TGF-β2 (rTGF-β2) to milk prior to feeding. Milk-borne TGF-β bioactivity was measured by established luciferase reporter assays. Molecular interactions of TGF-β2 were investigated by nondenaturing gel electrophoresis and immunoblots, computational molecular modeling, and affinity capillary electrophoresis. Addition of rTGF-β2 (20–40 nM) to human preterm milk samples failed to increase TGF-β bioactivity in milk. Milk-borne TGF-β2 was bound to chondroitin sulfate (CS) containing proteoglycan(s) such as biglycan, which are expressed in high concentrations in milk. Chondroitinase treatment of milk increased the bioactivity of both endogenous and rTGF-β2, and consequently, enhanced the ability of preterm milk to suppress LPS-induced NF-κB activation in macrophages. These findings provide a mechanism for the normally low bioavailability of milk-borne TGF-β2 and identify chondroitinase digestion of milk as a potential therapeutic strategy to enhance the anti-inflammatory effects of preterm milk.
Keywords: breast milk, necrotizing enterocolitis, TGF-β2, inflammation, chondroitinase
human milk contains biologically important amounts of transforming growth factor-β (TGF-β), particularly the TGF-β2 isoform (12). Although the precise function of milk-borne TGF-β is unclear, orally ingested TGF-β is presumed to promote gut barrier function, immune tolerance, and mucosal repair in the neonatal gastrointestinal tract (8, 18, 19, 31). We have previously reported that TGF-β2 suppresses macrophage cytokine expression and mucosal inflammatory responses in the developing human intestine, and shown using murine models that enterally administered TGF-β2 can protect against intestinal injury similar to necrotizing enterocolitis (NEC), an inflammatory bowel necrosis of premature infants (22, 27).
We showed recently that preterm human milk shows minimal TGF-β bioactivity in the native state because most milk-borne TGF-β is in an inactive/latent form (26). Because of neurological immaturity, premature infants born prior to 32 wk of gestation frequently receive expressed breast milk by gavage, and fortification of mother's milk to increase caloric density and protein/mineral content is a common clinical practice in neonatal intensive care units. Therefore, we now asked whether TGF-β bioactivity in preterm human milk could be enhanced for potential therapeutic purposes by adding recombinant TGF-β2 (rTGF-β2) to milk prior to feeding. In the following sections, we demonstrate that TGF-β2 is sequestered in milk by chondroitin sulfate (CS) proteoglycans such as biglycan, and show that chondroitinase digestion of these glycosaminoglycans (GAGs) can restore the bioavailability of milk-borne TGF-β.
METHODS
Human milk.
Milk samples were collected at Rush University Medical Center, Chicago, IL, during the period January to March 2013; at Evanston Hospital, Evanston, IL, during the period October 2008 to September 2010; and at the University of Texas at San Antonio, TX, during January 2009 to January 2011. Deidentified samples were received at University of Illinois at Chicago (UIC). The study was approved by the Institutional Review Boards at each site. Mothers who delivered between 23 0/7 and 31 6/7 wk or at ≥37 wk of gestation were enrolled after informed consent. Mothers who delivered prior to term provided 2- to 5-ml milk samples at three time points after delivery: within 48 h (colostrum), on day 6–7 (1 wk), and on day 30–31 (1 mo). Mothers who delivered at term gestation provided samples within the first week. All samples were centrifuged at 13,000 g for 10 min at 4°C. After removing the fat layer, aqueous fractions and cell pellets were separated and stored at −80°C, and freeze-thaw cycles were minimized. Samples were handled according to the Biospecimen Reporting for Improved Study Quality guidelines (24). Premature infant formula (Similac Special Care) was purchased from Abbott Laboratories, Abbott Park, IL.
Reagents.
Human rTGF-β2 (12.7 kDa, active fragment) was purchased from R&D systems (Minneapolis, MN). Chondroitin sulfate (from bovine cartilage) and chondroitinase ABC (derived from Proteus vulgaris, specific activity 50–250 units/mg protein) were purchased from Sigma, St. Louis, MO. Chondroitinase ABC was reconstituted in 0.01% (wt/vol) bovine serum albumin (BSA) in ultrapure water to prepare a stock solution, which was diluted prior to each experiment in 50 mM Tris (pH 8.0) with 60 mM sodium acetate and 0.02% BSA.
TGF-β bioactivity.
Milk-borne TGF-β bioactivity was measured with one of two luciferase reporter cell lines: 1) mink lung epithelial (MLE) cells stably transfected with a luciferase construct containing the TGF-β-responsive plasminogen activator inhibitor-1 (PAI-1) promoter, and 2) RAW 264.7 cells stably transduced with a luciferase lentiviral construct containing a Smad-response element (Cignal SRE luc reporter kit, Qiagen, Valencia, CA). In some experiments, we used these reporter cells to measure milk-borne TGF-β activity after adding recombinant biglycan (My Bio-source, San Diego, CA) or chondroitin sulfate. After treatment with milk samples for 16 h, cell lysates (M-PER reagent, Thermo Scientific, Rockford, IL) were obtained and luciferase activity was measured with a commercially available kit (GloMax multi-detection system, Promega, Madison, WI). All assays were performed in triplicate.
Animals.
Murine intestine was harvested after euthanasia from pups on postnatal day 10 (P10; n = 6) as part of a protocol approved by Institutional Animal Care and Use Committee.
Denaturing and nondenaturing immunoblots.
To identify the inhibitor(s) of TGF-β2 in milk, we immunoprecipitated TGF-β2 from aqueous fractions of preterm milk (Novex Dynabeads Protein-A kit, Life Technologies, Grand Island, NY) and resolved the immune complexes in nondenaturing gels. These gels were stained separately with coomassie blue and alcian blue dyes by established methods (3, 6) and then probed for chondroitin sulfate with monoclonal mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA). To identify various constituents of the immune complex, we also used sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and these denaturing gels were probed for decorin and biglycan by using rabbit polyclonal IgG antibodies (Santa Cruz Biotechnology) with a standard immunoblotting protocol (22). To confirm biglycan-TGF-β2 binding, we pulled down biglycan and TGF-β2 from milk and then probed these complexes for each protein in nondenaturing and denaturing gels.
To measure the signaling effects of milk-borne TGF-β2 in intestinal tissue, we treated explanted murine neonatal intestine with milk samples and measured phosphorylated Smad2 (Ser465/467; rabbit polyclonal IgG from Santa Cruz Biotechnology) expression. To prepare these explants, murine neonatal intestine was opened longitudinally and rinsed gently in phosphate-buffered saline (PBS). Three to five square millimeter pieces of intestinal tissue were placed in serum-free RPMI 1640 in 5% CO2 at 37°C. Some explants were treated with milk or PBS diluted 1:1 in media for 25 min, and then homogenized in ice-cold lysis buffer (T-PER reagent containing protease and phosphatase inhibitors; Thermo Scientific).
