Abstract
Adiponectin (APN) is a cardioprotective molecule. Its reduction in diabetes exacerbates myocardial ischemia/reperfusion (MI/R) injury. Although APN administration in animals attenuates MI/R injury, multiple factors limit its clinical application. The current study investigated whether AdipoRon, the first orally active molecule that binds APN receptors, may protect the heart against MI/R injury, and if so, to delineate the involved mechanisms. Wild-type (WT), APN knockout (APN-KO), and cardiomyocyte specific-AMPK dominant negative (AMPK-DN) mice were treated with vehicle or AdipoRon (50 mg/kg, 10 min prior to MI) and subjected to MI/R (30 min/3–24 h). Compared with vehicle, oral administration of AdipoRon to WT mice significantly improved cardiac function and attenuated postischemic cardiomyocyte apoptosis, determined by DNA ladder formation, TUNEL staining, and caspase-3 activation (all P < 0.01). MI/R-induced apoptotic cell death was significantly enhanced in mice deficient in either APN (APN-KO) or AMPK (AMPK-DN). In APN-KO mice, AdipoRon attenuated MI/R injury to the same degree as observed in WT mice. In AMPK-DN mice, AdipoRon's antiapoptotic action was partially inhibited but not lost. Finally, AdipoRon significantly attenuated postischemic oxidative stress, as evidenced by reduced NADPH oxidase expression and superoxide production. Collectively, these results demonstrate for the first time that AdipoRon, an orally active APN receptor activator, effectively attenuated postischemic cardiac injury, supporting APN receptor agonists as a promising novel therapeutic approach treating cardiovascular complications caused by obesity-related disorders such as type 2 diabetes.
Keywords: reperfusion injury, diabetes, apoptosis, adipokines
ischemic heart disease is the single most important cause of death in developed countries. Accumulating evidence indicates that apoptosis, a gene-controlled programmed cell death pathway, contributes significantly to postischemic cardiomyocyte death, suggesting that therapeutic interventions inhibiting apoptotic cell death may attenuate ischemic/reperfusion-induced heart injury (10).
Adiponectin (APN) is an adipocyte-derived cytokine. Most adipokines (e.g., TNFα) are proinflammatory and significantly increased in diabetic patients. The majority of currently published studies support APN as a potent cardiovascular protective molecule, and its levels are markedly reduced in type 2 diabetic patients (18, 26, 28). APN reduces oxidative/nitrative stress, protects cells from apoptosis, inhibits leukocyte-endothelial interaction, and decreases smooth muscle proliferation (9). Two APN receptors, AdipoR1 and AdipoR2, have been cloned (37). Belonging to a new family of membrane receptors (i.e., the progestin and AdipoQ receptor superfamily) (7, 16, 29) predicted to contain seven transmembrane domains, the APN receptors are topologically distinct from G protein-coupled receptors (GPCR). Although numerous studies, done by others and us, demonstrate that exogenous recombinant APN supplementation significantly protects the heart from ischemia/reperfusion injury in experimental animals (9, 25, 31), clinical APN application is limited due to multiple factors such as the high cost of production.
An APN receptor agonist, AdipoRon was recently discovered by Okada-Iwabu et al. (19). This synthetic small molecule is orally active, binds to and activates both AdipoR1 and AdipoR2, ameliorates insulin resistance and type 2 diabetes, and prolongs the shortened lifespan of db/db mice. These results in sum suggest AdipoRon may be a novel therapeutic molecule that effectively treats type 2 diabetes. However, whether AdipoRon may possess cardioprotective properties, attenuating postischemic cardiomyocyte death and improving cardiac function, have not been previously investigated.
Therefore, the aims of the present study were 1) to determine whether oral administration of AdipoRon might attenuate postischemic cardiomyocyte apoptosis and improve cardiac function recovery and 2) if so, to investigate the underlying molecular mechanisms.
MATERIALS AND METHODS
Adult male WT mice and APN knockout (APN−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Cardiomyocyte-specific AMPK (5′-adenosine monophosphate-activated protein kinase)-α2 subunit mutant transgenic mice (AMPK-DN) were kindly provided by Dr. R. Tian (University of Washington). Generation, breeding, phenotype characteristics, and genotyping of AMPK-DN mice (>80% inhibition of cardiac AMPK activity) have previously been described in detail (36). The experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care.
Myocardial ischemia/reperfusion.
