Abstract
Humanin (HN) is an endogenous mitochondria-associated peptide that has been shown to protect against various Alzheimer's disease-associated insults, myocardial ischemia-reperfusion injury, and reactive oxygen species-induced cell death. We have shown previously that HN improves whole body glucose homeostasis by improving insulin sensitivity and increasing glucose-stimulated insulin secretion (GSIS) from the β-cells. Here, we report that intraperitoneal treatment with one of HN analogs, HNG, decreases body weight gain, visceral fat, and hepatic triglyceride (TG) accumulation in high-fat diet-fed mice. The decrease in hepatic TG accumulation is due to increased activity of hepatic microsomal triglyceride transfer protein (MTTP) and increased hepatic TG secretion. Both intravenous (iv) and intracerebroventricular (icv) infusion of HNG acutely increase TG secretion from the liver. Vagotomy blocks the effect on both iv and icv HNG on TG secretion, suggesting that the effects of HNG on hepatic TG flux are centrally mediated. Our data suggest that HN is a new player in central regulation of peripheral lipid metabolism.
Keywords: humanin, triglyceride secretion, hepatic microsomal triglyceride transfer protein, hypothalamus
nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis are burgeoning health problems that affect one-third of adults and an increasing number of children in developed countries (1, 6). NAFLD is associated with obesity, insulin resistance, diabetes mellitus, and metabolic syndrome (25). It is estimated that NAFLD affects ∼70–80% of obese people (3), 60–70% of people with diabetes (52), and virtually 100% of obese type 2 diabetic patients (53). Excess lipid accumulation in the liver could elicit an inflammatory response, thereby progressing to steatohepatitis, cirrhosis, and liver cancer. In addition, fatty liver contributes to insulin resistance (7) and diabetes (48), which in turn increases the risks for many diseases, including cardiovascular diseases (51), cancer (45), and Alzheimer's disease (AD) (11). Thus, reducing lipid accumulation in the liver could have favorable hepatic as well as systemic consequences.
NAFLD arises from the imbalance of triglyceride (TG) storage and clearance in the liver. TG is generated in the liver by coupling free fatty acids (FFA) derived from adipose tissue lipolysis, diet, or de novo lipogenesis to a glycerol via ester bonds. TG in the liver can undergo hydrolysis to release FFA that can be used to generate ATP through β-oxidation in the mitochondria or secreted from the liver in the form of very low-density lipoprotein (VLDL), thereby preventing hepatic TG accumulation. Microsomal TG transfer protein (MTTP) is the rate-limiting enzyme in the assembly and secretion of hepatic VLDL. Mice lacking the hepatic mttp gene show hepatic steatosis and a dramatic decrease in plasma lipid levels (39), whereas hepatic overexpression of mttp increases VLDL-TG secretion in vivo (4). In humans, mutations in MTTP cause abetalipoproteinemia, an autosomal recessive disorder characterized by the virtual absence of apolipoprotein B (apoB)-containing lipoproteins in plasma (34, 37). apoB is an obligatory structural component of VLDL, and individuals heterozygous for inactivating mutations in apoB produce fewer VLDL particles and have a threefold increase in hepatic TG content relative to healthy individuals (50).
Humanin (HN) is a 24-amino acid mitochondria-associated peptide that was identified from a cDNA library from the surviving neurons of human AD brain (15). Endogenous HN is both an intracellular and secreted protein and has been detected in heart, liver, muscle, ovary, pancreas, kidney, brain, colon, testis plasma, cerebral spinal fluid, and seminal fluid (31, 49). HN has a well-described role in neuroprotection against AD-associated cell death (15), prion-induced apoptosis (46), and chemically induced neuronal damage (12, 24). Analogs of HN, created by a single amino acid substitution, are reported to increase the stability and biological potency; one such analog is [Gly(14)]-humanin (HNG; with replacement of serine with glycine at position 14) (14). We have shown that, in addition to the neuroprotective effects, HNG also displays a cardioprotective effect in ischemia and the reperfusion mouse model (32). It was demonstrated recently that neuroprotective effects of HN are mediated through a trimeric cell surface receptor complex of CNTFR, WSX-1, and gp130 (13) and are associated with activation of STAT3 (26). Indeed, antiapoptotic action and neuronal protection by HN were lost in the presence of dominant negative STAT3 (16). We have shown that HN increases overall insulin sensitivity in rodents through the activation of the central STAT3 pathway (31). Very recently, our studies showed that HNGF6A, another analog of HN, increases insulin secretion in the cultured βTC3 pancreatic β-cell line, islets from wild-type and db/db mice, and rats in vivo, and these effects are mediated through increased glucose oxidation and ATP generation (21).
Since insulin resistance is associated with NAFLD, and HN increases insulin sensitivity, we postulated that HN will decrease hepatic TG accumulation, the pathological hallmark of NAFLD. We tested our hypothesis in wild-type and diet-induced obesity animal models where HNG was administered either centrally or peripherally.
MATERIALS AND METHODS
Animals
Male 12-wk-old C57BL/6J mice (The Jackson Laboratory) and Sprague-Dawley rats (Harlan Laboratory) were used in the present study. Mice were used for both acute and chronic studies, whereas rats were used for the acute studies that evaluated potential signaling pathways. We used rats to confirm that the results are reproducible in a different species. Animals were housed under a standard 12-h light-dark cycle (lights on at 7 AM) with access to food and water ad libitum.
Chronic studies.
For the chronic treatment study, mice were fed a high-fat diet (60% fat; Research Diets, New Brunswick, NJ) and received a once daily intraperitoneal (ip) injection of 2 mg/kg scrambled peptide (SP; FRGGETRARAMPLIDLSPLCLLKV) or HNG (MAPRGFSCLLLLTGEIDLPVKRRA, GenScript, Piscataway, NJ) for 4 wk.
Acute studies.
For the acute studies, depending on the study group, the animals were prepared with survival surgeries. For the acute intravenous (iv) studies, catheters were placed in the right internal jugular vein and left carotid artery, as described previously (28–31, 33). For the intracerebroventricular (icv) studies, 2 wk before the in vivo experiments, cannulas were implanted in the third cerebral ventricle, and after complete recovery, usually 1 wk, catheters were placed in the right internal jugular vein and left carotid artery, as described previously (28–31, 33). In the vagotomy group, hepatic vagotomy was performed as described previously (38) along with the catheter replacement. Briefly, mice were anesthetized, and laparotomy was performed. The stomach was exposed, and the hepatic branch of the abdominal vagus was identified. The hepatic nerve bundles of mice were isolated on a fine wire loop and rapidly heated until the loop cut through the nerve. Based on the study group, the animals received icv, iv, or ip administration of SP or HNG.