Immunohistochemistry.
Deidentified human mammary tissue (from biopsies) were immunostained as previously described to localize biglycan expression (26). Briefly, tissue sections were deparaffinized and antigen retrieval was achieved with the EZ-AR Common solution (Biogenex, San Remon, CA) per manufacturer's protocol. These sections were then treated with Proteinase K (20 μg/ml) (Promega, Madison, WI) for 10 min at room temperature, rinsed in PBS, blocked (SuperBlock T20 blocking buffer; Thermo Scientific) for 30 min, and then incubated overnight at 4°C with rabbit antihuman biglycan (Santa Cruz Biotechnology). Secondary staining was performed at room temperature for 1 h with Alexa Fluor 488-conjugated chicken antihuman antibody (Invitrogen, San Diego, CA). Nuclear staining was obtained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Fluorescence imaging was performed with a Zeiss LSM 710 confocal microscope.
Reverse transcriptase-quantitative polymerase chain reaction.
Biglycan expression in the cellular fractions of milk was measured by using a SYBR green-based reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) protocol (33). The primer sequences were, forward: ACACCATCAACCGCCAGAG; reverse: GCCACCGACCTCAGAAGC. Data were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the groups were compared by the 2-ΔΔCT method.
Enzyme-linked immunosorbent assay.
Biglycan concentrations in milk were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits (My Bio-source). Optical densities and standard concentrations were log transformed and a linear equation was obtained (acceptable r2 ≥ 0.95). Analyte concentrations in test samples were calculated by regression. The linear range of measurement was 0.31–20 ng/ml.
Computational studies.
The crystal structure of human TGF-β2 was obtained from the RCSB Protein Data Bank (PDB ID: 1TFG; 2.2 Å resolution) and prepared for modeling with SYBYL-X, v2.0 (Certara, St. Louis, MO), which included removal of water molecules, adjustment of protonation states of amino acids to physiological conditions, addition of hydrogen atoms, and Powell minimization. Potential sites for binding to chondroitin sulfate sequences were identified by analyzing the protein's electrostatic potential in PyMOL Molecular Graphics System, v1.5.0.4 (Schrödinger, New York, NY). Two potential binding regions were identified, including residues Lys25, Arg26, Lys31, His34, Lys37, Lys94, and Lys97 (Site 1) and residues His58, Arg60, and Lys110 (Site 2; Fig. 2B), and all residues within 16 Å of centroid of each site were identified as part of the binding site. The coordinates for chondroitin sulfate oligosaccharide sequences were generated in a combinatorial manner in SYBYL-X from a previously described set of 24 known chondroitin sulfate disaccharides (13), which resulted in 192 tetrasaccharide and 1,536 hexasaccharide sequences. The structure optimization parameters used in modeling these chondroitin sulfate oligosaccharides followed our previous work on other glycosaminoglycans (29). A “rigid backbone” approach was used to restrict the conformational flexibility of chondroitin sulfate sequences, as suggested earlier (29). The average ΦH and ΨH values for the interglycosidic torsion angles used in docking were derived from literature (25). Molecular docking of each oligosaccharide sequence at the predicted binding sites (Site 1 and Site 2) was performed with GOLD v5.1 (Cambridge Crystallographic Data Centre, Cambridge, UK) by using our previously described dual filter strategy (29). Best sequences were identified as those with the highest GOLDScore and the lowest root-mean-square deviation between top six solutions derived from a triplicate docking run.
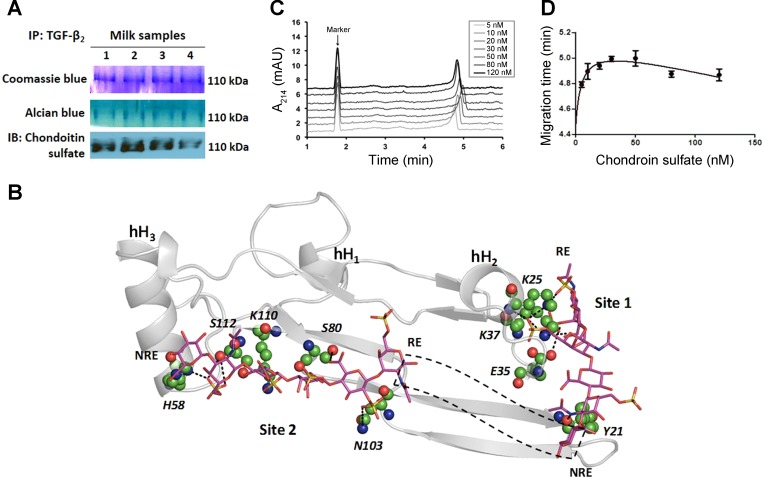
Fig. 2.
TGF-β2 is bound to chondroitin sulfate proteoglycans in milk. A: we immunoprecipitated TGF-β2 and resolved the immune complexes in nondenaturing gels. Staining with coomassie blue (top) or alcian blue (middle) dyes showed a prominent 110-kDa band. The same band also showed immunoreactivity for chondroitin sulfate. Data represent preterm milk samples from four mothers, tested in at least three separate runs. B: computational model predicts the binding of a chondroitin sulfate hexasaccharide sequence to TGF-β2 at two potential binding sites (Site 1, Site 2). Chondroitin sulfate sequence is depicted in a sticks form, whereas TGF-β2 is depicted in grey ribbons with individual residues predicted to be involved in some interaction at an atomic level in ball and stick form. Helices are denoted as hH1, hH2, and hH3. Site 1 residues include Lys25, Lys37, Glu35, and Tyr91 and Site 2 residues include His58, Ser80, Asn103, Lys110, and Ser112. Dotted black box depicts the possible orientation of intervening chondroitin sulfate residues connecting the reducing end (RE) of Site 2-bound chain to the nonreducing end (NRE) of Site 1-bound chondroitin sulfate chain. C: affinity capillary electrophoresis demonstrates biophysical interaction between chondroitin sulfate and TGF-β2. Panel shows electropherogram of 0.5 μM TGF-β2 in sodium phosphate buffer containing different concentrations of chondroitin sulfate. Dimethyl sulfoxide is the marker. Data represent findings from three separate experiments. D: binding curve of chondroitin sulfate-TGF-β2 system, fitted for a one-site total binding equation.
Affinity capillary electrophoresis.
