Abstract
Loss of significant intestinal length from congenital anomaly or disease may lead to short bowel syndrome (SBS); intestinal failure may be partially offset by a gain in epithelial surface area, termed adaptation. Current in vivo models of SBS are costly and technically challenging. Operative times and survival rates have slowed extension to transgenic models. We created a new reproducible in vivo model of SBS in zebrafish, a tractable vertebrate model, to facilitate investigation of the mechanisms of intestinal adaptation. Proximal intestinal diversion at segment 1 (S1, equivalent to jejunum) was performed in adult male zebrafish. SBS fish emptied distal intestinal contents via stoma as in the human disease. After 2 wk, S1 was dilated compared with controls and villus ridges had increased complexity, contributing to greater villus epithelial perimeter. The number of intervillus pockets, the intestinal stem cell zone of the zebrafish increased and contained a higher number of bromodeoxyuridine (BrdU)-labeled cells after 2 wk of SBS. Egf receptor and a subset of its ligands, also drivers of adaptation, were upregulated in SBS fish. Igf has been reported as a driver of intestinal adaptation in other animal models, and SBS fish exposed to a pharmacological inhibitor of the Igf receptor failed to demonstrate signs of intestinal adaptation, such as increased inner epithelial perimeter and BrdU incorporation. We describe a technically feasible model of human SBS in the zebrafish, a faster and less expensive tool to investigate intestinal stem cell plasticity as well as the mechanisms that drive intestinal adaptation.
Keywords: intestinal stem cell, adaptation, short bowel syndrome, intestinal failure, Egf receptor, Igf
severe reduction in intestinal length results in profound malabsorption leading to malnutrition, dehydration, failure to thrive, and death (3). This condition, known as short bowel syndrome (SBS), is a leading cause of intestinal failure. The most common etiologies in children are midgut volvulus, gastroschisis, and necrotizing enterocolitis. However, this condition also affects adult patients suffering from inflammatory bowel disease and cancer, among other rare diagnoses (25, 44). Current treatments are inadequate and associated with significant morbidity: the five-year mortality rate is greater than 30% (40, 48). Long-term supplementation with intravenous nutrition (total parenteral nutrition, TPN) is associated with hepatic failure, sepsis, and death (34). Intestinal transplant is currently the treatment of choice but is associated with lifelong immunosuppression, rejection, infection, and donor scarcity and is practiced only in specialized centers. The 2011 annual data report by the scientific registry of transplant recipients lists intestinal transplant 5-year survival at just over 60% and 5-year graft survival at 56% (14). A 2006 review of 141 intestinal transplants in 123 children revealed a 1-year patient survival between 44 and 83% and a 3-year survival between 32 and 60% (20). The yearly cost of intravenous nutrition is as high as $390,000. Approximately 20,000 patients in this country are on home TPN. A reduction of just 10% would result in estimated savings of $780,000,000 (27).
The high morbidity and mortality of SBS are partially offset by intestinal adaptation, a poorly understood response in which the epithelial surface area is expanded. This is a critical response for human patients to enhance the absorption of enteral nutrition and to reduce the need for TPN, but in many patients adaptation is inadequate. In vivo modeling of adaptation is described for mice and larger mammals (45). These models involve massive small bowel resection and are complicated, time consuming, and associated with low survival and high cost. Long-term end points are difficult to achieve. For these reasons, adaptation is still poorly understood. An improved model of intestinal adaptation would allow the identification of key factors for future human therapies.
Zebrafish (Danio rerio) is an ideal organism for the study of vertebrate intestine and is equivalent to the mouse with regards to precise genome modification (58). Zebrafish intestine is subdivided into seven segments of equal length that are strikingly similar to mammalian correlates. The proximal five subdivisions (S1–S5) are homologous to the regions of human small intestine and express genes related to metabolism of fatty acid, organic acid, lipid, vitamins, and carbohydrates as well as genes associated with the hydrolase and transferase activity of absorption. The distal segments (S6, S7) demonstrate ion transport and fecal dehydration as in mammalian colon and rectum (51). In contrast to mammalian intestine, zebrafish lack a stomach and the defensin-secreting Paneth cell (29, 49). The zebrafish also lacks the fingerlike projections and microvilli of mammalian villi. Instead, S1–S5 contain wide folds called villus ridges that are composed of enterocytes, goblet cells, and enteroendocrine cells in similar ratios to human mucosa. The zebrafish intestinal stem cells are found in the valleys between villus ridges, known as the intervillus pockets (49).
To date, few techniques have been described for the manipulation or response to injury of the adult zebrafish. Success with tail fin amputation, retroorbital injections, pancreatectomy, and injection of cells directly into the kidney via lateral incision have been accomplished (1, 7, 28, 37). However, survival surgery following resection and diversion of the adult intestine has not been reported. Our method is novel in modeling human SBS and as a proof of the feasibility of adult zebrafish intraperitoneal surgery for investigation of a human disease that demands improved therapies.
MATERIALS AND METHODS
The Children's Hospital Los Angeles Animal care facility and IACUC approved all protocols.
Generation of short bowel syndrome.
One-year-old, male zebrafish (wild type, Ekk) were obtained for surgery. Each fish was anesthetized for 2–5 min in 0.02% tricaine until swimming motions ceased and opercular movements became slow and regular. The anesthetized fish was then placed supine (dorsal fin down) on a moistened operating sponge under a stereomicroscope (Olympus SZX9), and the surgery was performed as described in results. Operative times were recorded. Fish were maintained individually as needed.
Determination of weight.
Sham control (n = 43) and SBS (n = 65) fish were anesthetized, patted dry, and placed on the balance. Weights were recorded weekly until euthanasia. Fish were euthanized in 0.02% tricaine for a period of 10 min after opercular movements stopped. Eighteen surviving sham and 24 SBS fish were euthanized at 2 wk. An additional cohort of sham (n = 13) and SBS (n = 13) fish were euthanized at 4 wk. Weights (mg) were compared as a percentage of preoperative weight. The change in body weight was expressed as percentage of preoperative weight ± SE.
Villus epithelial perimeter determination.
At 2 and 4 wk, the fish were euthanized. Under a dissecting microscope, the peritoneal cavity was entered and the proximal segment was harvested. The tissue was fixed in buffered formalin for 24 h and embedded in paraffin. Paraffin blocks were sectioned at 5 μm and stained with hematoxylin and eosin. Villus epithelial perimeter was measured manually by outlining the luminal surface of the epithelium from one junction of the villus with the intervillus pocket to the next. Quantifications were carried out with ImageJ software (NIH.gov) by a trained, blinded observer.
Micro-CT of zebrafish intestine.
One week postoperatively, sham (n = 3) and SBS (n = 3) fish were placed in a tank consisting of 400 ml of system water and 100 ml of Omnipaque 300 (Iohexol/Omnipaque 300, GE Healthcare) The fish were allowed to swim in the Omnipaque mixture for 7 days. The fish were euthanized in 0.02% tricaine and placed in a solution consisting of 10 mg strychnine (Sigma) and 1 mg dantrolene (Sigma) in 100 ml system water. After 15 min in solution, the fish were immediately imaged by using a Skyscan 1,172 micro-CT (Bruker). The optimal settings were determined to be 36 kVP, 161 μAmp, 0.5° steps for 360°, and two averages with an aluminum filter at a resolution of 2.07 μm. The fish were scanned for 90 min. The Bruker Skycan 1172 software was used for reconstruction, by a Feldkamp-type algorithm running on an array of processors supplied by Bruker. After assessment of images, 3D reconstruction was performed with Imiris (Imiris), Amira (FEI) and Lightwave (Newtek) software running on dedicated Windows-based workstations.
Determination of BrdU incorporation.