Intravenous studies.
A total of 2 mg/kg SP and HNG was infused through the jugular vein from the −60-min time point. One-third of the total amount (0.67 mg/kg) was given as a bolus in first 3 min and the remainder at the rate of 5.5 μg·kg−1·min−1 over the duration of the study. The above dose was chosen based on our previous experience in vivo (31). A single dose injection of 600 mg/kg iv tyloxapol, an inhibitor of lipoprotein lipase (LPL) that blocks degradation of TG in the plasma, was administered at the 0-min time point.
Intracerebroventricular studies.
On the day of the experiments, SP and HNG (bolus of 36 μg·kg−1·min−1 for 3 min, followed by an infusion at 0.9 μg·kg−1·min−1) were started at −60 min and infused icv throughout the duration of the study. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY-294002 [3-μl bolus of 1 mM and thereafter 2.5 μl/h for 5 h (40)], the melanocortin-3/4 receptor antagonist SHU9119 [0.66 nmol/3-μl bolus and thereafter 1.33 nmol/5 h (30)] (EMD Millipore, Darmstadt, Germany), or a STAT3 inhibitor (31) (Genemed Synthesis, San Antonio, TX) was infused to the third ventricle along with HNG or SP, depending on the study group. A single-dose injection of 600 mg/kg iv tyloxapol, an inhibitor of LPL that blocks degradation of TG in the plasma, was administered at the 0-min time point.
Blood samples were collected for the assessment of serum TG levels at 0, 60, 120, 150, and 180 min after the iv injection of tyloxapol. TG secretion rate was calculated by the slope of the rise in serum TG over time. At the end of the study, the animals were euthanized, the abdomen was quickly opened, and visceral fat pads were carefully dissected, weighed, and snap-frozen in liquid nitrogen. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committees of the Albert Einstein College of Medicine and University of Pittsburgh.
Indirect Calorimetry
Twelve-week-old weight-matched male mice were housed singly in metabolic chambers. Oxygen consumption, locomotor activity, and respiration exchange ratio were measured continuously for 2 wk during 12:12-h light-dark cycles using a CLAMS (Columbus Instruments, Columbus, OH) open-circuit indirect calorimetry system. SP and HNG were given by ip injection daily.
Isolation and Culture of Primary Hepatocytes
Primary hepatocytes were isolated from 12-wk-old adult male C57/B6 mice by the collagenase perfusion method, as described previously (44), with modifications. In brief, livers were washed with Hanks' calcium- and magnesium-free buffer for 3 min, followed by a collagenase buffer (0.5 mg/ml) for 10 min at a perfusion rate of 7 ml/min. After the perfusion, livers were rapidly excised and transferred to a sterile Petri dish. Hepatocytes were released by disrupting the liver capsule. The cells were separated from undigested tissue with a sterile 50-μm mesh nylon filter and cultured in DMEM supplied with 10% FBS and grown on type I collagen-coated plates. Primary cells were incubated with 150 μM oleic acid or palmitic acid overnight and then treated with SP or HNG for 4 h. Intracellular TG and secreted TG levels were assessed by TG quantification kit (Abcam, Cambridge, MA) and normalized to cell number (per million cells). For MTTP activity assay, cells were treated with SP or HNG for 0.5, 1, or 3 h and then harvested for the assay.
Biochemical Measurements
Insulin was detected using an ELISA kit (Crystal Chem, Downers Grove, IL). Blood glucose levels were measured by a OneTouch glucometer (LifeScan, Milpitas, CA). FFAs were measured by a colorimetric assay (Wako Chemicals, Richmond, VA). Tissue and serum TG levels were determined using a colorimetric assay (Thermo-Fisher Scientific, Middletown, VA). Liver TG was extracted using the Folch method. Briefly, liver tissue was homogenized with chloroform-methanol at a ratio of 2:1. The homogenate was centrifuged, and the liquid phase was recovered and washed twice with saline. After centrifugation and siphoning of the upper phase, the chloroform phase containing lipids was evaporated under a nitrogen stream, and lipids were dissolved in isopropanol alcohol. Because of the high concentration of TG in the serum in acute in vivo studies, samples were diluted by serum dilution buffer (25 mM HEPES, 150 mM NaCl, and 1 mM CaCl2, pH 7.4). All other chemicals were purchased from Sigma-Aldrich, unless otherwise stated.
Lipoprotein Fractions
Serum samples from five mice in each group were pooled, and fast protein liquid chromatography (FPLC) was performed to separate the lipoprotein fractions. TG levels in each fraction were determined using the TG kit (Thermo-Fisher Scientific).
RNA extraction and real-time PCR.
Liver RNA was extracted using the RNeasy purification kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from 1 μg of RNA (AMV First Strand cDNA Synthesis Kit; New England Biolabs, Ipswich, MA) according to the manufacturer's instructions. Real-time PCR was carried out in a 10-μl reaction mixture (LightCycler DNA master SYBR green; Roche) containing 1 μl of the diluted (1:10) first-strand cDNA, and the results were normalized to GAPDH using ΔΔCT. The sequences of the primers used for amplification of mouse Cpt-1a, Srebp-1c, Fasn, Dgat-2, mttp, apoB, and GAPDH are listed in Table 1.
Table 1.
Real-time PCR primers
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | aactttggcattgtggaagg | acacattgggggtaggaaca |
| Cpt-1a | tccctaagcagtgccagttt | gaacttgcccatgtccttgt |
| Fasn | caccctgatttctgccatct | aatgtgcttggcttggtagc |
| Mtpp | tcctctatgcctgtggcttt | tgcagccttcattctgacac |
| Srebp-1 | aagagccctgcacttcttga | ccacaaagaaacggtgacct |
| Dgat2 | gcacagaggccacagaagtg | ccctaacacaggcatcg |
| apoB | atgtctgattacccccagca | gggctcacattattggctgt |
| Pepck | cttctctgccaaggtcatcc | ttttggggatgggcac |
| HL | atgggaaatcccctccaaatct | gtgctgaggtctgagacga |
Cpt-1a, carnitine palmitoyl transferase-1a; Fasn, fatty acid synthase; Mttp, microsomal triglyceride transfer protein; Sprebp-1, sterol regulatory element-binding protein-1; Dgat2, diglyceride acyltransferase 2; apoB, apolipoprotein B; Pepck, phosphoenolpyruvate carboxykinase; HL, hepatic lipase.