The interaction of TGF-β2 with chondroitin sulfate was studied by using a Beckman P/ACE MDQ CE system (Fullerton, CA). The length of the uncoated fused-silica capillary (internal diameter = 75 μm) was 30 cm long, with an effective length of 20 cm from the injection point to the detection window. The capillary was activated by washing with 1 M HCl for 10 min, ddH2O for 3 min, 1 M NaOH for 10 min, and ddH2O for 3 min at 20 psi. Electrophoresis was performed at 25°C with a constant voltage of 8 kV. Before each electrophoretic run, the capillary was rinsed with the run buffer for 3 min at 20 psi. To ascertain TGF-β2 binding to chondroitin sulfate, run electrolytes containing varying concentrations of chondroitin sulfate were prepared in 20 mM of sodium phosphate buffer, pH 7.4. Dimethyl sulfoxide (DMSO) was used as an internal marker. The injection sample containing 0.5 μM TGF-β2 and 0.01% DMSO was injected at the anodic end for 3 s at 0.4 psi, and TGF-β2 was detected at the cathodic end using a 214 nm filter.
Nuclear factor-kappa B activation.
Nuclear factor-kappa B (NF-κB) activation was measured in stably transfected RAW 264.7 reporter cells that express NF-κB-driven secreted alkaline phosphatase (SEAP) (NF-κB SEAPorter Assay Kit, Imgenex, San Diego, CA) as previous described. Briefly, 250 μl of culture supernatant was first diluted 1:10 in assay buffer and then equal volumes of sample and H2O were incubated at 65°C for 30 min to inactivate endogenous alkaline phosphatase. One hundred microliters of a colorigenic substrate (p-nitrophenyl phosphate, 1 mg/ml) were added to each well and incubated for 1 h. Absorbance was read at 405 nm after 1 h (predetermined optimum duration). The linear standard range of measurement of all assays was 3.1–200 ng/ml.
To confirm the effect of chondroitinase-treated preterm milk on LPS-induced NF-κB activation in RAW264.7 cells, we also performed immunocytochemistry for phosphorylated NF-κB p65 (Ser 536). Briefly, cells grown on cover slips in serum-free media (Dulbecco's modified Eagle's media) were treated with preterm milk for 24 h and then with Escherichia coli LPS (0.5 μg/ml; Sigma) for 2 h. After rinsing with ice-cold PBS, cells were fixed with ice-cold methanol and acetone (1:1, vol/vol) for 10 min at −20°C, blocked (SuperBlock T20 blocking buffer, Thermo Scientific) for 30 min, and then incubated overnight at 4°C with primary mouse antiphospho NF-κB p65 (Ser 536) (Santa Cruz Biotechnology). Secondary staining was performed with Alexa Fluor 546-conjugated goat antimouse IgG (Invitrogen) for 1 h at room temperature. Nuclear staining was obtained with DAPI (Invitrogen). Fluorescence imaging was performed with a Zeiss LSM 710 confocal microscope.
Statistical methods.
Parametric and nonparametric tests were applied with Sigma Stat 3.1.1 software (Systat, Point Richmond, CA). For PCR data, crossing-threshold (ΔΔCT) values for genes with greater than or equal to twofold change were compared by the Mann-Whitney U test. Comparison across multiple groups was performed by the Kruskall-Wallis H test. In all tests, P < 0.05 was accepted as significant.
RESULTS
Addition of rTGF-β2 fails to increase TGF-β bioactivity in preterm milk.
We first measured TGF-β bioactivity in preterm milk samples in the native state and after adding 20–40 nM rTGF-β2 by using the milk lung cell bioassay. As seen in Fig. 1, the addition of rTGF-β2 failed to increase TGF-β bioactivity in these milk samples. In contrast, the addition of rTGF-β2 to infant formula increased TGF-β bioactivity as expected (Fig. 1, inset). These differences in TGF-β bioactivity were confirmed with a second reporter cell line, the RAW 264.7 SRE reporter cells (data not shown).
Fig. 1.
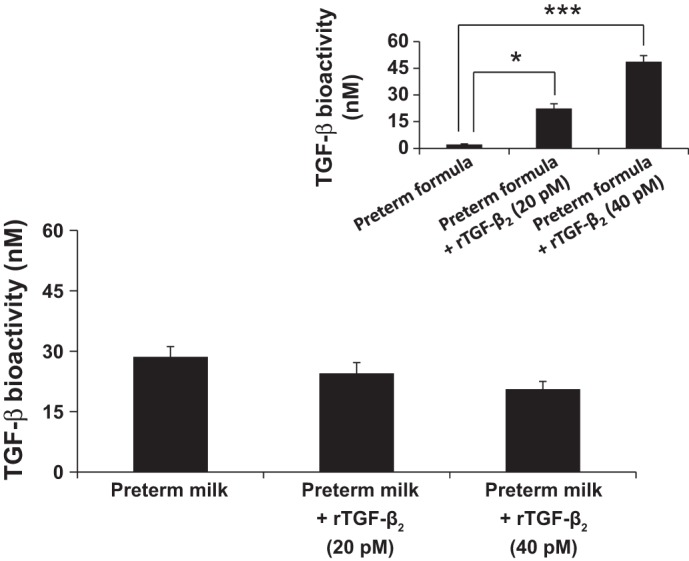
Addition of recombinant transforming growth factor-β2 isoform (TGF-β2) fails to increase TGF-β bioactivity in preterm milk. Bar diagram (means ± SE) shows TGF-β bioactivity (pM) in human preterm milk in the native state and after addition of recombinant TGF-β2 (20–40 pM). TGF-β bioactivity was measured with the mink lung epithelial (MLE) reporter cell line. Inset: bar diagram shows increased TGF-β bioactivity in preterm infant formula after addition of recombinant TGF-β2 (20–40 pM). Data represent experiments using milk samples from four different mothers. *P < 0.05, ***P < 0.001.
TGF-β2 is bound to chondroitin sulfate proteoglycans in milk.
To identify the inhibitor(s) of TGF-β activity in human preterm milk, we pulled down TGF-β2 (the predominant TGF-β isoform in milk) and resolved the immune complexes in nondenaturing gels. A prominent 110-kDa band was seen upon staining with coomassie blue or alcian blue dyes, indicating the presence of one or more proteoglycans containing acidic GAGs (6) in the immune complexes. We next probed this band by using antibodies against chondroitin sulfate and heparan sulfate, the two most abundant GAGs in human milk (4, 5). We detected strong immunoreactivity for chondroitin sulfate (Fig. 2A), but none for heparan sulfate (not depicted).
Computational analysis of chondroitin sulfate binding to TGF-β2.