Mice were anesthetized with 2% isoflurane. Myocardial ischemia/reperfusion (MI/R) was induced by temporarily exteriorizing the heart via a left thoracic incision, and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery. Ten minutes before coronary occlusion, animals were randomized to receive either vehicle or AdipoRon (50 mg/kg; Calbiochem, cat no. 509104) via a gavage tube. This dose was selected from previously published results demonstrating that maximal blood concentration was achieved 30 min after a single oral dose of AdipoRon (19). After 30 min of MI, the slipknot was released. The myocardium was reperfused for either 3 h (for all assays excluding cardiac functional measurement) or 24 h (for cardiac functional assay). All assays were performed utilizing tissue from the ischemic/reperfused area, i.e., the area at risk (AAR) identified by Evans blue negative staining. Sham-operated control mice (Sham MI/R) underwent the same surgical procedure, except the suture placed under the left coronary artery was not tied. Cardiac function was determined by echocardiography and left ventricular (LV) catheterization methods 24 h after reperfusion before thoracotomy, as described in our previous study (34).
Assessment of cardiomyocyte apoptosis.
Cardiomyocyte apoptosis was determined by DNA ladder formation, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, and caspase-3 activity, as reported in our previous study (31). For the DNA fragmentation assay, total DNA was isolated with the Gentra Puregene Tissue DNA Isolation Kit (QIAGEN, Valencia, CA) per manufacturer's instructions. DNA (10 μg) was loaded into 1.8% agarose gel containing 0.5 μg/ml ethidium bromide. DNA electrophoresis commenced at 60 V for 1–2 h. DNA ladder formation, a “hallmark” of tissue apoptosis, was visualized under ultraviolet light and photographed for permanent documentation.
TUNEL staining was performed via an In Situ Cell Death Detection Kit (Roche Diagnostics, Manheim, Germany) per manufacturer's protocol. In brief, cardiomyocytes from at least four random slides per block were evaluated immunohistochemically to determine the number and percentage of cells exhibiting apoptotic positive staining. The slides were covered with the mounting medium containing DAPI for total nuclei detection. By ×20 objective, the entire ischemic/reperfused area was digitally photographed with a QICAM-Fast digital camera mounted atop an Olympus BX51 fluorescence microscope. Total nuclei (blue) and the TUNEL-positive nuclei (green) were counted by IP Lab Imaging Analysis software (v. 3.5; Scanalytics, Fairfax, VA) with a custom-made script (by Ken Anderson, Bio Vision Technologies, North Exton, PA). The index of apoptosis (number of TUNEL-positive nuclei/total number of nuclei × 100) was automatically calculated and exported to Microsoft Excel for further analysis. Results from different fields taken from the same animal were averaged and counted as one sample. The caspase-3 activity was determined by utilizing the fluorogenic substrate DEVD-7-amino-4-trifluoromethyl-coumarin (AFC). Briefly, cells or mouse heart tissue were lysed with 1× caspase-3 lysis buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 5 mM DTT, 0.1 mM EDTA, 0.1% Triton X-100), and total protein concentration was determined by the Bradford method (Bio-Rad). To each well of a 96-well plate, supernatant containing 50 μg/50 μl protein was loaded and incubated with 3.645 μg of Ac-DEVD-AFC (Biomol, P-409) in 50 μl of 2× assay buffer (100 mM HEPES, 200 mM NaCl, 0.2% CHAPS, 2 mM EDTA,10% glycerol, 10 mM DTT, pH7.4) at 37°C for 1.5 h. AFC was cleaved from Ac-DEVD-AFC by activated caspase-3, and the free AFC was quantified with a Spectra Max M5 fluorescence microplate reader (excitation wavelength, 400 nm, emission wavelength, 508 nm; Molecular Devices, Sunnyvale, CA) by AFC standard curve (Biomol, KI-108). Caspase-3 activity was expressed as nanomoles of AFC formation per hour per milligram of protein.
Quantification of superoxide production.
Myocardial superoxide content (in the AAR) was determined by lucigenin-enhanced luminescence as previously described (34). Approximately 30 mg of protein of the ischemic LV region was separated, immediately minced, and incubated in 5 ml of oxygen-equilibrated Krebs-Henseleit solution containing 10 mM HEPES-NaOH (pH 7.4) for 20 min at room temperature. The samples were placed into glass tubes containing 10 μM lucigenin in a final volume of 1 ml of Krebs-Henseleit solution. Superoxide production was expressed as relative light units (RLU) per second per milligram of heart weight (RLU·s−1·mg wet tissue−1). The cellular origin of reactive oxygen species was determined by dihydroethidium (DHE) staining per manufacturer's protocol (Molecular Probes, Carlsbad, CA).
Western blot analysis.
Proteins were separated on SDS-PAGE gels, transferred to polyvinylidene difluoride membranes, and incubated with primary antibodies against acetyl-CoA carboxylase (ACC) phosphorylated ACC (pACC), gp91phox, or GAPDH (Cell Signaling Technology, Danvers, MA) followed by HRP-conjugated secondary antibody. The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL). The band was visualized by a Kodak Image Station 4000R Pro (Rochester, NY).