Immunoblotting
Livers were homogenized in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, and 0.1% SDS) containing proteinase inhibitors (Roche). Twenty micrograms of protein was resolved on precast gradient 4–12% SDS-PAGE and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBS-T) for 1 h at room temperature. Antibodies (Akt, p-Akt Ser473, and GAPDH; Cell Signaling Technologies, Cambridge, MA) diluted in TBS-T containing 5% of BSA were added to the membranes and incubated overnight at 4°C. The membranes were washed three times with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Signals detected using the SuperSignal West Dura extended the duration substrate (Thermo Scientific, Rockford, IL).
MTTP Activity Assay
MTTP activity was measured using an MTTP assay Kit according to the manufacturer's instructions (Roar Biomedical, New York, NY).
Oil Red O Staining
Liver sections were fixed in 10% formalin and then stained with Oil Red O solution at 60°C for 10 min. Images were taken by Olympus Light station 2.
Alanine Aminotransferase Activity Assay
Liver Alanine aminotransferase (ALT) activity assay was done using a colorimetric assay kit from Biovision (Milpitas, CA) according to the manufacturer's instructions.
Statistical Analysis
All values shown are expressed as means ± SE. When comparing two groups between SP and HNG, independent two-tailed t-test was used. When dynamic comparisons for variables such as TG secretion were made, two-way ANOVA (group × time) was used. For each statistically significant F value observed for the main effect or interaction, a two-tailed post hoc test (Tukey's) was applied to determine individual differences between means. Differences were considered to be statistically significant at P ≤ 0.05 for primary outcomes and P ≤ 0.01 for secondary outcomes.
RESULTS
Phenotype and Basic Metabolic Profile in HNG-Treated High-Fat Diet-Induced Obese Mice
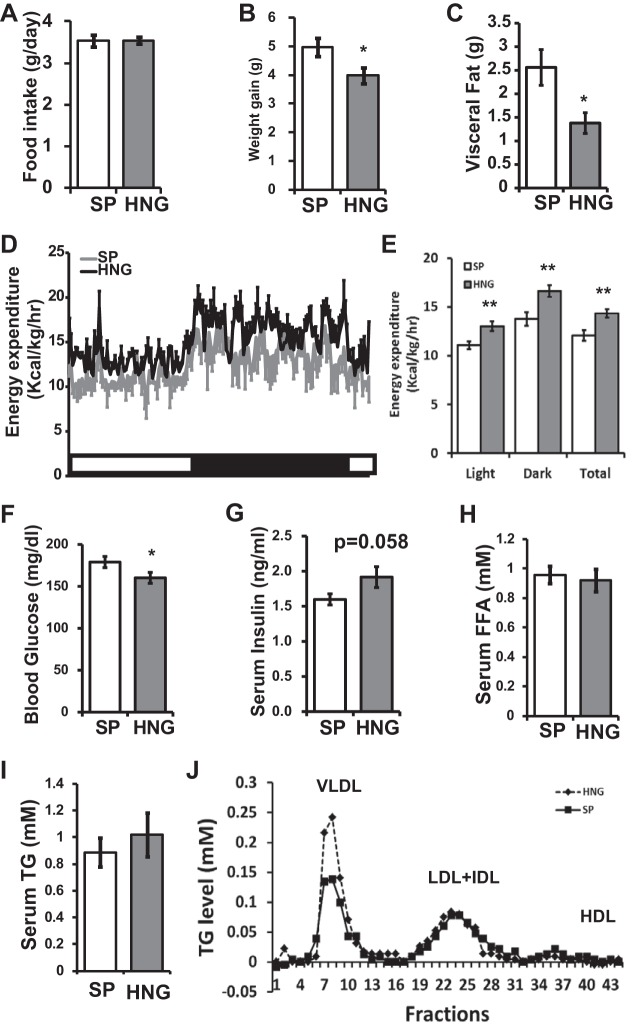
Twelve-week-old C57BL/6J mice were fed 60% high-fat diet and received a once daily ip administration of either SP or HNG (2 mg/kg) for 4 wk. Whereas no difference was observed in the food intake between the groups (Fig. 1A), there was an ∼20% less body weight gain in HNG-treated mice (P < 0.05; Fig. 1B). HNG treated mice had less visceral fat (P < 0.05; Fig. 1C). Increased energy expenditure was noted in HNG-treated group on CLAMS (P < 0.01; Fig. 1, D and E). There was no preferential substrate oxidation between the groups (respiratory quotient: 0.91 ± 0.01 vs. 0.92 ± 0.01 in SP vs. HNG, not significant). Fasting glucose was significantly decreased, and insulin levels tended to increase (P = 0.058) in the HNG-treated group (Fig. 1, F and G). No significant differences were found in serum FFA (Fig. 1H) or total TG levels (Fig. 1I) in HNG-treated mice. On fast protein liquid chromatography (FPLC) analysis of lipoprotein fractions from pooled samples, VLDL fraction was increased twofold in the HNG-treated group (Fig. 1J). There were no differences in LDL or HDL fractions.
Fig. 1.
Effects of [Gly(14)]-humanin (HNG) on phenotype and basic metabolic profile in high-fat diet (HFD)-induced obese mice. A and B: food intake (A) and body weight gain (B) in mice fed HFD and treated with scrambled peptide (SP; open bars) or HNG (gray bars) for 4 wk (n = 15 for SP and 17 for HNG). C–E: visceral fat (C) and 24-h and average energy expenditure levels (n = 3 in each group; D and E). F–J: fasting glucose (F), fasting insulin (G), serum free fatty acids (FFA; H), serum triglyceride (TG) levels (I), and lipoprotein fractions (J) in HFD mice treated with SP or HNG. *P < 0.05; **P < 0.01.