We next used computational models to investigate chondroitin sulfate binding to bind to TGF-β2. The amino acid structure of TGF-β2 shows two highly electropositive regions rich in basic amino acids, which we labeled as Site 1 and Site 2, respectively (Fig. 2B). To investigate the hypothesis that polymeric chondroitin sulfate recognizes one or both of these sites, we used our computational docking protocols established for other sulfated GAGs (29, 30) and GAG-like molecules (9). In this technique, a library of GAG oligosaccharides is prepared combinatorially to ensure that all GAG structures likely to be present in nature are considered, and each oligosaccharide sequence is then docked to the target site with a genetic algorithm to identify sequences that optimally fit the binding site. In this study, we used existing information on 24 naturally occurring chondroitin sulfate oligosaccharides (13) to generate 192 possible tetrasaccharide and 1536 hexasaccharide sequences in silico and investigated their interaction with Site 1 and Site 2 by using a protein ligand docking software program (GOLD v5.1). Studies with the tetrasaccharide library identified seven chondroitin sulfate sequences with strong probability of interaction with Site 1 and eight other tetrasaccharides that consistently interacted with Site 2 (not depicted). These sequences displayed a RMSD < 2.5 Å indicating that their interactions with TGF-β2 were highly selective. Yet, detailed analysis of the binding modes of each sequence indicated that the longer chondroitin sulfate sequences may be more preferred by TGF-β2. To assess this reasoning, we evaluated the interaction of chondroitin sulfate hexasaccharide sequences with Site 1 and Site 2, where two and five sequences, respectively, were predicted to interact with a high score and RMSD < 2.5 Å. Simultaneous analysis of these sequences with LigPlot+ (17) indicated the presence of multiple hydrogen bonds in both sites, particularly with Lys25, Lys37, Glu35, and Tyr91 of Site 1 and His58, Ser80, Asn103, Lys110, and Ser112 of Site 2 (Fig. 2B). The results suggested that oversulfated chondroitin sulfate oligosaccharides, such as the 4,6-disulfated ones, are more likely to interact with TGF-β2. In addition, the orientation of binding of selected chondroitin sulfate hexasaccharides suggested a plausible mode of binding of the longer chondroitin sulfate chain that could simultaneously engage both sites. Modeling predicted that the minimum chondroitin sulfate chain length necessary to engage both sites was ∼15–16 residues. Thus computational molecular modeling predicted strong interaction between chondroitin sulfate and TGF-β2.
Chondroitin sulfate-TGF-β2 binding shows dual-interaction characteristics.
To confirm the predicted interaction between TGF-β2 and chondroitin sulfate, we added rTGF-β2 to incremental concentrations of chondroitin sulfate (5–120 nM) in a capillary electrophoresis system. TGF-β2-chondroitin sulfate interaction and consequent changes in electrophoretic mobility was detected as altered migration times. Injection of TGF-β2 into a capillary filled with chondroitin sulfate changed its electrophoretic mobility as a function of chondroitin sulfate concentrations (Fig. 2C), confirming strong molecular interaction between the two analytes. TGF-β2 migration was delayed in the 5- to 50-nM range of chondroitin sulfate concentrations and was reversed at higher concentrations. These findings supported a dual-interaction model, where TGF-β2 and chondroitin sulfate showed one specific and highly potent interaction, and a second, less specific and less potent interaction. To derive quantitative information on this system, the migration time of TGF-β2 was plotted as a function of chondroitin sulfate concentrations (Fig. 2D). The characteristic saturation of the binding profile fitted a dual-site binding equation. The equilibrium dissociation constant for the chondroitin sulfate-TGF-β2 complex was calculated to be 4.8 nM, which indicates a high-affinity interaction. Decreased migration times at higher chondroitin sulfate concentrations in this curve suggested an additional interaction that is much less specific.
Human milk contains important amounts of biglycan.
To identify the individual chondroitin sulfate proteoglycans bound to TGF-β2 in milk, we immunoprecipitated TGF-β2 and resolved the immune complexes by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In these gels, several distinct bands were seen in the 40- to 70-kDa range (Fig. 3A). Evaluating major chondroitin sulfate proteoglycans (CSPG), CSPG1-CSPG8, phosphacan, decorin, and biglycan, CSPG5, we identified decorin and biglycan to have molecular weights in this range. Because both decorin and biglycan have been shown to bind TGF-β in the extracellular matrix (ECM) (11), we again pulled down TGF-β2 from milk and sought these two proteins in milk by SDS-PAGE. Although both decorin and biglycan were detected, biglycan was clearly more abundant of the two (P < 0.01; Fig. 3B). To confirm biglycan binding to TGF-β2, we resolved the immune complexes in nondenaturing gels and were able to detect both biglycan and TGF-β2 in the 110-kDa complex (Fig. 3C). Biglycan bound both the latent (∼55 kDa) and active (12.5 kDa) forms of TGF-β2 (Fig. 3D).
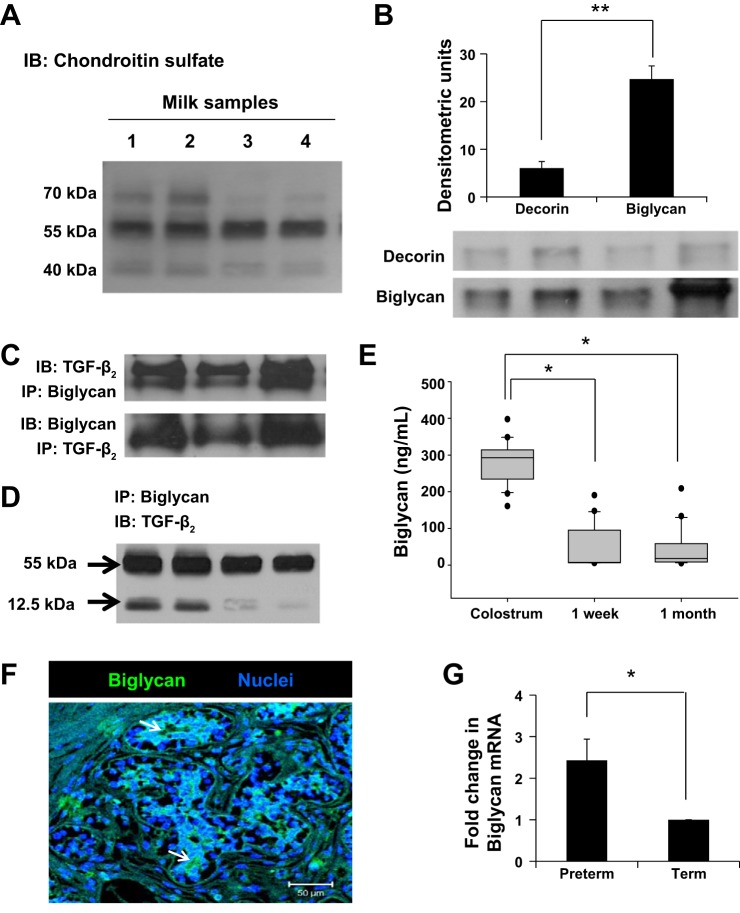
Fig. 3.