Determination of cardiac function.
Cardiac function was determined by echocardiography and LV catheterization methods 24 h after reperfusion, prior to chest reopening, as described previously (31, 32).
Statistical analysis.
All data in the text and figures are presented as means ± SE of n independent experiments. Hemodynamic data were analyzed by two-way ANOVA. All other data were analyzed by one-way ANOVA followed by the Bonferroni correction for post hoc t-tests (GraphPad Prism, San Diego, CA). Probabilities of 0.05 or less with Bonferroni correction were considered statistically significant.
RESULTS
AdipoRon treatment significantly improved cardiac functional recovery after reperfusion.
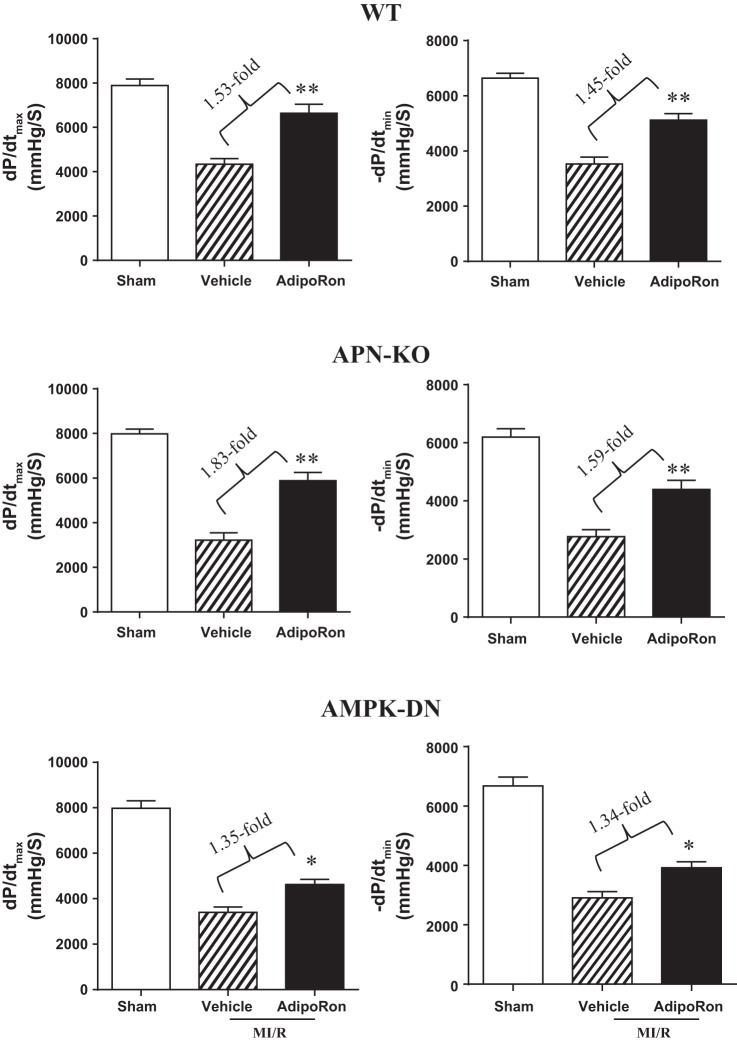
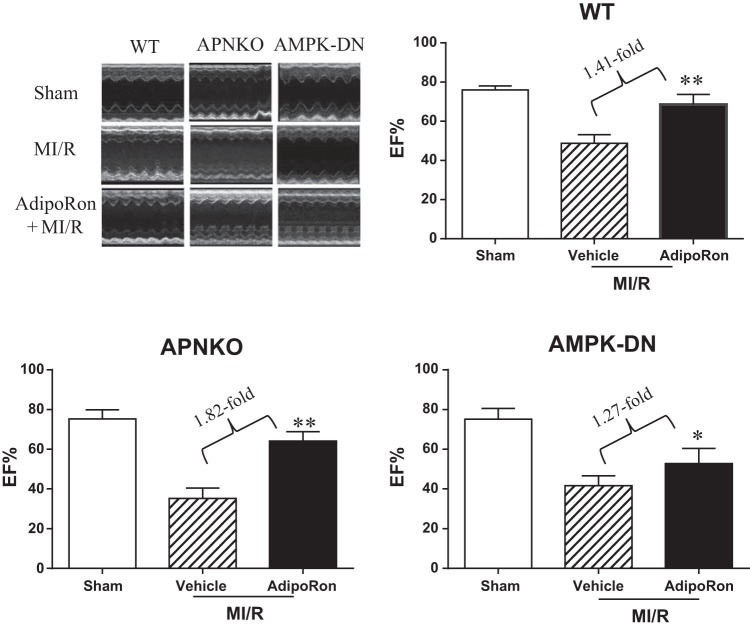
MI/R causes severe cardiac functional impairment 24 h after reperfusion (Figs. 1 and 2, WT). Treatment with AdipoRon significantly improved cardiac functional recovery, as evidenced by improved maximal positive and negative dP/dt (Fig. 1) and increased LV ejection fraction (EF%, Fig. 2 WT). Mean arterial blood pressure was slightly decreased in the MI/R group 24 h after reperfusion compared with the Sham-MI group. However, the difference was not statistically different. There was no difference in heart rate among the three groups studied (data not shown).