At 2 wk, sham (n = 9) and SBS (n = 14) fish underwent intraperitoneal injection with 30 μl of 2.5 mg/ml bromodeoxyuridine (BrdU) (Sigma Life Sciences B5002-1G) and were euthanized after 4 h. At 4 wk, sham (n = 13) and SBS (n = 13) were similarly injected. S1 was harvested, fixed in formalin, and paraffin embedded. Antigen retrieval was performed by boiling the slides for 12 min in 10 mM Na-citrate (pH 6.0). Samples were incubated overnight at 4°C with primary antibody anti-BrdU (1/100, BD Bioscience). Slides were washed and incubated with secondary antibody Cy3 goat anti-mouse IgG (1/200, Life Technologies), then counterstained with …4,6-diamidino-2-phenylindole (DAPI; Vector), mounted with ProLong Gold (Life Technologies), and imaged under an upright immunofluorescent microscope. All nuclei positive for BrdU incorporation were counted per hemi-intervillus pocket to the tip of the villus ridge and represented as a percentage of all DAPI-stained epithelial cell nuclei. All areas with a complete intervillus pocket and villus ridge were included for analysis. Counts were performed on sham and SBS fish by a single trained, blinded observer to prevent interobserver variations. Data were expressed as a percentage of BrdU-positive cells per total epithelial cells ± SE (significance, P < 0.05).
Igf1r inhibition experiment.
To determine whether the inhibition of Igf1r reduced intestinal adaptation, sham and SBS fish were exposed to 5 mM concentration of the Igf1r inhibitor NVP-AEW541 (sham n = 21, SBS n = 17) (10) (Cayman Chemical 13641) or vehicle control DMSO (sham n = 18, SBS n = 14) (Sigma Life Sciences D2650) administered in system water and refreshed every 48 h for 2 wk following surgery. Intraperitoneal BrdU (Sigma Life Sciences B5002-1G) was injected 4 h prior to euthanasia. S1 was collected for histology and quantitative PCR to determine the relative expression of the ligands igf1a, igf1b, igf2a, and igf2b, as well as the receptor igf1r. Based on hematoxylin and eosin staining on complete cross-sectional samples, the inner epithelial perimeter and circumference were measured manually by outlining the luminal surface of the epithelium and the outer serosal portion of the sample, respectively, with ImageJ by a trained, blinded observer.
Determination of Alcian blue quantification.
Slides were stained with Alcian blue and counterstained with Nuclear fast red [0.2 g Nuclear fast red (Kernechtrot), 10 g of aluminum sulfate (Sigma-Aldrich), 100 ml deionized water]. A single trained, blinded observer counted the total number of nuclei and the number of Alcian blue-positive cells per hemi-intervillus pocket to the tip of the villus ridge within a complete cross section of S1 for each fish. Data were expressed as means ± SE (significance, P < 0.05).
Quantitative real-time PCR.
In the Egf PCR, without Igf1r inhibitor, S1 segments were resected from sham (n = 6) and SBS (n = 7) fish at 2 wk postsurgery. In the Igf experiment, S1 segments were resected from sham (n = 9) and SBS (n = 19) fish at 2 wk postsurgery. The RNA was extracted by using Qiagen RNeasy mini kit. RNA concentration was determined by use of NanoDrop (Thermo Scientific). RNA integrity was determined on a bioanalyzer (Agilent technologies). All samples had a RNA integrity number of at least 8.1. One microgram of RNA was reverse-transcribed to cDNA and quantitative PCR (qPCR) was carried out with Roche LightCycler 480 reagents by using SYBR Green and the following primers: egfra F: 5′GAACAAGGCGTAAAAGAGTTGC, R: 5′GTCCCCACGTTACATAAATGGT; egf F: 5′TCTTACTTCTGCACCTGTCCTG, R: 5′ATGATCACAATCCACAGCTTTC; btc F: 5′CCCAGCGAATAGGACTGTGT, R: 5′TTTGGACAGGCAGAGAAGTGT; hbegfa F: 5′GATGATGATGTTGAAGAAGACGAG, R: 5′ACTTGGGTCTTTGGGTTGACT; hbegfb F: 5′ATTTCTGCATTCATGGAGTGTG, R: 5′GCAGGGTGAATACGTGACATCT; igf1a: F: 5′GGGCATTGGTGTGATGTCTT, R: 5′CCAGTGAGAGGGTGTGGGTA; igf1b: F: 5′CCAAAATCCTTAATGAGTAACTTAGCA, R: 5′AGACATTTTCAACAGGAAACAGC; igf2a: F: 5′TGAAGTCGGAGCGAGATGTT, R: 5′GGAGTACTTCACATTTATGGTGTCC; igf2b: F: 5′AGCTGGTGGACGCTCTACA, R: 5′GAGAACGTCGACTGTTTGACC; igf1r: F: 5′GCAACCTGCAAATCAACATC, R: 5′CTGGATCAGCCCCATGAA; ef1a (housekeeping): F: 5′CCTCTTTCTGTTACCTGGCAA, R: 5′CTTTTCCTTTCCCATGATTGA.
For all samples, the expression level was determined by using Roche LightCycler 480 software release 1.5.0. Each PCR reaction was run in triplicate, and single outliers that occurred in the technical replicates were removed for quantification. Relative expression of the genes investigated compared with the housekeeping gene ef1a was determined by 2−ΔΔCT algorithm. All the samples were normalized to the resected S2 segment.
Statistical analyses.
Quantifications were performed on sham and SBS fish by a single trained, blinded observer to prevent interobserver variations. Cell proliferation was expressed as a percentage of BrdU-positive cells per total epithelial cells per hemivillus ± SE. Goblet cell quantification was expressed as a percentage of Alcian blue-positive cells per total nuclei per hemivillus ± SE. Outliers were determined by the ROUT method with the value of Q set to 0.5%. Statistical significance was determined by one-way ANOVA or Student's t-test in Prism software.
RESULTS
Intestinal resection in the zebrafish model is fast and technically straightforward.
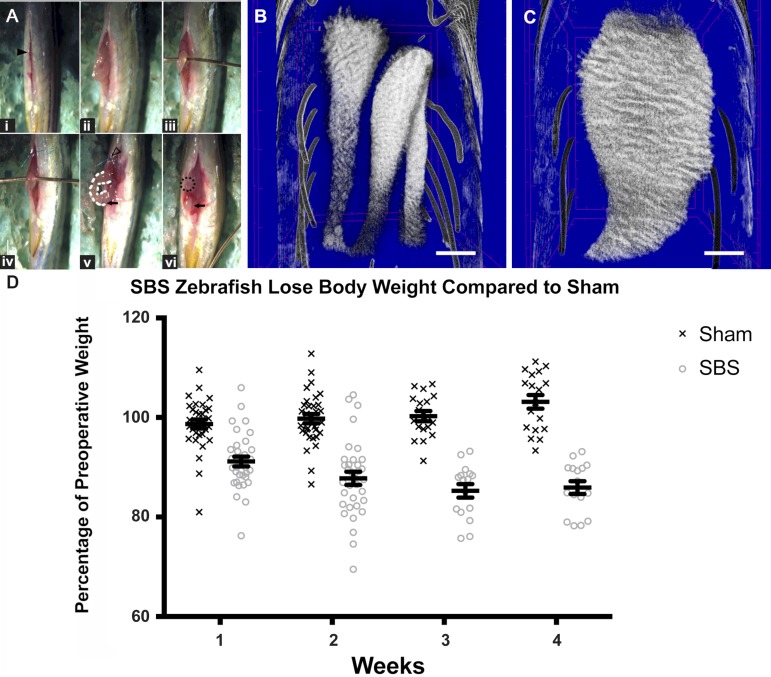
On average, the SBS procedure took 329.6 s (5.5 min) vs. 17.6 s for sham. The procedure is presented step by step in Supplementary Video S1 (Supplemental Material for this article is available online at the Journal website). Each fish was anesthetized in 0.02% tricaine diluted in system water for ∼3 min until fin motions ceased. The fish was then placed supine (dorsal fin down) on a moist operating sponge beneath a stereomicroscope. The peritoneal cavity was entered with microscissors through a 3-mm midline incision (laparotomy) started immediately cephalad to the origin of the anal fins (Fig. 1Ai; Supplemental Material for this article is available online at the Journal website). The liver overlying the intestine was gently swept cephalad and the segment 2 (S2) loop of intestine was grasped with microforceps (Fig. 1Aii). A 22-gauge bare copper wire was inserted underneath S2 to prevent reduction of intestine back into the abdomen (Fig. 1Aiii). The proximal intestine was sutured to the body wall with a single 10-0 monofilament polypropylene suture (Fig. 1Aiv) and the distal intestine was suture ligated (Fig. 1Av). The close anatomic proximity of the swim bladder to the distal intestine precludes complete resection of the distal intestine. S2 and S3 were excised, leaving behind the shortened proximal segment 1 (S1) as a functional proximal ostomy. The distal segment was reduced back into the abdomen (Fig. 1Avi). Sham operation consisted of laparotomy alone with no intestinal manipulation (Fig. 1Ai). The ventral laparotomy incision did not require surgical closure and healed around the open stoma (Fig. 2, A–C). Of note, in the subsequent inhibitor experiments, the incision did not heal in the sham or SBS groups by 2 wk, demonstrating the global inhibitor effect, but survival was not affected. Survival rates in these untreated groups were 90.7% for sham (N = 39/43) and 66.2% for SBS (N = 43/65), with deaths predominantly in the first week coincident with rapid weight loss. In an additional control group to verify that this is not a model of intestinal obstruction, the reversed surgery was performed in 10 fish: a proximal ligation and distal stoma. In these zebrafish, we caused complete obstruction of the intestine. We performed postmortem examinations on all zebrafish. Most zebrafish that are obstructed as a result of a proximal ligation die early in the postoperative period. These animals were all confirmed to be obstructed, with hugely dilated intestines (larger than in the SBS model at the same time point) and no fistula or connection to the distal bowel. In two animals that did survive complete obstruction through proximal ligation, a fistula that had formed to the distal bowel was identified. Obstruction and therefore elevated proximal pressure is a known risk factor for fistula formation. We do not identify fistula forming in the SBS model, which further validates that there is egress of intestinal contents via the stoma, and therefore nonpathological pressures in the proximal intestine.