Human milk contains important amounts of biglycan. A: representative Western blots on immune complexes obtained upon immunoprecipitation of TGF-β2 from milk showed three prominent bands in the 40- to 70-kDa range. Data show milk samples from four mothers. B: representative Western blots detected chondroitin sulfate proteoglycans decorin and biglycan attached to TGF-β2 in preterm human milk. These immunoblots were performed on protein complexes obtained by immunoprecipitation of TGF-β2. Bar diagram (means ± SE) summarizes densitometric data. C: nondenaturing gels following immunoprecipitation with anti-TGF-β2 or antibiglycan antibodies confirm that biglycan was bound to TGF-β2 in milk. Data represent milk samples from three different mothers. D: representative Western blots show that biglycan binds both latent (55 kDa) and active TGF-β2 (12.5 kDa) in milk. These immunoblots were performed on protein complexes obtained by immunoprecipitation of biglycan. E: box-whisker plots show temporal change in biglycan concentrations in preterm milk. Biglycan concentrations were measured by ELISA. N = 20 mothers; each sample tested in triplicate. *P < 0.05. F: immunofluorescence photomicrograph (magnification ×250) of human mammary tissue shows biglycan immunoreactivity (green) in epithelial cells. Nuclear staining (blue) was obtained with 4′,6-diamidino-2-phenylindole (DAPI). Data represent results in tissue from three different subjects. G: biglycan mRNA expression in the cellular fraction of preterm vs. term milk, normalized against GAPDH. Bar diagram (means ± SE) shows fold changes in biglycan mRNA expression in preterm milk over term milk. N = 8 each samples; tested in triplicate. *P < 0.05, **P < 0.01.
To define the longitudinal change in biglycan expression in preterm milk as a function of time elapsed since childbirth, we measured biglycan concentrations by ELISA in colostrum at 1 wk and at 1 mo after delivery (n = 20 mothers/group). As shown in Fig. 3E, colostrum contained higher concentrations (median 292.6, range 160.76–397.72 ng/ml) than at later time points (median 7.25, range 5.6–190.49 ng/ml at 1 wk and median 17.92, range 5.04–208.83 ng/ml at 1 mo, respectively; P < 0.05). To identify the cellular source of biglycan in milk, we performed immunohistochemistry on human mammary tissue. Biglycan was immunolocalized to mammary epithelial cells (Fig. 3F) in an apical staining pattern, consistent with vectorial discharge into the mammary glands (7). We also used RT-qPCR to measure biglycan expression in the cellular fractions from preterm and term milk samples; preterm milk showed a 2.88- ± 0.58-fold higher expression of biglycan transcripts than in term milk (P < 0.05; Fig. 3G).
Chondroitin sulfate inhibits the biological activity of TGF-β.
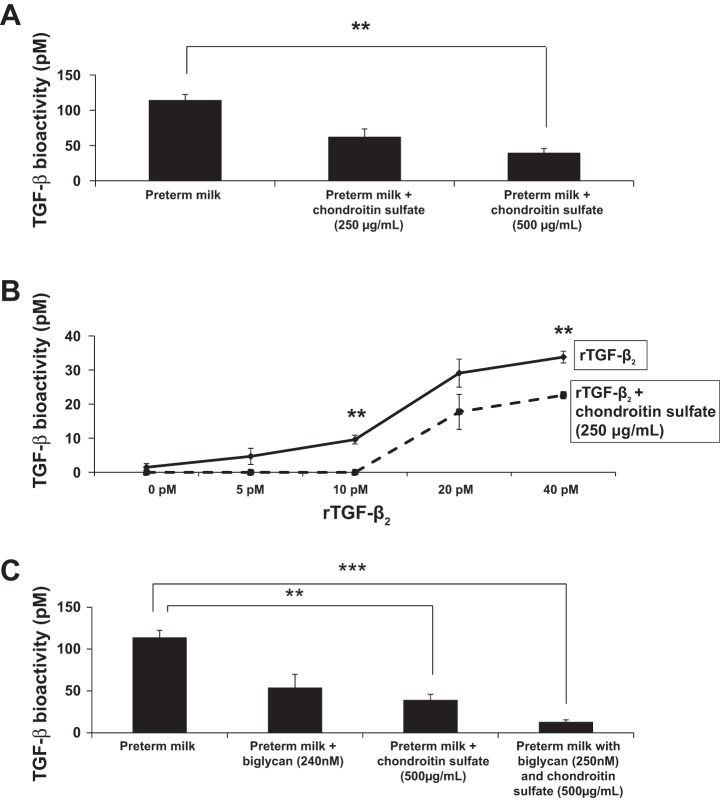
To determine the effect of chondroitin sulfate binding on the biological activity of TGF-β2 in milk, we added incremental amounts of chondroitin sulfate to human preterm milk and measured TGF-β bioactivity by using the MLE reporter cells. As shown in Fig. 4A, chondrotin sulfate inhibited the biological activity of endogenous TGF-β in a dose-dependent fashion. In another experiment, we preincubated rTGF-β2 with chondroitin sulfate in vitro (in PBS) before addition to MLE cells and detected decreased TGF-β bioactivity (Fig. 4B). The addition of recombinant biglycan (expressed in mammalian cells and, therefore, already decorated with GAGs) also inhibited TGF-β bioactivity in milk (Fig. 4C). Recombinant biglycan provided additional inhibition of TGF-β bioactivity in milk samples already “saturated” with chondroitin sulfate, indicating that the protein backbone of biglycan may also bind TGF-β2 independent of binding through chondroitin sulfate side chains (Fig. 4C).
Fig. 4.
Chondroitin sulfate inhibits TGF-β bioactivity in preterm human milk. A: chondroitin sulfate inhibited the biological activity of endogenous TGF-β in a dose-dependent fashion. Bar diagram (means ± SE) shows TGF-β bioactivity (pM) in human preterm milk measured by using the MLE reporter cells. Data represent milk samples from four different mothers. B: preincubation of rTGF-β2 with chondroitin sulfate in vitro (in phosphate-buffered saline) inhibited the biological activity of rTGF-β2. Line diagram (means ± SE) shows TGF-β bioactivity (pM; MLE cells). C: recombinant biglycan inhibited endogenous TGF-β bioactivity in milk. When added to milk samples already treated with chondroitin sulfate, recombinant biglycan provided additional inhibition of TGF-β bioactivity. Bar diagram (means ± SE) shows TGF-β bioactivity (pM) in human preterm milk from four mothers measured by using MLE cells. **P < 0.01, ***P < 0.001.