Fig. 1.
Oral AdipoRon administration significantly improved ±dP/dtmax in wild-type (WT), adiponectin knockout (APN−/−), and cardiomyocyte-specific AMPKα2 mutant transgenic mice (AMPK-DN); n = 14–16 animals/group. *P < 0.05, **P < 0.01 vs. MI/R + vehicle. P values between myocardiac ischemia/reperfusion (MI/R) vs. Sham are all < 0.01 (not labeled, the same for all figures).
Fig. 2.
Oral AdipoRon administration significantly enhanced left ventricular (LV) ejection fraction (EF%) determined by echocardiography in WT, APN−/−, and AMPK-DN mice. Note attenuated response to AdipoRon treatment in AMPK-DN mice vs. WT mice. However, a significant portion of cardioprotection is retained in AMPK-DN mice; n = 14–16 animals/group. *P < 0.05, **P < 0.01 vs. MI/R + vehicle.
AdipoRon significantly inhibited post-MI apoptosis.
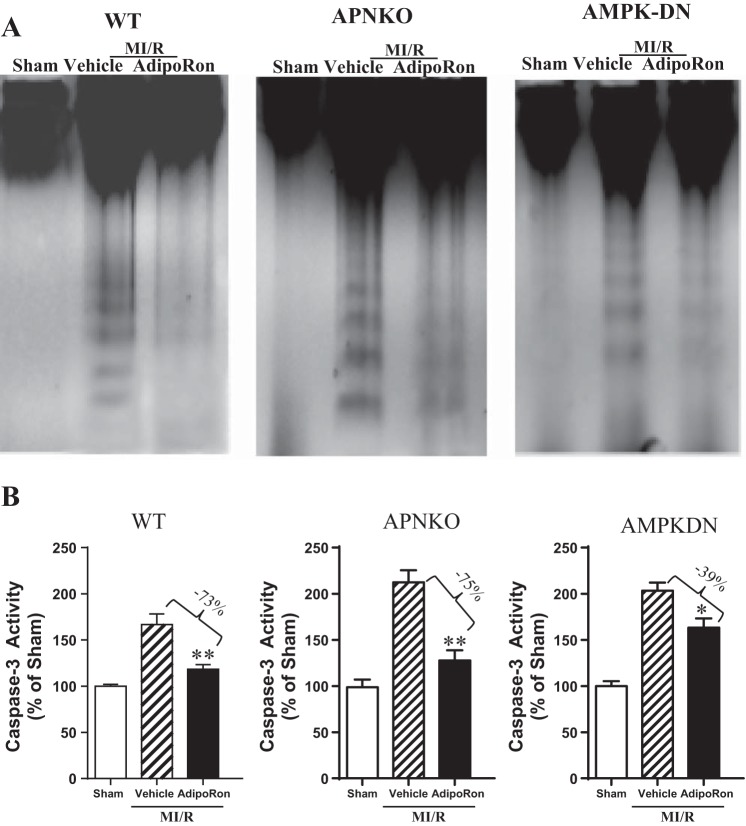
To determine the cellular mechanism responsible for cardioprotective effect of AdipoRon, we first determined the effect of AdipoRon treatment on cardiomyocyte apoptotic death by DNA ladder formation, a hallmark of apoptotic cell death. In myocardial tissue from Sham-MI hearts, no DNA ladder was detected (Fig. 3A, WT, lane 1). In contrast, the formation of DNA nucleosome ladders was clearly detected in myocardial tissues obtained from MI/R hearts receiving only vehicle (Fig. 3A, WT, lane 2). Most importantly, hearts treated with AdipoRon exhibited markedly decreased DNA fragmentation (Fig. 3A, WT, lane 3).
Fig. 3.
Oral AdipoRon administration significantly attenuated postischemic cardiac apoptosis determined by DNA ladder formation (A) and caspase-3 activation (B) in WT and APN−/− mice. AdipoRon also significantly inhibited DNA ladder formation and reduced caspase-3 activity in AMPK-DN mice, albeit to a lesser extent than seen in WT mice; n = 6–8 animals/group. *P < 0.05, **P < 0.01 vs. MI/R + vehicle.