Fig. 1.
Zebrafish with short bowel syndrome (SBS) lose significantly more weight compared with sham-operated fish. A: SBS operation. i: Ventral laparotomy incision (solid black arrowhead). The head of the fish is located at the top of the image, while the tail is oriented at the bottom. ii: segments 2 and 3 (S2 and S3) are delivered through the incision. iii: A 22-gauge copper wire inserted beneath the bowel loop prevents the intestine from retracting back into the abdomen. iv: One wall of the proximal intestine is sutured to skin with a blue stitch. v: The proximal intestine is held in place along the abdominal wall by the suture (hollow arrowhead). The midportion of intestine (white dotted line) is resected and the distal intestine is suture ligated (black arrow). vi: The distal intestine (black arrow) is reduced into the abdomen leaving behind proximal S1 as an ostomy (dotted circle). B: micro-CT intestine following sham operation with proximal intestine at the top and distal intestine at the bottom of the image. Sham intestine is intact with normal caliber. C: micro-CT of intestine 2 wk after SBS surgery. The ostomy is present at the bottom of the image with the proximal intestine profoundly dilated. Scale bar = 500 μm. D: percentages of preoperative weight were plotted at each weekly time point with the surviving fish euthanized at 2 and 4 wk. Zebrafish with SBS lose a significant amount of weight compared with sham-operated fish. Error bars indicate SE.
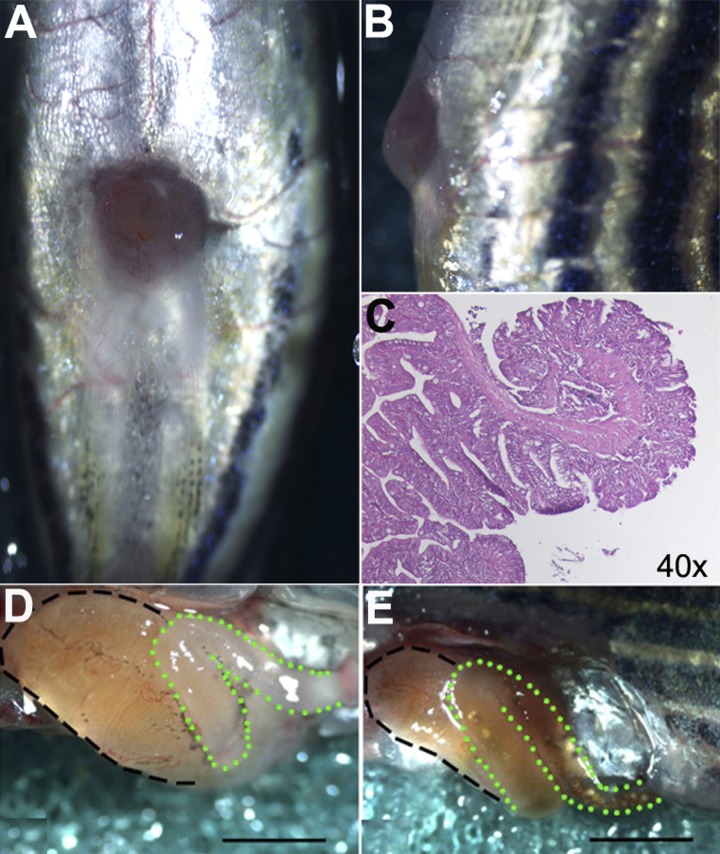
Fig. 2.
SBS fish demonstrate open and functional stomas and dilated proximal intestine at the time of euthanasia. A: ventral view demonstrates a patent ostomy and healed midline incision. B: stoma protrudes past the level of the skin on lateral view. C: hematoxylin and eosin stain on sagittal sectioning demonstrates the epithelial edge curling outward toward the skin. D: SBS fish demonstrate enlargement of the proximal intestinal segment S1 (black dashed line). The remaining distal intestine (green dotted line) is devoid of luminal content and feces, indicating that the distal intestine remains ligated and nonfunctional. E: sham-operation fish demonstrate a normal-appearing proximal segment (black dashed line) in continuity with distal intestine (green dotted line) evidenced by luminal content and feces from proximal segment to anus. Scale bar = 1 mm.
Response to intestinal truncation in zebrafish short bowel surgery is similar to that in humans.
At 2 wk following SBS surgery, the S1 segment becomes significantly more dilated in SBS (Fig. 1C) compared with sham (Fig. 1B) fish. In the 2–4 wk following surgery, the fish were weighed each week and monitored for loss of body weight, a common indication of malabsorption and malnutrition related to SBS. The change in body weight was expressed as percentage of the preoperative body weight ± SE. At 2 wk, SBS fish weighed 87.77 ± 1.33% of preoperative body weight compared with sham fish, which weighed 99.76 ± 0.94% of preoperative body wt (P < 0.01) (Fig. 1D). Following a period of 2 wk, the majority of fish were euthanized and dissected for morphological evaluation of the proximal intestine. Another large cohort of zebrafish were followed and euthanized at 4 wk. Whereas the sham fish gained weight over the 4-wk period, SBS fish continued to lose weight, reaching a nadir of 85.91 ± 1.28% preoperative body weight compared with sham fish that weighed an average of 103.14 ± 1.38% body wt (P < 0.01) (Fig. 1D). The remnant proximal intestine, or ostomy, at the ventral surface of the skin of the experimental group of fish was visible and patent as seen grossly and on histological cross section (Fig. 2, A–C).
Micro-CT was performed to allow for better visualization of the intestinal lumen with the sham fish having completely intact native intestine with normal caliber (Fig. 1B), whereas the SBS fish demonstrated profoundly dilated proximal intestine with an end stoma (Fig. 1C). Further evaluation of the SBS surgery model included three-dimensional reconstructions of the sham and SBS Micro-CT with renderings at various angles included in Supplemental Videos S2 and S3, respectively.