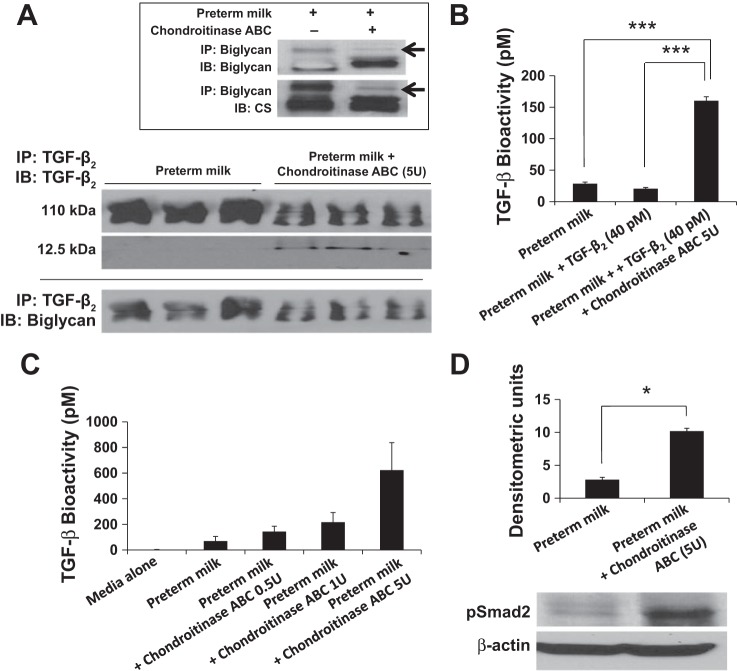
To confirm chondroitin sulfate inhibition of TGF-β bioactivity in milk, we next asked whether chondroitinase digestion of these GAGs could restore the bioactivity of TGF-β2 in milk. We pulled down TGF-β2 from control and chondroitinase-treated preterm milk samples and resolved the immune complexes in nondenaturing gels. Chondroitinase-treated milk showed decreased TGF-β2 in the biglycan-TGF-β complex (110 kDa), and a 12.5-kDa band corresponding to the active fragment of TGF-β2 was now seen (Fig. 5A). In milk samples treated with chondroitinase ABC, we detected increased electrophoretic mobility of biglycan with loss of higher molecular weight chondroitin sulfate (Fig. 5, inset), indicating that the enzyme worked as expected.
Fig. 5.
Chondroitinase ABC restores TGF-β bioactivity in preterm human milk. A: milk samples treated with chondroitinase ABC showed decreased TGF-β2 in the biglycan-TGF-β complex (110 kDa), and a 12.5-kDa band corresponding to the active fragment of TGF-β2 was now seen. Panel at bottom: the presence of biglycan in the 110-kDa complex was confirmed by probing with specific antibodies. Inset: representative Western blots show that chondroitinase ABC treatment of milk increased the electrophoretic mobility of biglycan with loss of higher molecular weight bands of chondroitin sulfate (arrow), indicating that the enzyme was functioning as expected. Data represent experiments using milk samples from four different mothers. B: TGF-β bioactivity (pM) in preterm milk, preterm milk spiked with rTGF-β2 (40 pM), and preterm milk spiked with rTGF-β2 and then treated with chondroitinase ABC (5 units). C: bar diagram (means ± SE) shows TGF-β bioactivity (pM) in preterm milk treated with chondroitinase ABC (0.5–5 units). Data represent experiments with milk from four different mothers. D: representative Western blots show that chondroitinase ABC treatment of preterm human milk increased the ability of milk to promote Smad2 phosphorylation in explanted murine neonatal intestinal tissue. Bar diagram (means ± SE) summarizes the densitometric data (normalized against β-actin). Data represent experiments with milk from three mothers, each tested in triplicate. *P < 0.05, ***P < 0.001.
Chondroitinase treatment of milk increased the bioactivity of endogenous TGF-β in a dose-dependent fashion (Fig. 5B) and also restored the bioactivity of rTGF-β2 to expected levels (Fig. 5C). To confirm the relevance of these findings in the neonatal intestine, we treated explanted intestinal tissue from P10 mouse pups with control and chondroitinase-treated preterm human milk. The neonatal intestine expresses TGF-β receptors at lower levels than the adult (26), and, therefore, it was important to confirm that the developing intestine is not already saturated for TGF-β signaling and is capable of responding to increased milk-borne TGF-β activity. As shown in Fig. 5D, chondroitinase treatment of milk increased Smad2 phosphorylation in intestinal explants treated with these milk samples, indicating that increased bioavailability of milk-borne TGF-β2 could potentially enhance TGF-β effects in the neonatal intestine.
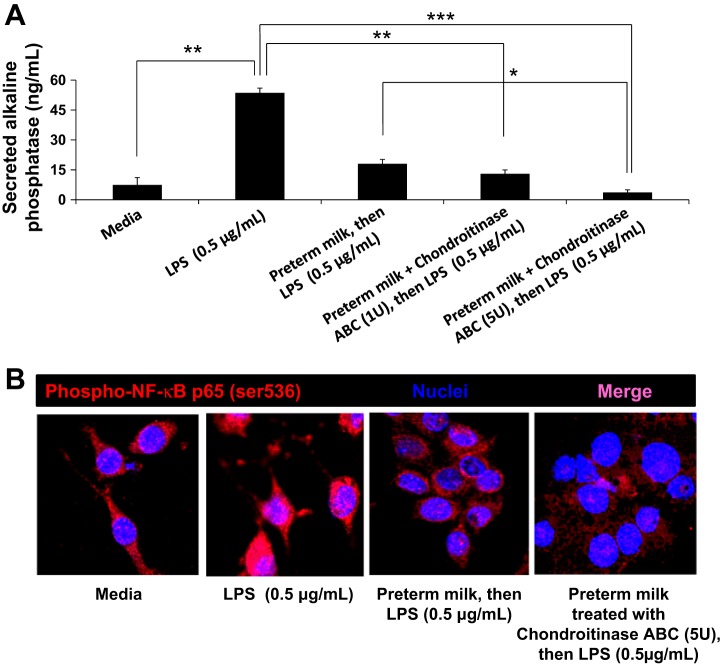
Finally, we asked whether chondroitinase-restored TGF-β bioactivity augmented the anti-inflammatory effects of milk. Because TGF-β is a potent inhibitor of macrophage inflammatory responses (22), we asked whether chondroitinase ABC treatment of milk can improve its ability to suppress NF-κB signaling in macrophages. We pretreated RAW 264.7 NF-κB/SEAP reporter cells with preterm human milk for 24 h and stimulated these cells with LPS. As depicted in Fig. 6A, addition of chondroitinase ABC (5 units) to preterm human milk increased its ability to suppress LPS-induced NF-κB activation (measured as NF-κB-driven expression of secreted embryonic alkaline phosphatase/SEAP) in a dose-dependent fashion. Further confirmation was obtained by immunocytochemistry for phosphorylated NF-κB p65 (ser 536), where treatment with preterm milk plus chondroitinse suppressed the nuclear localization of phospho-NF-κB p65 (Fig. 6B).