HNG Decreases Hepatic Lipid Accumulation in HFD-Induced Obese Mice
Oil Red O staining of liver sections demonstrated a significant decrease in liver steatosis in HNG-treated mice (Fig. 2A). This was confirmed by liver TG levels (Fig. 2B); HNG-treated mice showed decreased hepatic TG accumulation (P = 0.011; Fig. 2B). There were no differences in liver ALT activity between SP and HNG-treated mice (Fig. 2C).
Fig. 2.
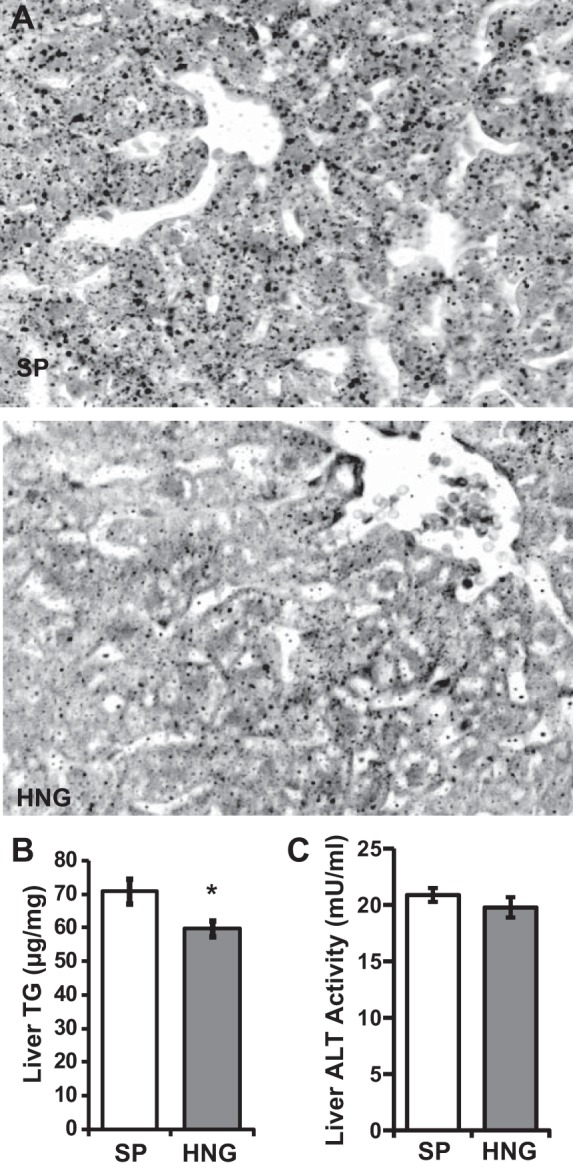
Effects of HNG on hepatic steatosis. A: representative images for Oil Red O staining of liver from mice fed HFD and treated with SP or HNG. Liver TG (B) and liver alanine aminotransferase (ALT; C) activity of HFD mice treated with SP (open bars) or HNG (gray bars) for 4 wk (n = 6 in each group).
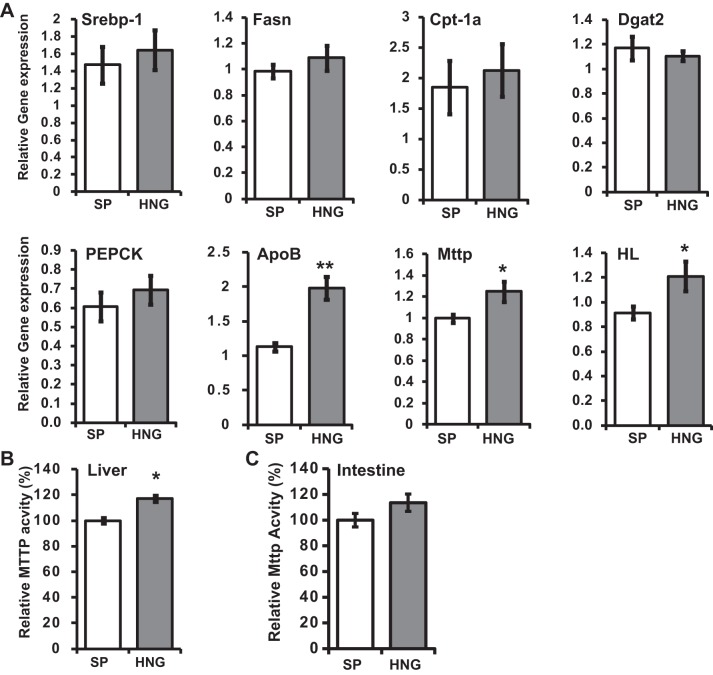
HNG Increases Gene Expression of apoB and mttp and Increases the Activity of MTTP
To further analyze the role of HNG in attenuation of hepatic TG accumulation, we analyzed the expression level of genes involved in TG synthesis [diglyceride acyltransferase 2 (DGAT2)], FFA synthesis [sterol regulatory element-binding protein (SREBP-1c), fatty acid synthase (FASN)], fatty acid β-oxidation [carnitine palmitoyltransferase (CPT-1a)], gluconeogenesis [phosphoenolpyruvate carboxykinase (PEPCK)], and VLDL packaging and secretion (apoB and MTTP) in the liver. HNG significantly increased the mRNA levels of apoB and mttp, genes involved in VLDL packaging and secretion (Fig. 3A). HNG also increased the mRNA level of hepatic lipase (HL; Fig. 2A), which hydrolyzes TG and phospholipids in chylomicron remnants, interediate-density lipoprotein, and HDL (42). There were no changes in the mRNA levels of DGAT2, SREBP-1c, FASN, CPT-1a, and PEPCK (Fig. 3A). Along with the increase in expression, there was a ∼20% increase in the MTTP enzyme activity in the liver in HNG-treated group (Fig. 3B), and an upward trend in the intestinal MTTP activity (P = 0.15; Fig. 3C).
Fig. 3.
Effects of HNG on hepatic gene expression and activity of microsomal TG transfer protein (MTTP) in HFD mice. A: relative gene expression levels for sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (Fasn), carnitine palmitoyl transferase-1a (CPT-1a), diglyceride acyltransferase 2 (DGAT2), phosphoenolpyruvate carboxykinase (PEPCK), apolipoprotein B (apoB), mttp, and hepatic lipase (HL) in SP- (open bars) or HNG-treated (gray bars) HFD mice (n = 6 each group). B and C: activity of hepatic (B) and intestinal (C) MTTP in HFD mice treated with SP or HNG for 4 wk. *P < 0.05; **P < 0.01.
