Abstract
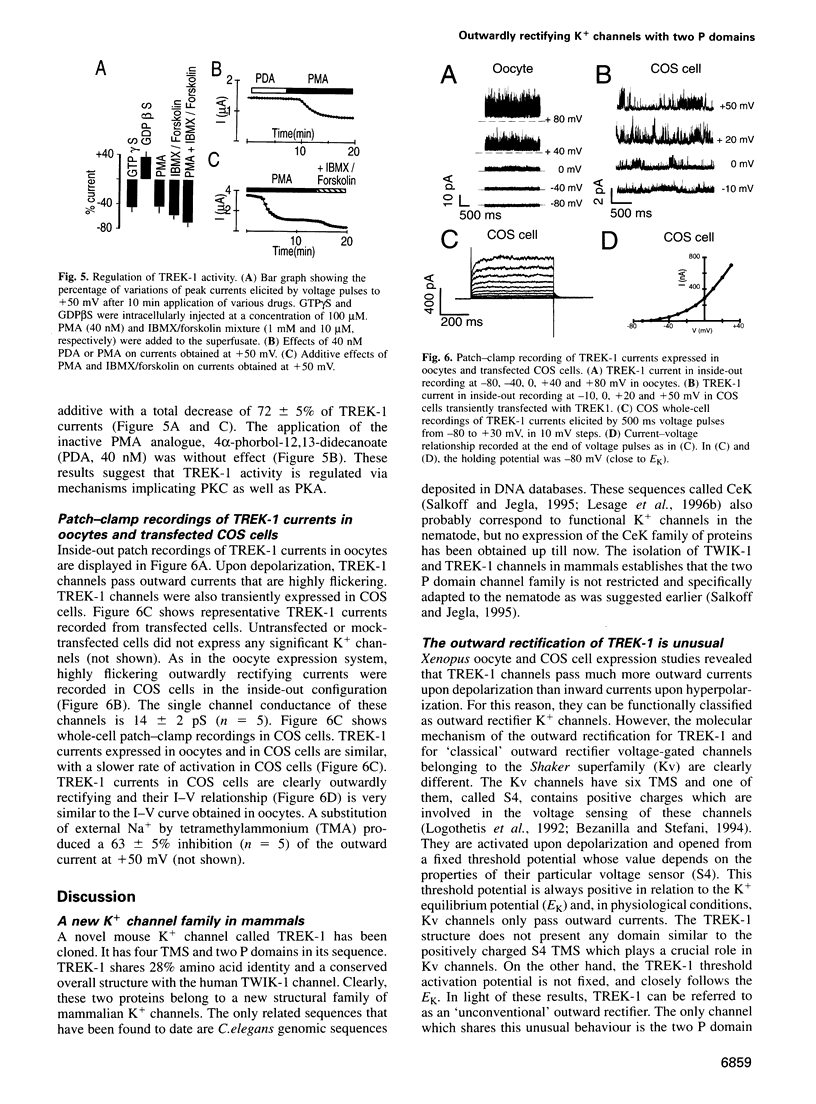
Human TWIK-1, which has been cloned recently, is a new structural type of weak inward rectifier K+ channel. Here we report the structural and functional properties of TREK-1, a mammalian TWIK-1-related K+ channel. Despite a low amino acid identity between TWIK-1 and TREK-1 (approximately 28%), both channel proteins share the same overall structural arrangement consisting of two pore-forming domains and four transmembrane segments (TMS). This structural similarity does not give rise to a functional analogy. K+ currents generated by TWIK-1 are inwardly rectifying while K+ currents generated by TREK-1 are outwardly rectifying. These channels have a conductance of 14 pS. TREK-1 currents are insensitive to pharmacological agents that block TWIK-1 activity such as quinine and quinidine. Extensive inhibitions of TREK-1 activity are observed after activation of protein kinases A and C. TREK-1 currents are sensitive to extracellular K+ and Na+. TREK-1 mRNA is expressed in most tissues and is particularly abundant in the lung and in the brain. Its localization in this latter tissue has been studied by in situ hybridization. TREK-1 expression is high in the olfactory bulb, hippocampus and cerebellum. These results provide the first evidence for the existence of a K+ channel family with four TMS and two pore domains in the nervous system of mammals. They also show that different members in this structural family can have totally different functional properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. doi: 10.1146/annurev.bb.23.060194.004131. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. K+ channels in cardiac cells: mechanisms of activation, inactivation, rectification and K+e sensitivity. Pflugers Arch. 1989;414 (Suppl 1):S88–S92. doi: 10.1007/BF00582254. [DOI] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Guillemare E., Honoré E., Pradier L., Lesage F., Schweitz H., Attali B., Barhanin J., Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992 Dec 15;31(49):12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994 Apr;66(4):1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U. Excitatory amino acids and epilepsy-induced changes in extracellular space size. Adv Exp Med Biol. 1986;203:449–460. doi: 10.1007/978-1-4684-7971-3_34. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Potassium channels and their evolving gates. Nature. 1994 Sep 8;371(6493):119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Jurman M. E., Boland L. M., Liu Y., Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994 Nov;17(5):876–881. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Ketchum K. A., Joiner W. J., Sellers A. J., Kaczmarek L. K., Goldstein S. A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995 Aug 24;376(6542):690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lesage F., Attali B., Lazdunski M., Barhanin J. Developmental expression of voltage-sensitive K+ channels in mouse skeletal muscle and C2C12 cells. FEBS Lett. 1992 Sep 28;310(2):162–166. doi: 10.1016/0014-5793(92)81320-l. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. A pH-sensitive yeast outward rectifier K+ channel with two pore domains and novel gating properties. J Biol Chem. 1996 Feb 23;271(8):4183–4187. doi: 10.1074/jbc.271.8.4183. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996 Mar 1;15(5):1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E., Voilley N., Waldmann R., Lazdunski M., Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993 Feb 22;318(1):95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Movahedi S., Satler C., Lindpaintner K., Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron. 1992 Mar;8(3):531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994 Sep 15;371(6494):243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Pore loops: an emerging theme in ion channel structure. Neuron. 1995 May;14(5):889–892. doi: 10.1016/0896-6273(95)90327-5. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annu Rev Physiol. 1991;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Makhina E. N., Pearson W. L., Sha Q., Lopatin A. N. Inward rectification and implications for cardiac excitability. Circ Res. 1996 Jan;78(1):1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer-Linn C. L., Perlman I., Lasater E. M. Sodium dependency of the inward potassium rectifier in horizontal cells isolated from the white bass retina. Brain Res. 1995 Dec 1;701(1-2):81–88. doi: 10.1016/0006-8993(95)00964-4. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Pongs O. Structure-function studies on the pore of potassium channels. J Membr Biol. 1993 Oct;136(1):1–8. doi: 10.1007/BF00241484. [DOI] [PubMed] [Google Scholar]
- Reid J. D., Lukas W., Shafaatian R., Bertl A., Scheurmann-Kettner C., Guy H. R., North R. A. The S. cerevisiae outwardly-rectifying potassium channel (DUK1) identifies a new family of channels with duplicated pore domains. Receptors Channels. 1996;4(1):51–62. [PubMed] [Google Scholar]
- Ritchie J. M. Voltage-gated ion channels in Schwann cells and glia. Trends Neurosci. 1992 Sep;15(9):345–351. doi: 10.1016/0166-2236(92)90052-a. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Jegla T. Surfing the DNA databases for K+ channels nets yet more diversity. Neuron. 1995 Sep;15(3):489–492. doi: 10.1016/0896-6273(95)90137-x. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995 Apr 21;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflugers Arch. 1992 Feb;420(2):180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- Zhou X. L., Vaillant B., Loukin S. H., Kung C., Saimi Y. YKC1 encodes the depolarization-activated K+ channel in the plasma membrane of yeast. FEBS Lett. 1995 Oct 9;373(2):170–176. doi: 10.1016/0014-5793(95)01035-d. [DOI] [PubMed] [Google Scholar]