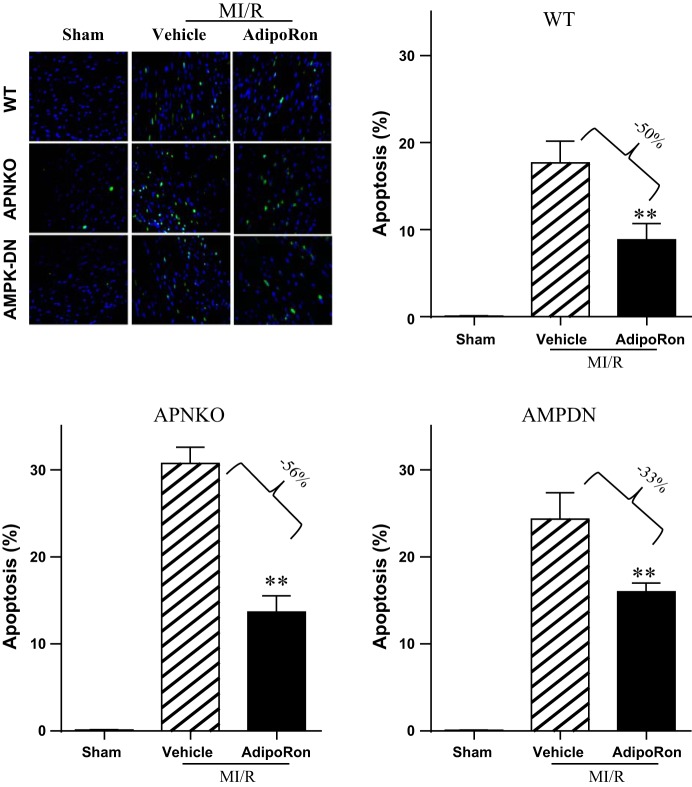
To determine the effect of AdipoRon on apoptosis in a quantitative manner, caspase-3 activation and TUNEL staining were performed. AdipoRon treatment markedly reduced ischemia/reperfusion-induced caspase-3 activation (Fig. 3B, WT). In Sham-MI hearts, an extremely low level of TUNEL-positive cells (Fig. 4, WT) was observed. In contrast, tissues from ischemic-reperfused hearts receiving only vehicle manifested prevalent TUNEL-positive nuclei (Fig. 4, WT). AdipoRon treatment reduced the number of TUNEL-positive cells (Fig. 4, WT).
Fig. 4.
Oral AdipoRon administration significantly attenuated postischemic cardiac apoptosis determined by TUNEL staining in WT and APN−/− mice. AdipoRon also significantly inhibited TUNEL staining in AMPK-DN mice, albeit to a lesser extent than seen in WT mice; n = 6–8 animals/group. *P < 0.05, **P < 0.01 vs. MI/R + vehicle.
Enhanced cardiomyocyte apoptosis in APN-deficient mice is rescued by AdipoRon administration.
To determine whether AdipoRon is effective in rescuing the heart from enhanced MI/R injury in APN-deficient animals, the effect of AdipoRon on cardiac dysfunction and cardiomyocyte apoptosis was determined in APN−/− mice. AdipoRon significantly improved cardiac function (Figs. 1 and 2, APN−/−) and reduced post-MI cardiomyocyte apoptosis, as evidenced by attenuated ladder formation (Fig. 3A, APN−/−), reduced caspase-3 activity (Fig. 3B, APN−/−), and decreased TUNEL-positive cells (Fig. 4, APN−/−).
Antiapoptotic effect of AdipoRon is attenuated but not lost in AMPK-DN mice.