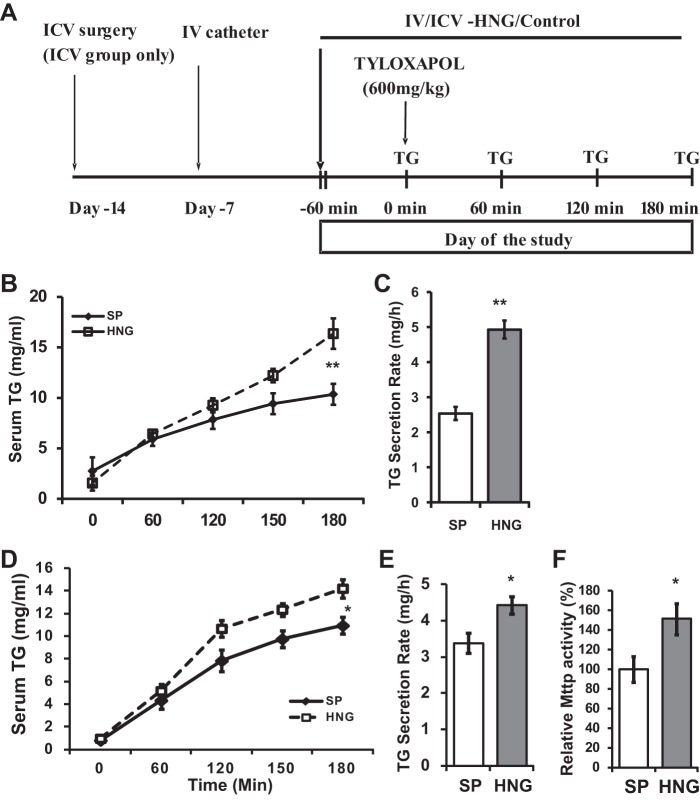
Acute Effects of HNG on Hepatic TG Flux
To test whether HNG increases hepatic TG secretion in vivo, we infused SP or HNG iv, along with a single dose of tyloxapol through the right internal jugular vein at 0 min, to block the degradation of VLDL-TG in the circulation (experimental design shown in Fig. 4A). Our results show that TG levels increased steadily in the serum after the administration of tyloxapol in both groups, and serum TG levels were significantly higher in the group that received an iv infusion of HNG compared with SP (Fig. 4B). Liver TG secretion rate was increased in HNG treated mice (Fig. 4C).
Fig. 4.
Acute effects of HNG on TG secretion in WT mice. A: timeline for the acute experiments. B and C: dynamic TG secretion (B) and TG secretion rate over 3 h (C) in mice that received acute intravenous (iv) infusion of SP (open bars) or HNG (gray bars) for 3 h. D and E: dynamic TG secretion (D) and TG secretion rate over 3 h (E) in mice that received acute intracerebroventricular (icv) infusion of SP (open bars) or HNG (gray bars) for 3 h. F: relative hepatic MTTP activity in icv SP or HNG mice. *P < 0.05; **P < 0.01.
To test whether the effect of HNG on hepatic TG accumulation is mediated via the hypothalamus, we infused HNG or SP directly into the third ventricle (icv) and examined the acute TG secretion from the liver in mice. Similar to the iv infusion study, we found that icv HNG increased TG accumulation in the circulation (Fig. 4D) and that the calculated liver TG secretion rate was increased in the icv HNG group (Fig. 4E). Consistent with the increased TG secretion rate, liver MTTP activity was increased by almost 50% in icv HNG-treated mice compared with those treated with SP (Fig. 4F).
HNG Does Not Directly Affect TG Flux in Primary Hepatocytes
We then challenged the isolated primary hepatocytes overnight with FFAs (oleic acid or palmitic acid) and treated them with either SP or HNG for 4 h to examine whether the HNG directly affects hepatic TG accumulation. All cells had significantly higher intracellular TG concentration after the treatment of FFAs, but there were no difference in the intracellular TG concentration (Fig. 5A) or the level of secreted TG in the media (Fig. 5B) between SP and HNG-treated groups. We also measured the activity of MTTP, key regulator of VLDL assembly, and found no difference during 3 h of HNG treatment (Fig. 5C). Although overnight incubation with FFAs increased the expression levels of MTTP and apoB, there were no differences between the SP and HNG-treated groups. These studies indicate that the effects of HNG on TG secretion are not mediated through direct actions on the liver.
Fig. 5.
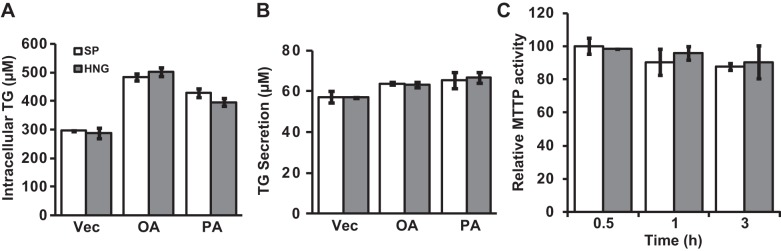
Effects of HNG on TG secretion in primary hepatocytes. A and B: intracellular TG (A) and TG secreted into the media (B) from the hepatocytes that were challenged with vehicle (Vec), palmitic acid (PA), or oleic acid (OA) overnight and then treated with SP (open bars) or HNG (gray bars) for 4 h. C: MTTP activity in primary hepatocytes treated with SP or HNG for 0.5, 1, and 3 h.