Following dissection of the abdominal cavity at 2 wk, S1 of the SBS fish was considerably hypertrophied and dilated compared with the controls. In contrast, the distal bowel in SBS fish was small in size and had no visible luminal content (Fig. 2D). In addition to the proximal segment dilation, lack of luminal content in the distal remnant intestine confirmed that the intestine had not reconnected, or formed a fistula, following the operation. All SBS fish demonstrated this proximal dilation and remnant decompression at the time of dissection. The intestine of sham fish remained normal in caliber with food content visible throughout (Fig. 2E).
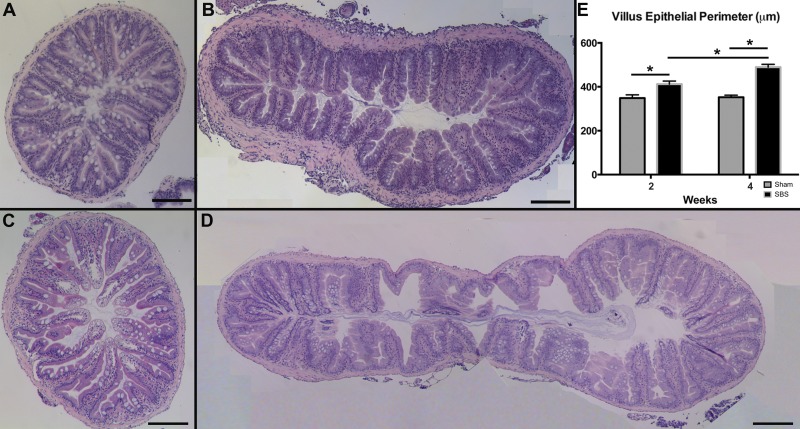
Hematoxylin and eosin staining demonstrated increased complexity of each villus ridge in SBS compared with sham (Fig. 3, A–D), contributing to a greater villus epithelial perimeter (Fig. 3E). At 2 wk, the villus epithelial perimeter was significantly longer in the SBS group compared with sham (412.36 ± 13.76 vs. 348.85 ± 14.64 μm; P < 0.01, Fig. 3E). Similarly, the inner epithelial perimeter was significantly longer in the SBS group compared with sham (11,919.68 ± 1,899.65 vs. 7,146.05 ± 369.88 μm; P < 0.01). At 4 wk, the villus epithelial perimeter, the inner epithelial perimeter, and the overall circumference of the proximal intestine were significantly increased in the SBS compared with sham fish. Each individual villus was also more complex in the SBS fish at 4 wk with a mean villus epithelial perimeter of 486.40 ± 12.10 μm compared with 352.36 ± 9.50 μm in sham fish (P < 0.01) (Fig. 3E). Similarly, the inner epithelial perimeter was also significantly greater in SBS fish compared with sham fish, with means of 13,265.39 ± 1,429.89 vs. 8,337.63 ± 338.313 μm, respectively (P < 0.01). The mean circumference of the proximal intestine was 4,038.98 ± 255.492 μm for SBS fish vs. 2,719.66 ± 118.971 μm in sham fish (P < 0.01).
Fig. 3.
The villus epithelial perimeter is significantly increased in SBS fish, suggesting increased adaptation. The sham fish at 2 wk (A) and 4 wk (C) postoperatively vs. SBS fish at 2 wk (B) and 4 wk (D) postoperatively. S1 segments were harvested and stained with hematoxylin and eosin, and composite ×10 images were tiled for a complete cross section. E: quantification of villus epithelial perimeter (μm) of the sham and SBS groups at 2 and 4 wk. Results are reported as means ± SE. Scale bar = 200 μm. *P < 0.01.
Increased proliferation in the stem cell zone following intestinal resection is associated with increased villus epithelial perimeter.
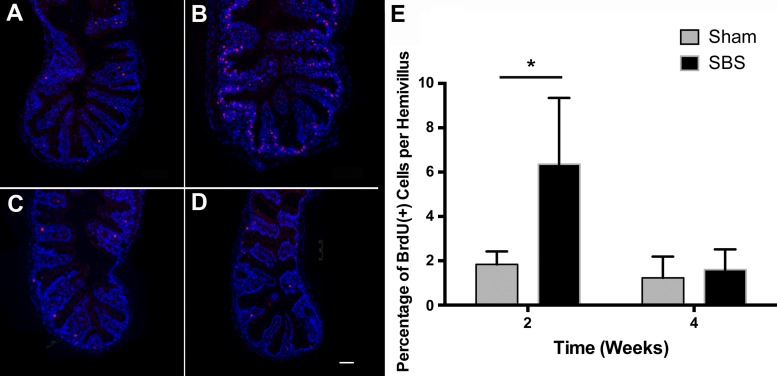
The intervillus pocket is the intestinal stem cell zone of the zebrafish, corresponding to the human intestinal crypt (49). Severely decreased intestinal length available to absorb nutrition through the creation of a proximal stoma led to a significant increase in the villus epithelial perimeter of the proximal intestine. To investigate whether there was an increase in the number of proliferating cells, epithelial cells positive for BrdU incorporation, injected 4 h prior to harvest, were quantified as a percentage of total epithelial cells along the hemi-intervillus pocket to tip of villus ridge axis. The percent of BrdU-positive epithelial cells was tripled in SBS (Fig. 4B) compared with sham (Fig. 4A) fish at 2 wk (6.36 ± 0.80 vs. 1.84 ± 0.19%; P < 0.01, Fig. 4E) and localized predominantly to the intervillus stem cell zone. The percent of BrdU-positive epithelial cells did not remain increased at the 4-wk time point. At 4 wk, there was no statistically significant difference in BrdU-positive cells per hemivillus in sham (Fig. 4C) compared with SBS fish (Fig. 4D) (1.60 ± 0.26 vs. 1.23 ± 0.27%; P = 0.34, Fig. 4E).
Fig. 4.
Cell proliferation increased 2 wk after SBS. A–D: immunofluorescence (IF) staining showing bromodeoxyuridine (BrdU)-labeled cells (in red) in sham fish at 2 wk (A) and 4 wk (C) postoperative vs. SBS fish at 2 wk (B) and 4 wk (D) postoperative. E: quantification of BrdU-positive cells as a percentage of total epithelial cells per hemivillus ridge at 2 wk and 4 wk postoperative. Results are reported as means ± SE. Scale bar = 50 μm. *P < 0.01.
Intestinal adaptation following short bowel resection is associated with increased mRNA expression of epidermal growth factor receptor, as well as its ligands epidermal growth factor and betacellulin.
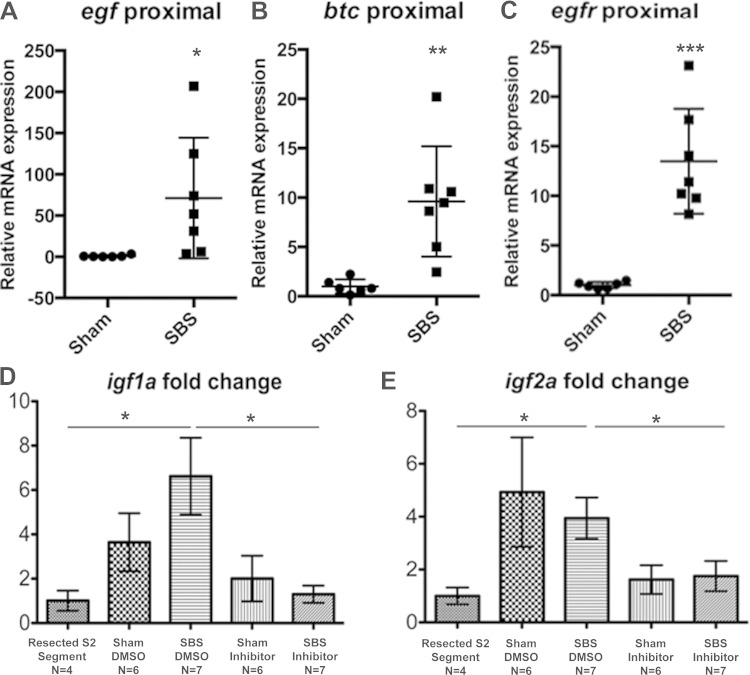
Studies in rodent models show that epidermal growth factor (EGF) receptor (EGFR) signaling plays a critical role in intestinal adaptation following either resection or fasting/refeeding (4, 8, 16) EGFR ligand expression increases following resection in mammals, and exogenous EGFR ligand administration amplifies intestinal adaptation (6, 31). To test whether this ligand-receptor system is also regulated during adaptation in zebrafish, RNA isolated from the proximal intestine of sham and SBS zebrafish was subjected to RT-qPCR analysis for egfr and the ligands egf, btc, hb-egfa, and hb-egfb. There was a 13.5-fold increase in expression of egfr in the SBS proximal intestine compared with sham fish (P < 0.01) (Fig. 5A). We also found 71.2-fold and 9.6-fold increases in expression of egf and btc (P < 0.05 and P < 0.01), respectively (Fig. 5, A–C); in contrast, there were no differences in hb-egfa or hb-egfb expression with experimental SBS (data not shown).