Fig. 6.
Chondroitinase ABC enhances the ability of preterm milk to suppress LPS-induced nuclear factor-kappa B (NF-κB) activation in macrophages. A: addition of chondroitinase ABC to preterm human milk increases its ability to suppress LPS-induced NF-κB activation in macrophages. NF-κB activation was measured in RAW264.7 NF-κB/SEAP reporter cells; bar diagram (means ± SE) shows the concentrations of secreted embryonic alkaline phosphatase (SEAP) (μg/ml) in culture supernatants. B: fluorescence photomicrographs (magnification ×630) show immunoreactivity (red) for phosphorylated NF-κB p65 (ser 536) in RAW 264.7 macrophages cultured in media alone, stimulated with LPS (0.5 μg, 1 h), treated with preterm milk overnight before LPS stimulation, and treated with preterm milk plus chondroitinse ABC (5 units) before LPS stimulation, respectively. Nuclear staining (blue) was obtained with DAPI. Data represent three separate experiments, where each sample was tested in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
This is the first study to show that TGF-β2 is sequestered in human preterm milk by chondroitin sulfate proteoglycans, and that chondroitinase treatment of milk can release TGF-β2 from this compartment to restore its bioactivity. We also show that human milk contains biologically relevant amounts of the small leucine-rich proteoglycans (SLRPs) biglycan and decorin. SLRPs can influence diverse biological processes such as hormone-receptor interaction, enzyme inhibition, cell adhesion, and cellular trafficking, and the detection of these proteins in milk raises the possibility of interesting developmental implications in the neonatal intestine (15).
Our findings of chondroitin sulfate binding to TGF-β2 in milk are consistent with existing information on TGF-β binding by sulfated GAGs and hyaluronic acid in the ECM (10, 21). Although chondroitin sulfate clearly inhibits the biological activity of TGF-β2 in milk, GAG interactions with TGF-β can be isoform specific and may also vary with the source and sulfation status of the GAGs (10, 21). TGF-β1 has been shown to bind chondroitin sulfate, heparan sulfate, and hyaluronic acid, but information on TGF-β2 and TGF-β3 remains limited (10, 20, 21). In one study, highly sulfated heparan sulfate from rat liver potentiated the effects of TGF-β1 but did not affect TGF-β2, whereas heparan sulfate GAGs with lower degrees of sulfation from the porcine gut mucosa failed to augment the biological activity of either isoform (21). In human milk, TGF-β binding to chondroitin sulfate is highly plausible because human milk is exceptionally rich in sulfated GAGs (4) and chondroitin sulfate is the most abundant GAG in human milk. Preterm milk contains even greater concentrations of GAGs, with a total GAG content up to 3–10 times that in term human milk (5). In this study, we show that TGF-β2 contains two highly electropositive regions that may bind chondroitin sulfate through the formation of multiple hydrogen bonds, particularly with Lys25, Lys37, Glu35, Tyr91 of Site 1 and His58, Ser80, Asn103, Lys110, and Ser112 of Site 2. In support of these data, we have demonstrated the biophysical interactions between chondroitin sulfate and TGF-β2 with dual-site binding characteristics, and also shown that treatment of preterm milk with chondroitinase restored the biological activity of TGF-β in milk.
We have shown for the first time that human milk contains biologically important amounts of biglycan and decorin. The core protein in both SLRPs contain several leucine-rich repeats of 20–24 amino acids (28). Decorin and biglycan are ubiquitous proteoglycans; biglycan is often localized in the immediate vicinity of cells, whereas decorin is expressed more extensively in the ECM and binds type I collagen (1, 2, 35). Similar to the effects of chondroitin sulfate GAGs, biglycan inhibited the biological activity of milk-borne TGF-β2 in our study. However, existing literature shows conflicting reports on the effects of decorin and biglycan on TGF-β activity. Both decorin and biglycan inhibit TGF-β activity in the ECM (16, 37), but may potentiate the effects of TGF-β in specific in vitro conditions, in the bone matrix, and in the fetal membranes during pregnancy (10, 34, 36). Although some of this functional variability likely emanates from differences in the GAGs decorating these proteoglycans in various organs systems, the effects of biglycan and decorin may also vary with individual TGF-β isoforms (10). For instance, chondroitin sulfate GAGs associated with decorin and biglycan may not block the biological activity of TGF-β1 (37), which contrasts with our findings of chondroitin sulfate inhibition of TGF-β2 in milk.
In our study, chondroitinase digestion of milk was effective not only in restoring the biological activity of rTGF-β2, but also activated endogenous TGF-β2 in preterm milk. We have previously shown that preterm milk contains a large pool of latent TGF-β2, which can be activated by exogenous neuraminidase (26). Our findings that milk-borne TGF-β is normally sequestered by chondroitin sulfate proteoglycans help reconcile the presence of latent TGF-β2 in milk alongside several known activators of TGF-β2 such as neuraminidase, plasmin, and matrix metalloproteinases (26). We speculate that chondroitinase treatment released TGF-β2 from the GAGs and made it accessible to these milk-borne TGF-β activators. Although we used chondroitinase ABC as a tool to investigate the contribution of chondroitin sulfate GAGs to inhibit milk-borne TGF-β, these findings raise important possibilities about the effects of chondroitinases produced by commensal bacteria present in the gut lumen. Chondroitinases as widely expressed in nature by a wide range of bacteria, including those present in the intestine such as Aeromonas, Vibrio, Flavobacteria, Proteus, Micrococcus, and Bacteroides (14).
In conclusion, we have shown that milk-borne TGF-β2 is normally sequestered by chondroitin sulfate GAGs associated with proteoglycans such as biglycan, and identified chondroitinase treatment as a potential strategy to enhance TGF-β bioactivity in milk. This study has limitations of small sample size and paucity of clinical/demographic information from mothers who donated milk samples. Our study was also limited to the aqueous fraction of milk and did not explore the fat compartment, which may contain important amounts of TGF-β2 (23). However, a major strength of this study is the potential for knowledge translation. Because of neurological immaturity, premature infants born prior to 32 wk of gestation frequently receive expressed breast milk by gavage, and fortification of mother's milk to increase caloric density, protein content, and the amount of minerals in milk is a common practice. In this context, the possibility of using a bacterial/recombinant chondroitinase as a prefeed fortification agent to activate milk-borne TGF-β in either mother's own milk or commercially available human milk-derived fortifiers to enhance the anti-inflammatory effects raises exciting therapeutic possibilities (32). These options may also be valid when mother's own milk is not available, and pasteurized and fortified donor human milk is considered as a substitute. However, there is a need for further study to evaluate the effects of storage and processing on chondroitin sulfate concentrations and the bioavailability of TGF-β in these samples.