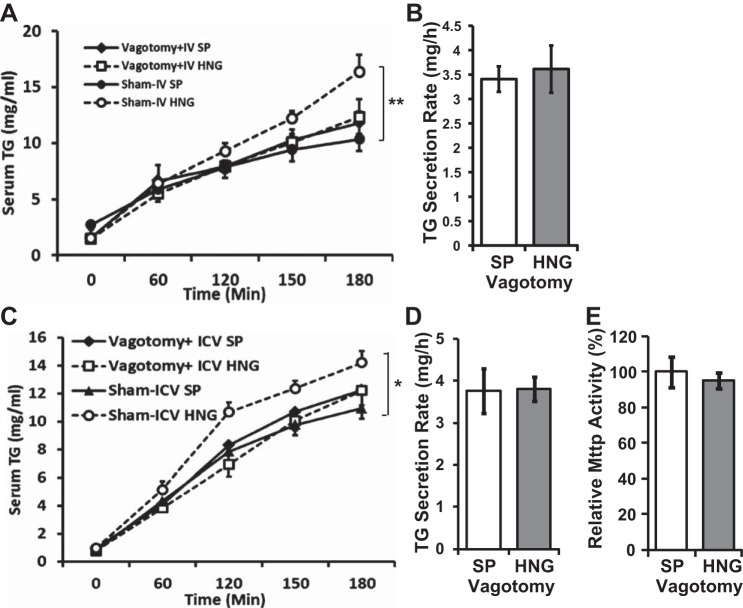
Effects of HNG on Hepatic TG Secretion are Lost After Vagotomy
To study the role of vagus nerve as the efferent signal from the hypothalamus in mediating the effects of HNG on hepatic TG secretion, we infused SP and HNG iv to animals that had sham surgery or vagotomy. Intravenous HNG markedly increased serum TG levels compared with the SP treatment group in the sham surgery mice, whereas vagotomy completely abolished this effect (Fig. 6, A and B). We also infused HNG icv and measured the hepatocyte TG secretion and MTTP activity after vagotomy. Again, vagotomy completely abolished the effects of icv HNG on hepatic TG secretion (Fig. 6C), TG secretion rate (Fig. 6D), and MTTP activity (Fig. 6E). Taken together with direct studies on the isolated primary hepatocytes, these data suggest that HNG affects hepatocyte lipid metabolism through the central pathway(s).
Fig. 6.
Acute effects of HNG on hepatic TG secretion in vagotomized mice. A and B: dynamic TG secretion following sham surgery or vagotomy (A) and TG secretion rate over 3 h (B) in vagotomized mice that received acute iv infusion of SP (open bars) or HNG (gray bars) for 3 h. C and D: dynamic TG secretion following sham surgery or vagotomy (C) and TG secretion rate over 3 h (D) in vagotomized mice that received acute icv infusion of SP (open bars) or HNG (gray bars) for 3 h. E: relative hepatic MTTP activity in vagotomized mice following icv SP or HNG. *P < 0.05; **P < 0.01.
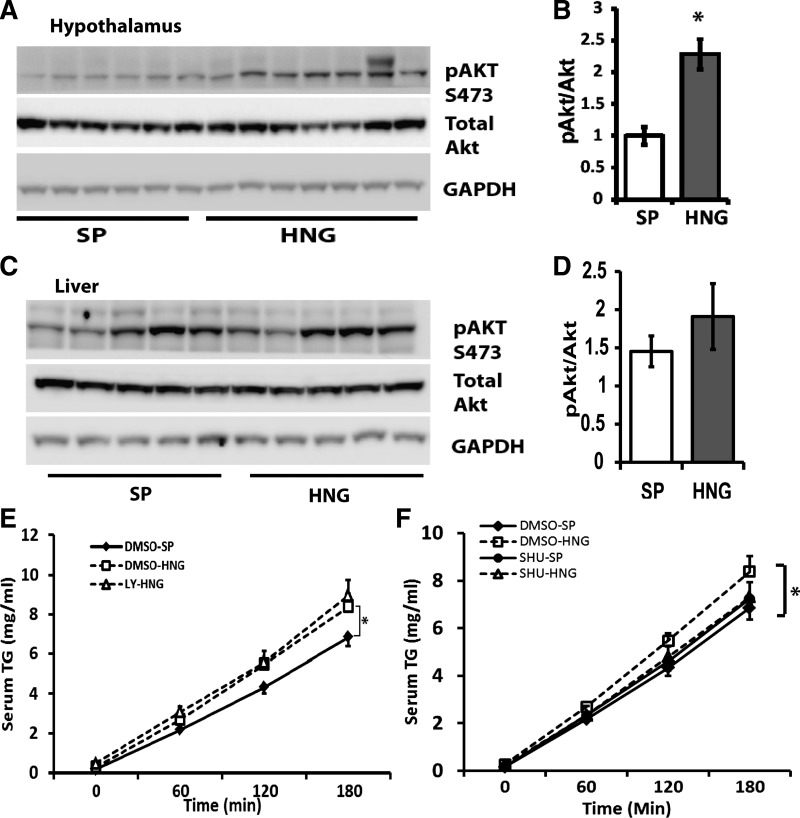
Effects of HN on TG Flux are Independent of Hypothalamic STAT3 and Insulin-Signaling Pathways
We have shown previously that HN regulates whole body glucose homeostasis through activation of the central STAT3 signaling pathway (31). Blockage of the STAT3 pathway in the hypothalamus by infusion of STAT3 inhibitors into the third ventricle abolished HN's effects on glucose homeostasis. To examine whether the effect of HNG on hepatic TG secretion is also through the hypothalamic STAT3-signaling pathway, cell-permeable STAT3 inhibitor was infused icv 1 h before the infusion of HNG in rats. Our results showed that HNG increased TG secretion from the liver in rats, replicating our results seen in mice. Furthermore, infusion of a STAT3 inhibitor did not block the effects of HNG on hepatic TG secretion. We found that icv HNG increases the phosphorylation of Akt2 in the hypothalamus (Fig. 6, B and C) but not in the liver (Fig. 7, C and D). However, we failed to block the effects of HNG on hepatic TG secretion by co-infusion of the PI3K inhibitor LY-294002 into the third ventricle (Fig. 7E), suggesting that the activation of hypothalamic insulin signaling is not involved in the effects of HNG on TG flux.
Fig. 7.
Effects of HNG on hepatic TG secretion are independent of central insulin signaling. A and B: levels of GAPDH, total Akt, and phosphorylated (p)-Akt [Ser473 (S473)] in the hypothalamus by immunoblot (A) and quantification (B). C and D: levels of GAPDH, total Akt, and p-Akt (Ser473) in the liver by immunoblot (C) and quantification (D). E: serum TG levels in mice treated with SP (DMSO-SP; solid line with ⧫), HNG (DMSO-HNG; dashed line with □), and HNG + LY-294002 (LY-HNG; dashed line with △). F: serum TG levels in DMSO-SP (solid line with ⧫), DMSO-HNG (dashed line with □), SP + SHU9119 (SHU-SP; solid line with ●), and HNG + SHU-9119 (SHU-HNG; dashed line with △). *P < 0.05.