Fig. 5.
SBS fish show increased expression of adaptation-associated growth factor pathways. A–C: RNA from proximal intestinal segments of sham and SBS fish was subjected to RT-quantitative PCR (qPCR) analysis for egf, btc, and egfr. Each point represents 1 fish. D and E: RNA from sham and SBS fish treated with either DMSO vehicle or Igfr inhibitor was subjected to RT-qPCR analysis for igf1a and igf2a. Results reported as ratio of ef1a, housekeeping gene. Error bars indicate SE. *P < 0.05; **P < 0.01; ***P < 0.0001.
Intestinal adaptation following short bowel syndrome is associated with increased mRNA expression of insulin-like growth factor receptor (Igf1r) ligands igf1a and igf2a, and Igf1r inhibitor abrogates intestinal adaptation after intestinal resection in zebrafish.
Insulin-like growth factor (Igf) has been shown in previously described animal models to promote intestinal adaptation (23, 47). To investigate whether Igf was an effector of the phenotype observed in our model, we treated sham or SBS fish with the Igf1r inhibitor NVP-AEW541, with the required solvent DMSO alone applied to the control sham and SBS fish. RT-qPCR results were measured as a ratio of the housekeeping gene, ef1a, demonstrating that there were no differences in Igf receptor or ligand expression in sham fish with or without inhibitor treatment. For SBS fish, there was no significant difference between SBS/inhibitor and SBS/DMSO-treated fish in the mRNA quantification of ligands igf1b, and igf2b, or the receptor igf1r by RT-qPCR analysis (data not shown). Compared with the intestine resected during the surgery to create the SBS fish (S2 and S3), SBS/DMSO fish had a 6.6-fold change increase in igf1a expression (P < 0.05, Fig. 5D) and a 3.9-fold increase in igf2a expression (P < 0.05, Fig. 5E) compared with S2 and S3. This difference was not observed in SBS fish when treated with inhibitor. Compared with SBS/DMSO, SBS/inhibitor fish had decreased igf1a (P < 0.01, Fig. 5D) and igf2a (P < 0.05, Fig. 5E) expression; in the presence of the inhibitor, igf1a and igf2a expression were not increased. In addition, fish did not heal their laparotomy wound in the presence of the inhibitor, whereas those only exposed to DMSO did close their wounds, indicating a definite biological effect. Consistent with the initial experiments, inhibitor-treated SBS fish demonstrated greater weight loss than inhibitor-treated sham fish (losing 23.9 vs. 5.2% initial body weight). SBS fish treated with inhibitor also lost more weight than SBS fish exposed only to the vehicle control DMSO (losing 12.9 vs. 5.2% initial body weight) (Fig. 6E).
Fig. 6.
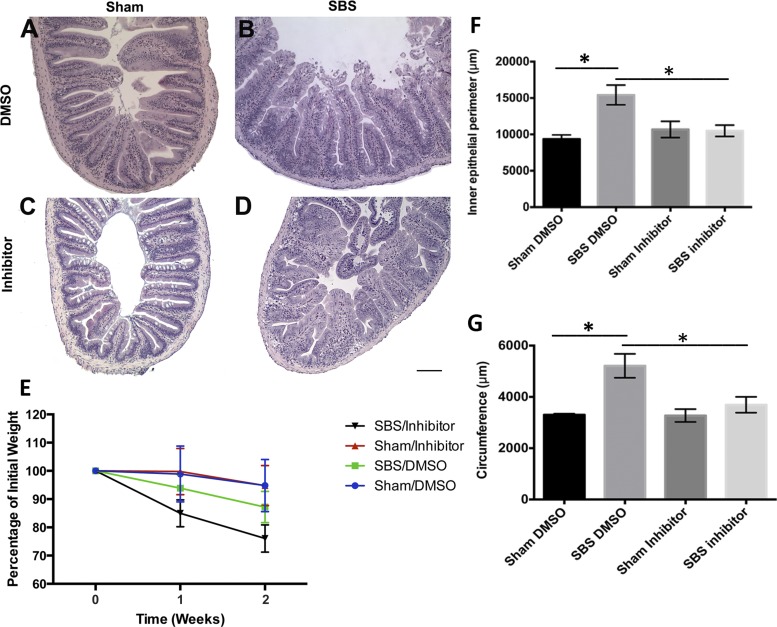
Adaptation, as evidenced by an increase in inner epithelial perimeter, is increased in SBS/DMSO fish but inhibited by Igf receptor inhibitor NVP-AEW541. Sham fish exposed to DMSO (A) or inhibitor (C) postoperatively vs. SBS fish exposed to DMSO (B) or inhibitor (D) postoperatively. E: percentage of initial weight at each week was plotted, with the SBS/inhibitor group losing a significant amount of weight compared with SBS/DMSO group. Quantification of the inner epithelial perimeter (F) and circumference (G) of the sham and SBS groups exposed to DMSO or inhibitor. Results are reported as means ± SE. Scale bar = 100 μm. *P < 0.05.
In addition to visually dilated bowel, increased villus ridge complexity resulted in an increased inner epithelial perimeter in SBS/DMSO (Fig. 6B) compared with sham/DMSO (Fig. 6A) (15,423 ± 1,362 vs. 9,369 ± 559 μm, P < 0.01, Fig. 6F) and SBS/DMSO (Fig. 6B) compared with SBS/inhibitor (Fig. 6D) (15,423 ± 1,362 vs. 10,498 ± 777 μm, P = 0.01, Fig. 6F). This increase was not demonstrated in measurements of the inner epithelial perimeter for the SBS/inhibitor zebrafish compared with sham/inhibitor. The serosal circumference is significantly increased in SBS/DMSO compared with sham/DMSO fish (5,213 ± 465 vs. 3,303 ± 46 μm, P < 0.05, Fig. 6G). With the addition of Igf inhibitor, this effect is blunted and there is no significant increase in circumference of the intestine between SBS/inhibitor and sham/inhibitor (3,694 ± 308 vs. 3,274 ± 249 μm, P > 0.05, Fig. 6G).