GRANTS
This work was supported by National Institutes of Health Grants R01 HD-059142 (to A. Maheshwari) and P01 HL-107152 (to U. Desai), the Robert Wood Johnson Foundation Research Grant 67067, UT Health Science Center at San Antonio–Clinical & Translational Science Award UL1RR025767, the American Diabetes Association Grant ID7-11-BS13 (to C. Blanco), and a grant from the Gerber Foundation (to B. Frost).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.N., U.R.D., and A.M. conception and design of research; K.N., H.P.C., N.V.S., Y.J., K.M., B.L.F., C.L.B., A.L.P., P.P.M., and S.A.G. performed experiments; K.N., H.P.C., N.V.S., Y.J., K.M., S.A.G., U.R.D., and A.M. analyzed data; K.N., N.V.S., Y.J., K.M., B.L.F., C.L.B., A.L.P., P.P.M., S.A.G., U.R.D., and A.M. interpreted results of experiments; K.N., U.R.D., and A.M. prepared figures; K.N. and A.M. drafted manuscript; K.N., H.P.C., N.V.S., Y.J., K.M., B.L.F., C.L.B., A.L.P., P.P.M., S.A.G., U.R.D., and A.M. edited and revised manuscript; K.N., H.P.C., N.V.S., Y.J., K.M., B.L.F., C.L.B., A.L.P., P.P.M., S.A.G., U.R.D., and A.M. approved final version of manuscript.
REFERENCES
- 1.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem 38: 1549–1563, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix 9: 468–478, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Brunelle JL, Green R. Coomassie blue staining. Methods Enzymol 541: 161–167, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Coppa GV, Gabrielli O, Buzzega D, Zampini L, Galeazzi T, Maccari F, Bertino E, Volpi N. Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology 21: 295–303, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Maccari F, Buzzega D, Galeotti F, Bertino E, Volpi N. Glycosaminoglycan content in term and preterm milk during the first month of lactation. Neonatology 101: 74–76, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Cowman MK, Slahetka MF, Hittner DM, Kim J, Forino M, Gadelrab G. Polyacrylamide-gel electrophoresis and alcian blue staining of sulphated glycosaminoglycan oligosaccharides. Biochem J 221: 707–716, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke WW, Heid HW, Grund C, Winter S, Freudenstein C, Schmid E, Jarasch ED, Keenan TW. Antibodies to the major insoluble milk fat globule membrane-associated protein: specific location in apical regions of lactating epithelial cells. J Cell Biol 89: 485–494, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkes JS, Bryan DL, James MJ, Gibson RA. Cytokines (IL-1beta, IL-6, TNF-alpha, TGF-beta1, and TGF-beta2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res 46: 194–199, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Henry BL, Connell J, Liang A, Krishnasamy C, Desai UR. Interaction of antithrombin with sulfated, low molecular weight lignins: opportunities for potent, selective modulation of antithrombin function. J Biol Chem 284: 20897–20908, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302 (Pt 2): 527–534, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-beta function. J Biochem 152: 321–329, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalliomaki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-beta in breast milk: a potential regulator of atopic disease at an early age. J Allergy Clin Immunol 104: 1251–1257, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Karamanos NK, Syrokou A, Vanky P, Nurminen M, Hjerpe A. Determination of 24 variously sulfated galactosaminoglycan- and hyaluronan-derived disaccharides by high-performance liquid chromatography. Anal Biochem 221: 189–199, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Kitamikado M, Lee YZ. Chondroitinase-producing bacteria in natural habitats. Appl Microbiol 29: 414–421, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11: 725–732, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol 280: L1327–L1334, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51: 2778–2786, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Lebman DA, Edmiston JS. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect 1: 1297–1304, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science 264: 1936–1938, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Locci P, Marinucci L, Lilli C, Martinese D, Becchetti E. Transforming growth factor beta 1-hyaluronic acid interaction. Cell Tissue Res 281: 317–324, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem 272: 18000–18006, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. TGF-beta(2) Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. Gastroenterology 140: 242–253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson RJ, Wagner CL. TGFβ2 distribution in aqueous and fat compartments of human milk. Pediatr Res 41: 85-85, 1997. [Google Scholar]
- 24.Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, Hayes DF, Hainaut P, Kim P, Mansfield E, Potapova O, Riegman P, Rubinstein Y, Seijo E, Somiari S, Watson P, Weier HU, Zhu C, Vaught J. Biospecimen reporting for improved study quality (BRISQ). J Proteome Res 10: 3429–3438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulloy B. Progress in the structural biology of chondroitin sulfate. Adv Pharmacol 53: 49–67, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Namachivayam K, Blanco CL, Frost BL, Reeves AA, Jagadeeswaran R, MohanKumar K, Safarulla A, Mandal P, Garzon SA, Raj JU, Maheshwari A. Preterm human milk contains a large pool of latent TGF-beta, which can be activated by exogenous neuraminidase. Am J Physiol Gastrointest Liver Physiol 304: G1055–G1065, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namachivayam K, Blanco CL, MohanKumar K, Jagadeeswaran R, Vasquez M, McGill-Vargas L, Garzon SA, Jain SK, Gill RK, Freitag NE, Weitkamp JH, Seidner SR, Maheshwari A. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 304: G167–G180, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patthy L. Detecting homology of distantly related proteins with consensus sequences. J Mol Biol 198: 567–577, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Raghuraman A, Mosier PD, Desai UR. Finding a needle in a haystack: development of a combinatorial virtual screening approach for identifying high specificity heparin/heparan sulfate sequence(s). J Med Chem 49: 3553–3562, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghuraman A, Mosier PD, Desai UR. Understanding dermatan sulfate-heparin cofactor II interaction through virtual library screening. ACS Med Chem Lett 1: 281–285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rautava S, Nanthakumar NN, Dubert-Ferrandon A, Lu L, Rautava J, Walker WA. Breast milk-transforming growth factor-beta(2) specifically attenuates IL-1beta-induced inflammatory responses in the immature human intestine via an SMAD6- and ERK-dependent mechanism. Neonatology 99: 192–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves AA, Johnson MC, Vasquez MM, Maheshwari A, Blanco CL. TGF-β2, a protective intestinal cytokine, is abundant in maternal human milk and human-derived fortifiers but not in donor human milk. Breastfeed Med 8: 496–502, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaik SS, Soltau TD, Chaturvedi G, Totapally B, Hagood JS, Andrews WW, Athar M, Voitenok NN, Killingsworth CR, Patel RP, Fallon MB, Maheshwari A. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38{beta} MAPK-NF-{kappa}B pathway. J Biol Chem 284: 5945–5955, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J Biol Chem 269: 32634–32638, 1994. [PubMed] [Google Scholar]
- 35.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223: 587–597, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346: 281–284, 1990. [DOI] [PubMed] [Google Scholar]