It has been reported that the central melanocortin system is critical for the control of lipid metabolism in the periphery, including liver and fat tissues (35). To investigate whether the effects of HN on hepatic TG flux are mediated through the central melanocortin system, we infused SHU9119, a melanocortin-3/4 receptor antagonist into the third ventricle, 1 h prior to the infusion of HNG. Although SHU9119 alone did not induce any changes in TG levels in the serum, the accumulation of serum TG in response to HNG is greatly inhibited in the presence of SHU9119, suggesting that the effect of HNG on hepatic TG secretion could involve the central melanocortin system (Fig. 7F).
DISCUSSION
In studies presented here, we show that HNG, a potent analog of HN, decreases hepatic TG levels in high-fat diet-induced obesity and increases hepatic MTTP activity and TG secretion, and these effects are mediated through the hypothalamus.
Previously, we have shown a role for HN and its analogs in glucose homeostasis (31). We have demonstrated that HN and analogs improve hepatic and skeletal muscle insulin sensitivity (31) and that HN increases insulin secretion through direct effects on β-cells (21). Consistent with those observations, we show here that a 4-wk ip injection of HNG in an obese mouse model leads to a decrease in fasting glucose levels and a trend toward an increase in insulin levels (P = 0.058; Fig. 1, F and G) compared with controls. Importantly, we report here that HNG treatment reduces hepatic TG accumulation in obese mice (Fig. 2B). The demonstration of a twofold increase in VLDL by FPLC analysis (Fig. 1J) suggests increased hepatic TG efflux. However, the decrease in hepatic TG accumulation in the chronic study model could be multifactorial and attributable to a variety of factors, including improved insulin sensitivity from decreased visceral fat and an increase in energy expenditure as well as increased hepatic TG efflux as a result of increased MTTP activity, a key regulator of the VLDL-TG secretion from the liver. Overall, the end result of decreased hepatic lipid accumulation, especially in the setting of a high-fat diet, could have significant hepatic and systemic consequences such as improvement in insulin sensitivity. A lack of changes in the expression level of genes involved in de novo lipogenesis by HNG (Fig. 2A) and the observation that HNG does not affect phosphorylation of ACC (data not shown) suggest that HNG does not directly affect lipid deposition and utilization in the liver. Overproduction of TG and hypersecretion of VLDL-TG from the liver increases the risk for dyslipidemia and cardiovascular disease in vivo (23, 54). The significant reductions in liver fat content (Fig. 2B), an increase in serum VLDL (Fig. 1J) along with a reduction in adipose tissue LPL (data not shown), and a decrease in visceral fat (Fig. 1C) suggest that HNG may influence lipid metabolism in other tissues and require further investigation.
One of the regulators of VLDL-TG secretion is insulin (19). Insulin controls the expression level of mttp and the degradation of apoB (5, 19). However, phosphorylation of Akt2 was unchanged in the liver in the HNG-treated mice (Fig. 7, C and D), suggesting that the effects noted are not secondary to increase hepatic insulin signaling. Furthermore, our findings that HNG did not lower the intracellular or TG levels in the media or increase MTTP activity or mRNA levels in primary hepatocytes (Fig. 5) exclude the possibility of direct actions of HNG on the liver.
The hypothalamus plays central roles in energy and glucose homeostasis (8). We and others have demonstrated that the neurons in the arcuate nucleus (ARC) of the hypothalamus are able to sense the nutrient levels and integrate hormonal signals such as insulin, IGF-I, and leptin and ultimately control food intake and hepatic glucose production (HGP) (41). Indeed, hypothalamic insulin receptor disruption is sufficient to reduce the insulin-induced suppression of HGP (36), and adenovirus delivery of IRS-2 in the ARC slowed the rise in blood glucose levels in a mouse model of type 1 diabetes (9). Hypothalamic leptin signaling is also involved in regulating hepatic insulin sensitivity, HGP, and whole body glucose homeostasis through both the JAK-STAT and PI3K pathways (10, 17, 20, 27). Beyond HGP, hypothalamus also plays a role in adipose tissue metabolism. Mediobasal hypothalamus infusion of insulin in Sprague-Dawley rats increases white adipose tissue lipogenic protein expression, inactivates hormone-sensitive lipase and adipose tissue triglyceride lipase, and suppresses lipolysis (43). On the other hand, mice that lack the neuronal insulin receptor exhibit unrestrained lipolysis and decreased de novo lipogenesis in WAT (43). These indicate that hypothalamus is a critical organ for regulating whole body fat metabolism.
Our data in in vivo models demonstrating that the effects of both iv and icv HNG on hepatic TG secretion were lost in the mice after vagotomy (Fig. 6) suggest a central role for hypothalamus and vagus nerve in mediating the effects of HNG on hepatic TG secretion. Our studies point to the vagus nerve as the efferent carrying the signals from the hypothalamus to the liver, as effects of icv and iv HNG on hepatic TG flux were lost in the mice postvagotomy. Also, the significant increase in hepatic MTTP activity in response to hypothalamic HNG was lost postvagotomy, providing evidence for the first time that HNG regulates hepatic MTTP activity through the hypothalamus. Previous studies have shown that hypothalamic leptin signaling controls intestinal MTTP activity and lipid absorption (18). However, MTTP activity in the intestine in the high-fat diet-fed mice were slightly but not significantly increased with HNG treatment (Fig. 3C), suggesting that HNG increases hepatic TG flux without affecting lipid absorption in vivo. We acknowledge that in our experimental model, vagotomy would have resulted in transection of both sympathetic and parasympathetic nerves, and further studies are necessary to delineate the components of autonomic nervous system that are involved in the effects of HNG on VLDL-TG secretion.