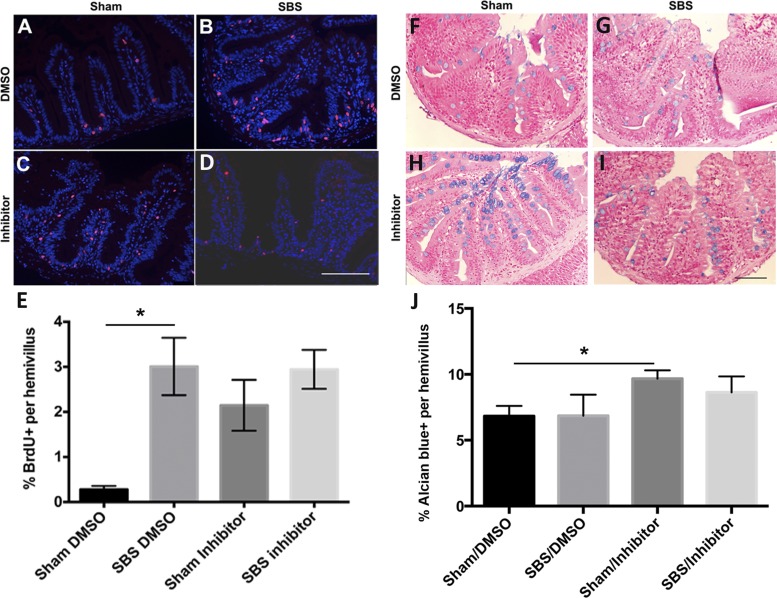
As in the initial experiments conducted in water alone, the percentage of BrdU-positive cells increased significantly in SBS/DMSO (Fig. 7B) compared with sham/DMSO (Fig. 7A) fish (3.9 vs. 0.5%, P = 0.01, Fig. 7E). There was also a significant increase in sham/inhibitor (Fig. 7C) compared with sham/DMSO (Fig. 7A) fish (2.1 vs. 0.5%, P < 0.05, Fig. 7E). However, there was no significant difference in the percentage of BrdU-positive cells between the SBS/DMSO (Fig. 7B) and SBS/inhibitor (Fig. 7D). There was also no significant difference between the sham/inhibitor (Fig. 7C) and SBS/inhibitor (Fig. 7D) groups, indicating that the inhibitor blocked adaptation in the SBS group.
Fig. 7.
Igf receptor inhibition blocks the increase in cell proliferation observed after SBS. IF staining showing BrdU-labeled cells (in red) with sham fish exposed to DMSO (A) or inhibitor (C) postoperatively vs. SBS fish exposed to DMSO (B) or inhibitor (D) postoperatively. E: quantification of percentage of BrdU-positive cells per hemivillus of the sham and SBS groups exposed to DMSO or inhibitor. Alcian blue staining of the goblet cells (in blue) with sham fish exposed to DMSO (F) or inhibitor (H) postoperatively vs. SBS fish exposed to DMSO (G) or inhibitor (I) postoperatively. J: quantification of the percentage of Alcian blue-positive cells per hemivillus of the sham and SBS groups exposed to DMSO or inhibitor. Results are reported as means ± SE. Scale bar = 50 μm. *P = 0.01.
There was no significant change in the percentage of Alcian blue-positive epithelial cells in SBS/DMSO (Fig. 7G) compared with sham/DMSO (Fig. 7F) (6.8 ± 1.6 vs. 6.8 ± 0.8%; P = 0.99, Fig. 7J). There was a significant increase in percentage of Alcian blue-positive cells per hemivillus in sham/inhibitor (Fig. 7H) compared with sham/DMSO (Fig. 7F) (9.7 ± 0.6 vs. 6.8 ± 0.8%; P = 0.01, Fig. 7J). The SBS/inhibitor (Fig. 7I) appeared to demonstrate an increased percentage of Alcian blue-positive cells per hemivillus compared with SBS/DMSO (Fig. 7G), though this was not significant (8.6 ± 1.2 vs. 6.9 ± 1.6%; P = 0.38, Fig. 7J).
DISCUSSION
We describe a novel model of SBS and intestinal adaptation that closely recapitulates the physiological effects seen in the human correlate condition. The small size of the adult zebrafish may appear to preclude attempts to surgically modify intraperitoneal organs, but this model is straightforward and reproducible and demonstrates a number of the characteristics necessary to study intestinal adaptation. With an average operative time of 329.6 s (5.5 min) for SBS and 17.6 s for sham operation, these procedures are much faster than murine SBS surgery, and we were able to demonstrate long postoperative time points in high experimental numbers at low cost. The animals survive the brief operation and demonstrate weight loss in response to the severely truncated intestinal surface area available to absorb nutrition. Housed and fed together with their sham counterparts, SBS zebrafish have a lower survival rate and demonstrate significantly greater weight loss within the first 2 wk following surgery than the sham laparotomy controls. In the subsequent 2 wk, there is continued weight loss to ∼85% of preoperative weight. As in humans, the remaining proximal intestine is grossly dilated with increases in length and cross section. This adaptive response has been well described in mammalian models and humans with SBS and is characterized by compensatory dilation, thickening, and lengthening of the remaining small intestine (41, 55).
There is no single efficient and well-accepted model of SBS and intestinal adaptation. The majority of investigations involve 50% or greater small bowel resection (SBR) in mouse, rat, and pig models. Piglet models benefit from the larger size of the animals, which allows a technically easier bowel resection and anastomosis, and authors also cite more anatomical, physiological, and biochemical similarities to newborn human infants than rodent models (33). However, housing, procurement, and surgical supplies are more expensive, typically thousands of dollars per piglet. A technical review of 27 articles reporting porcine SBS models demonstrated great heterogeneity in age, weight, and sex of pigs, as well as percent and location of SBR performed. Experimental numbers were low, and mortality ranged from 6 to 18% where reported (52). One report of 75% SBR in 7-day-old piglets reported 8% mortality rate at 28 days; however, the animals were fed a standard amount of calories per body weight daily and were supplemented with electrolytes, additional costly and work-intensive interventions (15).
Rodent models offer more options for transgenic investigations than pigs, but experimental approaches are also diverse and a gold standard has not been achieved. Few authors include mortality data when describing their models, but it is understood through the few centers that publish data from these models that rodent bowel resection and anastomosis is a very specialized skill. After achieving only a 16% survival rate after 75% SBR, which closely mimics human disease, Warner and colleagues (45) modified their approach and developed a robust model of intestinal adaptation with 85% survival following 50% SBR with reanastomosis. Mouse models also report a similarly modest resection, likely due to prohibitively high mortality rates (32). It is important to note that clinical SBS does not result after 50% bowel resection in humans, and adaptation is typically seen at higher levels of intestinal loss. In addition to modeling adaptation in response to greater than 75% SBR by creation of a proximal stoma, our model anatomically recapitulates severe SBS and the early, rapid weight loss of the resultant intestinal failure, while allowing high-throughput investigations given the low cost, high survival rate, and reproducibility of the intestinal response. We are the first to describe the time per proximal diversion operation and, given our experience with intra-abdominal surgery in rodent and pig models, believe it to be a reasonable claim that it is in fact the shortest operative time of any existing SBS model despite the size of the zebrafish. Additional confounding interventions including parenteral nutritional support are not required, and not providing supplementary therapeutic agents will be less costly as well. Advances in zebrafish genetics make this a reasonable vertebrate model to develop possible future human therapies. In addition, slight variations in technique could facilitate more in-depth investigations of adaptation in other more distal regions of the intestine.
Histologically, human adaptation is associated with crypt proliferation with cells migrating at increased rates into the villus (53). There is a coincident increase in apoptosis, with the overall effect of increased intestinal mass, surface area, and hyperplasia of the muscularis propria (13, 42, 55). In the zebrafish, quantification demonstrates increased villus epithelial perimeter and greater than threefold increase in the number of cells incorporating BrdU in the fish with severely truncated intestine at 2 wk. These proliferating cells were primarily located in the intervillus pocket, the zebrafish intestinal epithelial stem cell zone. In the zebrafish, the increase in BrdU-positive cells was demonstrated only at the 2-wk time point following the SBS operation and did not persist at the 4-wk time point. In humans, intestinal adaptation is noted mainly in the first 1–2 years following massive small bowel resection, although large cohorts with replicated time points are of course not possible. Future identification of these proliferating cells may be informative about the mechanisms that underpin adaptation.
Multiple factors contribute to intestinal adaptation. The magnitude of the adaptation response in human patients is thought to correlate with extent of resection but is also altered by luminal factors in recovery after resection, including nutrition, pancreaticobiliary secretions, and the microbiome. EGF, glucagon-like peptide 2 (Glp2), growth hormone, and insulin-like growth factor (IGF) are among the nonnutritive factors that have been confirmed to stimulate adaptation in multiple rat, rabbit, and piglet studies (5, 11, 23, 26, 30, 43, 47, 50). A glucagon-like peptide 2 analog (GLP-2) was recently approved in Europe and the United States for the treatment of intestinal failure and is believed to exert its effect through IGF-1, IGF-2, and ERBB ligands (39). Experimental models in animals have focused on withdrawal or addition of these factors to investigate the mechanism underlying the adaptive response.