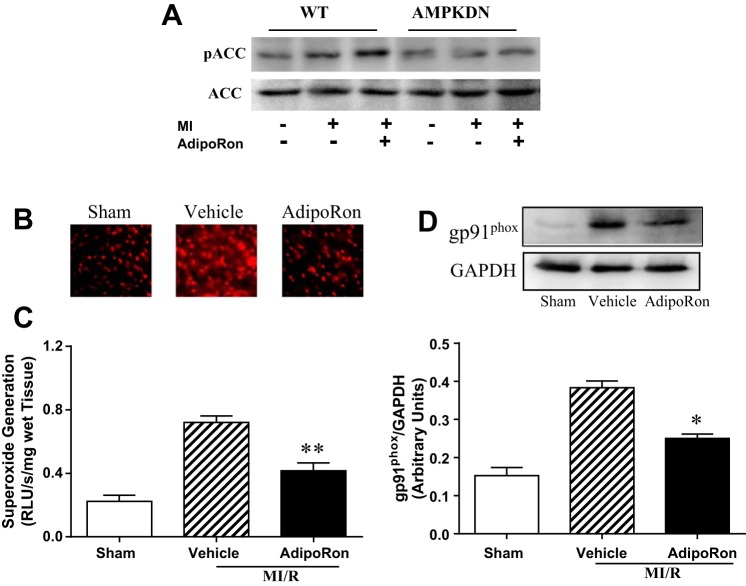
Compared with WT, cardiac dysfunction and apoptotic cell death caused by MI/R was increased in the AMPK-DN heart. We (34) reported previously that the cardioprotective effects of APN are only partially mediated by AMPK activation. In a similar fashion, the beneficial effects of AdipoRon on cardiac dysfunction are blunted (Figs. 1 and 2, AMPK-DN vs. WT) but not lost in AMPK-DN mice. Similarly, the antiapoptotic effect of AdipoRon is partially blocked but not completely lost in AMPK-DN mice. Specifically, administration of AdipoRon reduced caspase-3 activation by 39% (vs. 73% reduction in WT mice; Fig. 3) and TUNEL staining by 33% (vs. 50% reduction in WT mice; Fig. 4) in AMPK-DN mice. In the AMPK-DN heart, there was complete blockade of AdipoRon-induced phosphorylation of ACC (pACC, Fig. 5A). Therefore, the remaining portion of antiapoptotic effect of AdipoRon in AMPK-DN mice can be attributed to AMPK-independent signaling mechanisms.
Fig. 5.
A: AdipoRon activated AMPK signaling (determined by ACC phosphorylation) in WT but not in AMPK-DN animals. AdipoRon significantly inhibited ischemia/reperfusion-induced superoxide production, determined by DHE staining (B), lucigenin-enhanced luminescence assay (C), and gp91phox overexpression (D) in AMPK-DN mice; n = 6–8 animals/group for DHE staining and Western analysis; n = 9–11 animals/group for lucigenin-enhanced luminescence assay. *P < 0.05, **P < 0.01 vs. MI/R + vehicle.
AdipoRon significantly reduced NADPH oxidase expression and inhibited superoxide production in ischemic/reperfused heart.
Our previous study demonstrated that the antioxidant effect of APN is not mediated by AMPK (34). To determine whether AdipoRon might have any antioxidant effect (potentially contributive to the remaining antiapoptotic effect of AdipoRon observed in AMPK-DN mice), the effect of AdipoRon on postischemic superoxide production and gp91phox (the primary subform of NADPH oxidase expressed in adult cardiomyocytes) expression were determined in AMPK-DN mice. As summarized in Fig. 5, AdipoRon administration significantly reduced superoxide production assessed by DHE staining (Fig. 5B) and lucigenin-enhanced luminescence assay (Fig. 5C). Moreover, AdipoRon treatment significantly attenuated ischemia/reperfusion-induced gp91phox overexpression (Fig. 5D).
DISCUSSION
Early reperfusion after coronary occlusion remains the most effective means of limiting ischemic myocardial injury. However, evidence from animal studies, as well as clinical observations, demonstrates that reperfusion itself may cause additional cell death, defined as “reperfusion injury” (6). Strong epidemiological evidence suggests that type 2 diabetes not only causes coronary vascular injury thereby increasing ischemic heart disease prevalence but also exacerbates cardiac injury after ischemia/reperfusion insult in these patients (4, 13, 17). APN is a protein hormone produced primarily by adipocytes (20). In contrast to the majority of adipokines (such as TNFα), which are proinflammatory and significantly increased in diabetic patients, APN is markedly reduced in diabetic patients and is potently protective of the vasculature (18, 26, 28). Plasma APN levels significantly decrease after tissue injury, such as acute lung injury caused by ovalbumin challenge (27). Numerous epidemiological studies reveal the association between hypoadiponectinemia and increased cardiovascular disease risk in obesity and diabetes (8, 12, 15, 38). Additionally, recent clinical observations demonstrate that post-MI plasma APN levels correlate positively with myocardial salvage index and ejection fraction recovery (23). Persistent plasma hypoadiponectinemia post-myocardial infarction is predictive of future adverse cardiac events (2). Moreover, recent experimental studies demonstrate that myocardial reperfusion injury is significantly enhanced in APN-KO mice. Replenishment of recombinant APN in APN-KO mice was cardioprotective and fully rescued phenotypic alteration (20–22, 24). Importantly, multiple investigations [including the seminal study by Walsh et al. (25), a large animal model study by Kondo et al. (14), and our recent study (31)] have documented that APN administration in WT mice and pigs significantly reduces infarct size and improves cardiac function. Despite clear experimental evidence that supplementation of recombinant human APN exerts significant antidiabetic and cardioprotective actions, APN's complex quaternary structure and rapid turnover are major disadvantages to producing and administering APN in the requisite amounts for appropriate clinical care. Thus, the field has been awaiting the advent of low-molecular-weight APN receptor agonists capable of overcoming such hindrances.