Neuropeptide Y (2) and glycine (54) have been shown to control hepatic VLDL-TG secretion through the hypothalamus. Our observation that inhibition of the melanocortin-3/4 receptor by infusion of SHU9119 into the third ventricle reversed the effects of HNG on hepatic TG secretion (Fig. 7F) is inconsistent with observations that acute activation or inhibition of the melanocortin pathway did not affect TG secretion from the liver (47). This could be due to the species and strains differences in the study (Sprague-Dawley rats instead of Long-Evans rats were used in our study) and merits further investigation using genetic models of altered melanocortin signaling.
In summary, we demonstrate that HNG, an analog of HN, increases TG flux from the liver through activation of hepatic MTTP, and these effects are mediated through the hypothalamus, with the vagus nerve serving as the efferent from hypothalamus to the liver. In addition to the described role in glucose metabolism, these studies provide new evidence that HNG, a potent analog of the endogenous mitochondrial peptide humanin, is a novel central regulator for hepatic TG secretion.
GRANTS
This study was supported by the Department of Pediatrics of the University of Pittsburgh School of Medicine and by Grants K08-AG-027462, K08-AG-027462-03S1, and R01-AG-035114 to R. H. Muzumdar from the National Institutes of Health.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Z.G. and R.H.M. conception and design of research; Z.G., K.S., L.C., and T.Z. performed experiments; Z.G. and L.C. analyzed data; Z.G., H.H.D., and R.H.M. interpreted results of experiments; Z.G. prepared figures; Z.G. and R.H.M. drafted manuscript; Z.G., E.T., H.H.D., S.Y., and R.H.M. edited and revised manuscript; T.Z., H.H.D., and R.H.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gary J. Schwartz (Albert Einstein College of Medicine) for the technical support of the vagotomy.
REFERENCES
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bruinstroop E, Pei L, Ackermans MT, Foppen E, Borgers AJ, Kwakkel J, Alkemade A, Fliers E, Kalsbeek A. Hypothalamic neuropeptide Y (NPY) controls hepatic VLDL-triglyceride secretion in rats via the sympathetic nervous system. Diabetes 61: 1043–1050, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 123: 134–140, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Newberry EP, Norris JY, Xie Y, Luo J, Kennedy SM, Davidson NO. ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. J Lipid Res 49: 2013–2022, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22: 353–363, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 332: 1519–1523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, Caprio S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33: 1817–1822, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci 36: 65–73, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG Jr, Rhodes CJ, Schwartz MW. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 3: 67–73, 2006. [DOI] [PubMed] [Google Scholar]
- 10.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150: 4502–4511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghareeb DA, Hafez HS, Hussien HM, Kabapy NF. Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab Brain Dis 26: 253–267, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423: 456–461, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell 20: 2864–2873, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci 21: 9235–9245, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA 98: 6336–6341, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci 77: 3092–3104, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150: 4874–4882, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC Jr, Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res 51: 1929–1942, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 118: 2347–2364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4: 123–132, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, Bastie C, Su K, Huffman D, Surana M, Barzilai N, Fleischer N, Muzumdar R. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J 27: 4890–4898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis GF, Steiner G. Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev 12: 37–56, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Mamiya T, Ukai M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol 134: 1597–1599, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka M, Hashimoto Y. Humanin and the receptors for humanin. Mol Neurobiol 41: 22–28, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Mauvais-Jarvis F, Ueki K, Fruman DA, Hirshman MF, Sakamoto K, Goodyear LJ, Iannacone M, Accili D, Cantley LC, Kahn CR. Reduced expression of the murine p85alpha subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J Clin Invest 109: 141–149, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes 53: 441–446, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Muzumdar R, Ma X, Atzmon G, Yang X, Barzilai N. Central resistance to the inhibitory effects of leptin on stimulated insulin secretion with aging. Neurobiol Aging 27: 1308–1314, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Muzumdar R, Ma X, Yang X, Atzmon G, Bernstein J, Karkanias G, Barzilai N. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB J 17: 1130–1132, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4: e6334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, Ji SY, Lavu M, Predmore BL, Lefer DJ. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol 30: 1940–1948, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muzumdar RH, Ma X, Fishman S, Yang X, Atzmon G, Vuguin P, Einstein FH, Hwang D, Cohen P, Barzilai N. Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action. Diabetes 55: 2788–2796, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Narcisi TM, Shoulders CC, Chester SA, Read J, Brett DJ, Harrison GB, Grantham TT, Fox MF, Povey S, de Bruin TW, Erekelens DW, Muller DP, Lloyd JK, Scott J. Mutations of the microsomal triglyceride-transfer-protein gene in abetalipoproteinemia. Am J Hum Genet 57: 1298–1310, 1995. [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi K, Ishibashi S, Osuga J, Tozawa R, Harada K, Yahagi N, Shionoiri F, Iizuka Y, Tamura Y, Nagai R, Illingworth DR, Gotoda T, Yamada N. Novel mutations in the microsomal triglyceride transfer protein gene causing abetalipoproteinemia. J Lipid Res 41: 1199–1204, 2000. [PubMed] [Google Scholar]
- 38.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Raabe M, Véniant MM, Sullivan MA, Zlot CH, Björkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest 103: 1287–1298, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 70: 513–535, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Santamarina-Fojo S, González-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler Thromb Vasc Biol 24: 1750–1754, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Scherer T, O'Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab 13: 183–194, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol 13: 29–83, 1976. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen HT, Mellemkjaer L, Jepsen P, Thulstrup AM, Baron J, Olsen JH, Vilstrup H. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol 36: 356–359, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci 25: 95–102, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Stafford JM, Yu F, Printz R, Hasty AH, Swift LL, Niswender KD. Central nervous system neuropeptide Y signaling modulates VLDL triglyceride secretion. Diabetes 57: 1482–1490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev 29: 939–960, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Tajima H, Niikura T, Hashimoto Y, Ito Y, Kita Y, Terashita K, Yamazaki K, Koto A, Aiso S, Nishimoto I. Evidence for in vivo production of Humanin peptide, a neuroprotective factor against Alzheimer's disease-related insults. Neurosci Lett 324: 227–231, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res 45: 941–947, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54: 3541–3546, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One 8: e57183, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12: 1106–1110, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Yue JT, Mighiu PI, Naples M, Adeli K, Lam TK. Glycine normalizes hepatic triglyceride-rich VLDL secretion by triggering the CNS in high-fat fed rats. Circ Res 110: 1345–1354, 2012. [DOI] [PubMed] [Google Scholar]