IGF-1 and -2 are produced in the liver, and locally in the intestine, and are important regulators of metabolism and organ regeneration. Whereas IGF-2 is primarily involved in growth during the fetal period (18), IGF-1 is thought to be one of the primary effectors of growth hormone; IGF-1 stimulates duodenal crypt cell proliferation in humans and increased intestinal adaptation when administered to rats after massive small bowel resection (18, 23, 47, 54, 56). IGF is preserved across vertebrate species. In the zebrafish, signaling occurs through four IGF genes: igf1a, -1b, -2a, and -2b. These genes encode four peptides that signal through the receptor, Igf1r (19, 57). Igfs are potent mitogens for zebrafish embryonic cells through activation of MAPK and PI3K signaling pathways and are involved in a variety of physiological processes including metabolism, growth, and reproduction (35, 38). Transcript levels are regulated tightly by hormonal and environmental factors such as feeding status (2). The role of Igf in intestinal regeneration in the zebrafish is undefined.
We investigated whether Igf contributed to intestinal adaptation in our model. By exposing sham or SBS fish to the Igf1r inhibitor NVP-AEW541 or vehicle control (DMSO), we demonstrated that inhibition reduced measurements associated with intestinal adaptation and prevented the increase in BrdU-marked intervillus pocket cells that is seen in SBS in the absence of NVP-AEW541. In the control group exposed to DMSO only, SBS fish demonstrated significantly increased BrdU incorporation compared with sham fish. This effect was not observed when inhibitor was added, suggesting that Igf signaling is important in the increase of marked progenitor cells associated with intestinal adaptation in our model. We noted an increase in goblet cells, as stained by Alcian blue, in the sham group exposed to Igfr1 inhibitor compared with the sham group exposed to vehicle. There was no difference in goblet cells per hemivillus between sham and SBS groups. Previous studies have reported significant increase in goblet cell density in mouse and pig SBS models (17, 33), but increases in goblet cells are also noted in response to ATOH1 and SPDEF (12, 21).
Although NVP-AEW541 is a kinase inhibitor reported to inhibit receptor activation and downstream signaling, likely to be most effective through these downstream pathways, we evaluated igf-1a, 1b, 2a, 2b, and 1r by RT-qPCR. There was no significant difference between SBS/inhibitor and SBS/DMSO fish in the mRNA quantitation of ligands igf1b, and igf2b, or the receptor igf1r. In SBS fish, igf1a and igf2a expression was increased relative to the sample of intestine (S2 and S3) immediately adjacent to the tested intestine (S1) resected at the time of initial surgery. There was a relative decrease in igf1a and igf2a expression in fish with SBS exposed to the inhibitor compared with those exposed to DMSO alone. We chose inhibition through NVP-AEW541 as an initial proof-of-concept of this SBS model because there are good data relating IGF to SBS in other animal models. Igf1 potently stimulates mucosal growth in uninjured and irradiated mouse small bowel. It has the ability to activate quiescent and constitutively active stem cell populations by different molecular mechanisms (46). However, addition of NVP-AEW541 to the tank water is a relatively blunt force approach, expected to have systemic effects as well as downstream signaling actions, and the modest mRNA changes are unlikely to be the major effector of the marked increase in BrdU-positive cells and intestinal villus epithelial perimeter identified in the SBS fish.
In further evaluation of SBS fish in the presence of NVP-AEW541, the histological changes associated with intestinal adaptation, including increased inner epithelial perimeter, were reduced in the presence of the inhibitor. However, all three groups of SBS fish, whether exposed to tank water alone, DMSO, or inhibitor, all demonstrated known characteristics of SBS as it is identified in human patients who have severely truncated small intestines. All of the SBS fish had an increased inner epithelial perimeter of the intestine with greater villus ridge complexity, dilated girth of the intestine, and increased serosal circumference. Linked to the increase in BrdU-positive cells identified in conjunction with these histological findings when not exposed to inhibitor, a stem/progenitor cell mechanism is a likely candidate.
Given the limitations of the Igf1r inhibitor, another molecular effector pathway of intestinal adaptation, EGFR, was evaluated for downstream changes within the proximal intestine of our zebrafish SBS model. The EGFR pathway is largely conserved between humans and zebrafish (9, 36). Zebrafish homologs have been isolated for direct EGFR ligands, including egf, btc, hb-egf, and tgfα. In the zebrafish SBS model, the proximal intestine demonstrated a significant increase in egf, btc, and egfr compared with the sham fish. These changes are consistent with data from rodent models of adaptation, further validating our model.
Shortening the bowel in zebrafish through the creation of a proximal stoma overcomes many of the limitations of mammalian models of SBS and intestinal adaptation such as high animal mortality, operative complexity and time, high cost, and low experimental numbers secondary to these factors. The model closely correlates to the human disease of SBS with the possibility for more rapid investigations of key morphogens that may eventually be translational targets. Our model can easily be modified to create more distal ostomies. Histological and biochemical changes may differ based on the level of ostomy creation and percentage of bowel resected and will be important to further understand intestinal adaptation.
This model demonstrates the feasibility and survivability of performing intraperitoneal microsurgery in the adult zebrafish. With advanced genetic and pharmacological tools now available, this approach also allows for drug and small molecule administration and may lead to progress in understanding the cellular and molecular mechanisms of intestinal adaptation to treat human disease.
GRANTS
This work was supported by the California Institute for Regenerative Medicine (CIRM) Grants RN2-00946-1 and RN3-06425 (T. C. Grikscheit) and National Institute of Diabetes and Digestive and Kidney Diseases award 5R01DK095004 (M. R. Frey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.A.S., K.A.H., C.N.G., D.E.L., E.R.T., A.M., A.D., and T.C.G. performed experiments; K.A.S., K.A.H., C.N.G., D.E.L., E.R.T., A.M., A.D., D.A.A., and T.C.G. analyzed data; K.A.S., K.A.H., C.N.G., D.E.L., E.R.T., H.A.P., R.A.M., M.R.F., D.A.A., and T.C.G. interpreted results of experiments; K.A.S., K.A.H., C.N.G., D.E.L., H.A.P., R.A.M., D.A.A., and T.C.G. prepared figures; K.A.S., K.A.H., C.N.G., D.E.L., and T.C.G. drafted manuscript; K.A.S., K.A.H., C.N.G., D.E.L., M.R.F., C.-L.E.L., and T.C.G. edited and revised manuscript; K.A.S., K.A.H., C.N.G., D.E.L., E.R.T., H.A.P., R.A.M., M.R.F., D.A.A., C.-L.E.L., and T.C.G. approved final version of manuscript; D.E.L. and T.C.G. conception and design of research.