In an effort to identify small synthetic molecules capable of activating the APN receptors (AdipoR1 and AdipoR2), Kadowaki et al. (19) recently screened a compound library and identified several molecules activating APN receptors, but they focused their in-depth analysis upon one, AdipoRon. AdipoRon binds, at a low micromolar concentration, to both AdipoR1 and AdipoR2. Like APN, in cultured mammalian cells it activates AMPK, an enzyme involved in many metabolic processes including insulin release, lipid synthesis inhibition, and glucose uptake stimulation. It also activates the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), which boosts mitochondrial proliferation and energy metabolism. Like APN, AdipoRon improved glucose metabolism, lipid metabolism, and insulin sensitivity in both cultured cells and mice, via APN receptor-dependent mechanisms. When db/db mice fed a high-fat diet were treated with AdipoRon, their metabolic improvements extended their life span. Furthermore, AdipoRon administration to chow-fed WT mice augmented exercise endurance capacity. Their study convincingly demonstrated the viable strategy of targeting APN receptors with low-molecular-weight agonists.
Strong clinical and experimental evidence supports the contribution of hypoadiponectinemia to enhanced cardiovascular injury in the diabetic population. We and others have previously demonstrated that MI/R injury is significantly exacerbated in APN-deficient mice, a phenotype fully rescued by recombinant APN replenishment (9, 25, 31). In the present study, AdipoRon administration rescued the pathological cardiac phenotype in APN-deficient mice, similarly to our previous report concerning APN administration (31). Moreover, our current study provides direct evidence that oral AdipoRon administration in WT mice also significantly improved postischemic cardiac function, as evidenced by increased ±dP/dtmax and enhanced LV ejection fraction. These results demonstrate that AdipoRon, an orally active small molecule previously shown to mimic the metabolic benefits of recombinant APN, is biologically active in protecting heart from ischemia/reperfusion injury.
Apoptotic cell death is the primary cell death pathway following MI/R and significantly contributes to postischemic cardiac dysfunction (3, 5). DNA ladder formation is highly specific for apoptotic cell death but lacks sensitivity and is difficult to quantify. In contrast, TUNEL staining of nuclei is extremely sensitive, but it is less specific for apoptosis, as some necrotic cells may stain TUNEL positive. Caspase-3 activation is the final common pathway leading to caspase-8- and caspase-9-induced apoptotic cell death. These three methods were used in combination to improve the accuracy and reliability of our results. AdipoRon reduced DNA ladder formation (Fig. 3A), inhibited caspase-3 activation (Fig. 3B), and decreased TUNEL staining (Fig. 4), indicating that AdipoRon possesses clear antiapoptotic property in ischemic/reperfused cardiomyocytes.
AMPK was once considered the most important downstream molecule mediating APN biological function. The effect of cardiac-specific AMPK inhibition upon the antiapoptotic effects of AdipoRon was determined. The beneficial effects of AdipoRon on cardiac dysfunction (Figs. 1 and 2) and apoptotis (Figs. 3 and 4) after MI/R were clearly blunted in AMPK-DN mice. These results indicate that AMPK activation contributes to the cardioprotective effect of AdipoRon. However, our results also clearly demonstrate that the cardioprotective effect of AdipoRon is not completely lost in AMPK-DN mice. Specifically, administration of AdipoRon in AMPK-DN increased ±dP/dtmax (1.35- and 1.34-fold), enhanced LV ejection fraction (1.27-fold), reduced caspase-3 activation (39%), and decreased TUNEL staining (33%). That AdipoRon retained a significant portion of antiapoptotic effect in AMPK-DN mice suggests the existence and contribution of mechanisms independent of AMPK signaling to AdipoRon-mediated antiapoptotic function in the ischemic/reperfused heart. This result is consistent with our previous study showing that the cardioprotective effect of APN is partially mediated by its AMPK-independent antinitrative action (35). Our present study provides supporting evidence that the remaining antiapoptotic action of AdipoRon in AMPK-DN mice is mediated by antioxidative effect. This notion is supported by the following three lines of evidence. First, oxidative stress plays critical causative roles in postischemic myocardial apoptosis and cardiac dysfunction (1, 30, 33); second, the antioxidative effect of APN is AMPK independent; and third, AdipoRon inhibits NADPH oxidase overexpression and superoxide overproduction in the ischemic/reperfused heart.
In summary, our study has demonstrated that the oral APN receptor agonist AdipoRon is effective in attenuating postischemic cardiac injury, indicating that APN receptor agonists are a promising novel therapeutic approach for treating cardiovascular complications caused by obesity-related disorders such as type 2 diabetes (11).
GRANTS
This research was supported by the following grants: National Institutes of Health HL-096686 and HL-123404, American Diabetes Association 7-11-BS-93 (X.-L. Ma), American Diabetes Association 1-14-BS-228, National Science Foundation of China 31322026 and 81170199 (Y.-J. Wang), and National Science Foundation of China 81270185 and 81470020 (J.-L. Zhao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z., J.Z., Rui Li, Y.-X.Y., B.L., Rong Li, and E.-H.G. performed experiments; Y.Z., J.Z., Rui Li, Y.-X.Y., and E.-H.G. analyzed data; W.B.L. and X.-L.M. interpreted results of experiments; W.B.L. and X.-L.M. edited and revised manuscript; W.J.K., X.-L.M., and Y.-J.W. conception and design of research; W.J.K., X.-L.M., and Y.-J.W. approved final version of manuscript; Y.-J.W. prepared figures.
ACKNOWLEDGMENT
We are greatly appreciative of Mr. Nadan Wang in the Center for Translational Medicine, Thomas Jefferson University, for expertise in evaluation of cardiac function by echocardiography.
REFERENCES
- 1.Ambrosio G, Zweier JL, Becker LC. Apoptosis is prevented by administration of superoxide dismutase in dogs with reperfused myocardial infarction. Basic Res Cardiol 93: 94–96, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Behrends M, Schulz R, Post H, Alexandrov A, Belosjorow S, Michel MC, Heusch G. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol 279: H1111–H1119, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res 61: 414–426, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Forrat R, Sebbag L, Wiernsperger N, Guidollet J, Renaud S, De Lorgeril M. Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res 27: 1908–1912, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Gao F, Tao L, Yan W, Gao E, Liu HR, Lopez BL, Christopher TA, Ma XL. Early anti-apoptosis treatment reduces myocardial infarct size after a prolonged reperfusion. Apoptosis 9: 553–559, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Dorado D. Myocardial reperfusion injury: a new view. Cardiovasc Res 61: 363–364, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Garitaonandia I, Smith JL, Kupchak BR, Lyons TJ. Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system. J Recept Signal Transduct Res 29: 67–73, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep 7: 25–33, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 6: 27–35, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann NY Acad Sci 874: 412–426, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Holland WL, Scherer PE. Cell biology. Ronning after the adiponectin receptors. Science 342: 1460–1461, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Jagasia D, McNulty PH. Diabetes mellitus and heart failure. Congest Heart Fail 9: 133–139, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv 3: 166–173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kupchak BR, Garitaonandia I, Villa NY, Smith JL, Lyons TJ. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFalpha and a ceramidase inhibitor. Biochemistry 48: 5504–5506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 45: 1172–1181, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Maruyoshi H, Kojima S, Otsuka F, Funahashi T, Kaikita K, Sugiyama S, Sakamoto T, Yoshimura M, Shimomura I, Ogawa H. Hypoadiponectinemia is associated with coronary artery spasm in men. Circ J 69: 1154–1156, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami KI, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503: 493–499, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med 16: 141–146, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology 151: 322–331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol 42: 1065–1074, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata R, Numaguchi Y, Matsushita K, Sone T, Kubota R, Ohashi T, Ishii M, Kihara S, Walsh K, Ouchi N, Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol 101: 1712–1715, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Ouchi N, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res 74: 471–479, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab 88: 3236–3240, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 118: 389–395, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF, Lam KSL. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab 89: 765–769, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol 61: 372–380, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Tao L, Gao E, Hu A, Coletti C, Wang Y, Christopher TA, Lopez BL, Koch W, Ma XL. Thioredoxin reduces post-ischemic myocardial apoptosis by reducing oxidative/nitrative stress. Br J Pharmacol 149: 311–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115: 1408–1416, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. PNAS 101: 11471–11476, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang QD, Pernow J, Sjoquist PO, Ryden L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res 55: 25–37, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation 119: 835–844, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Tao L, Yuan Y, Lau WB, Li R, Lopez BL, Christopher TA, Tian R, Ma XL. Cardioprotective effect of adiponectin is partially mediated by its AMPK-independent antinitrative action. Am J Physiol Endocrinol Metab 297: E384–E391, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative (alpha)2 subunit of AMP-activated protein kinase. J Biol Chem 278: 28372–28377, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 39: 1049–1059, 2004. [DOI] [PubMed] [Google Scholar]