Supplementary Material
REFERENCES
- 1.Akimenko MA, Johnson SL, Westerfield M, Ekker M. Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development 121: 347–357, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Amaral IP, Johnston IA. Insulin-like growth factor (IGF) signalling and genome-wide transcriptional regulation in fast muscle of zebrafish following a single-satiating meal. J Exp Biol 214: 2125–2139, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Andersson H, Bosaeus I, Brummer RJ, Fasth S, Hultén L, Magnusson O, Strauss B. Nutritional and metabolic consequences of extensive bowel resection. Dig Dis 4: 193–202, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447–2456, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Benhamou PH, Canarelli JP, Richard S, Cordonnier C, Postel JP, Grenier E, Leke A, Dupont C. Human recombinant growth hormone increases small bowel lengthening after massive small bowel resection in piglets. J Pediatr Surg 32: 1332–1336, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Chaet MS, Arya G, Ziegler MM, Warner BW. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg 29: 1035–1039, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Diep CQ, Davidson AJ. Transplantation of cells directly into the kidney of adult zebrafish. J Vis Exp 51: 2725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erwin CR, Helmrath MA, Shin CE, Falcone RA Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol Gastrointest Liver Physiol 277: G533–G540, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Frey MR, Goloyin A, Polk B. Epidermal growth factor-stimulated intestinal cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521, 2004. [DOI] [PubMed] [Google Scholar]
- 10.García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F. In vivo antitumor activity of NVP-AEW541—A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 5: 231–239, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gómez de Segura IA, Aguilera MJ, Codesal J, Codoceo R, De-Miguel E. Comparative effects of growth hormone in large and small bowel resection in the rat. J Surg Res 62: 5–10, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The Ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology 137: 1333–1345.e1–e3, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hanson WR, Osborne JW, Sharp JG. Compensation by the residual intestine after intestinal resection in the rat. II. Influence of postoperative time interval. Gastroenterology 72: 701–705, 1977. [PubMed] [Google Scholar]
- 14.Health Resources and Services Administration. Scientific Registry of Transplant Recipients: 2011 Annual Data Report. Minneapolis, MN: Scientific Registry of Transplant Recipients, 2011. [Google Scholar]
- 15.Heemskerk VH, van Heurn LW, Farla P, Buurman WA, Piersma F, ter Riet G, Heineman E. A successful short-bowel syndrome model in neonatal piglets. J Pediatr Gastroenterol Nutr 29: 457–461, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res 69: 76–80, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 292: G215–G222, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Herington AC. Insulin-like growth factors: biochemistry and physiology. Baillieres Clin Endocrinol Metab 5: 531–551, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Kamei H, Ding Y, Kajimura S, Wells M, Chiang P, Duan C. Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability. Development 138: 777–786, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Kato T, Tzakis AG, Selvaggi G, Gaynor JJ, David AI, Bussotti A, Moon JI, Ueno T, DeFaria W, Santiago S, Levi DM, Nishida S, Velasco ML, McLaughlin G, Hernandez E, Thompson JF, Cantwell P, Holliday N, Livingstone AS, Ruiz P. Intestinal and multivisceral transplantation in children. Ann Surg 243: 756–764; discussion 764–766, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazanjian A, Shroyer NF. NOTCH signaling and ATOH1 in colorectal cancers. Curr Colorectal Cancer Rep 7: 121–127, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen SLJ, Mac ASW, Henriksen L, van Deurs B, Grovdal LM. EGFR signaling patterns are regulated by its different ligands. Growth Factors 32: 155–163, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Lemmey AB, Martin AA, Read LC, Tomas FM, Owens PC, Ballard FJ. IGF-I and the truncated analogue des-(1–3)IGF-I enhance growth in rats after gut resection. Am J Physiol Endocrinol Metab 260: E213–E219, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Longshore SW, Wakeman D, McMellen M, Warner BW. Bowel resection induced intestinal adaptation: progress from bench to bedside. Minerva Pediatr 61: 239–251, 2009. [PubMed] [Google Scholar]
- 25.Lykins TC, Stockwell J. Comprehensive modified diet simplifies nutrition management of adults with short-bowel syndrome. J Am Diet Assoc 98: 309–315, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 286: G964–G972, 2004. [DOI] [PubMed] [Google Scholar]
- 27.McMellen ME, Wakeman D, Longshore SW, McDuffie LA, Warner BW. Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg 19: 35–43, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes 58: 1844–1851, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Dong PD, Stainier DY, Heath JK. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 286: 114–135, 2005. [DOI] [PubMed] [Google Scholar]
- 30.O'Loughlin E, Winter M, Shun A, Hardin JA, Gall DG. Structural and functional adaptation following jejunal resection in rabbits: effect of epidermal growth factor. Gastroenterology 107: 87–93, 1994. [DOI] [PubMed] [Google Scholar]
- 31.O'Louglin E, Winter M, Sun A, Hardin J, Gall DG. Structural and functional adaptatio following jejunal resection in rabbits: effect of epidermal growth factor. Gastroenterology 107: 87–93, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery 150: 217–223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira-Fantini PM, Thomas SL, Wilson G, Taylor RG, Sourial M, Bines JE. Short- and long-term effects of small bowel resection: a unique histological study in a piglet model of short bowel syndrome. Histochem Cell Biol 135: 195–202, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Peyret B, Collardeau S, Touzet S, Loras-Duclaux I, Yantren H, Michalski MC, Chaix J, Restier-Miron L, Bouvier R, Lachaux A, Peretti N. Prevalence of liver complications in children receiving long-term parenteral nutrition. Eur J Clin Nutr 65: 743–749, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Pozios KC, Ding J, Degger B, Upton Z, Duan C. IGFs stimulate zebrafish cell proliferation by activating MAP kinase and PI3-kinase-signaling pathways. Am J Physiol Regul Integr Comp Physiol 280: R1230–R1239, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Pruyot B, Cure Y, Dijotsa J, Voncken A, Muller M. Developmental defects in zebrafish for classification of EGF pathway inhibitors. Toxicol Appl Pharmacol 274: 339–349, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Pugach EK, Li P, White R, Zon L. Retro-orbital injection in adult zebrafish. J Vis Exp 34: 1645, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinecke M, Björnsson BT, Dickhoff WW, McCormick SD, Navarro I, Power DM, Gutiérrez J. Growth hormone and insulin-like growth factors in fish: where we are and where to go. Gen Comp Endocrinol 142: 20–24, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol 301: G1–G8, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol 17: 931–942, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MZ, Maeda K. Short bowel syndrome in infants and children. Pediatr Clin North Am 32: 1265–1279, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Scott RB, Sheehan A, Chin BC, Tan DT. Hyperplasia of the muscularis propria in response to massive intestinal resection in rat. J Pediatr Gastroenterol Nutr 21: 399–409, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Shulman DI, Hu CS, Duckett G, Lavallee-Grey M. Effects of short-term growth hormone therapy in rats undergoing 75% small intestinal resection. J Pediatr Gastroenterol Nutr 14: 3–11, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Stollman NH, Neustater BR, Rogers AI. Short-bowel syndrome. Gastroenterologist 4: 118–128, 1996. [PubMed] [Google Scholar]
- 45.Taylor JA, Martin CA, Nair R, Guo J, Erwin CR, Warner BW. Lessons learned: optimization of a murine small bowel resection model. J Pediatr Surg 43: 1018–1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Landeghem L, Santoro MA, Mah AT, Krebs AE, Dehmer JJ, McNaughton KK, Helmrath MA, Magness ST, Lund PK. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J 29: 1–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderhoof JA, McCusker RH, Clark R, Mohammadpour H, Blackwood DJ, Harty RF, Park JH. Truncated and native insulinlike growth factor I enhance mucosal adaptation after jejunoileal resection. Gastroenterology 102: 1949–1956, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Wales PW, Christison-Lagay ER. Short bowel syndrome: epidemiology and etiology. Semin Pediatr Surg 19: 3–9, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev 122: 157–173, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care 12: 526–532, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, Gong Z. Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11: 392, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weih S, Nickkholgh A, Kessler M, Frongia G, Hafezi M, Golriz M, Fard N, Holland-Cunz S, Mehrabi A. Models of short bowel syndrome in pigs: a technical review. Eur Surg Res 51: 66–78, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Welters CF, Dejong CH, Deutz NE, Heineman E. Intestinal adaptation in short bowel syndrome. ANZ J Surg 72: 229–236, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Wheeler EE, Challacombe DN. The trophic action of growth hormone, insulin-like growth factor-I, and insulin on human duodenal mucosa cultured in vitro. Gut 40: 57–60, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson RC. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med 298: 1444–1450, 1978. [DOI] [PubMed] [Google Scholar]
- 56.Wolvekamp MC, Heineman E, Taylor RG, Fuller PJ. Towards understanding the process of intestinal adaptation. Dig Dis 14: 59–72, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Zou S, Kamei H, Modi Z, Duan C. Zebrafish IGF genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One 4: e7026, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q, Zhu Z, Zhang B, Lin S. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10: 329–331, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.