Abstract
Exposure to chlorine (Cl2) damages airway and alveolar epithelia resulting in acute lung injury and reactive airway hyperresponsiveness (AHR) to methacholine. However, little is known about the effect of preexisting respiratory disease on Cl2-induced lung injury. By using a murine respiratory syncytial virus (RSV) infection model, we found that preexisting RSV infection increases Cl2 (187 ppm for 30 min)-induced lung inflammation and airway AHR at 24 h after exposure (5 days after infection). RSV infection and Cl2 exposure synergistically induced oxygen desaturation and neutrophil infiltration and increased MCP-1, MIP-1β, IL-10, IFN-γ, and RANTES concentrations in the bronchoalveolar lavage fluid (BALF). In contrast, levels of type 2 cytokines (i.e., IL-4, IL-5, IL-9, and IL-13) were not significantly affected by either RSV infection or Cl2 exposure. Cl2 exposure, but not RSV infection, induced AHR to methacholine challenge as measured by flexiVent. Moreover, preexisting RSV infection amplified BALF levels of hyaluronan (HA) and AHR. The Cl2-induced AHR was mitigated by treatment with inter-α-trypsin inhibitor antibody, which inhibits HA signaling, suggesting a mechanism of HA-mediated AHR from exacerbated oxidative injury. Our results show for the first time that preexisting RSV infection predisposes the lung to Cl2-induced injury. These data emphasize the necessity for further research on the effects of Cl2 in vulnerable populations and the development of appropriate treatments.
Keywords: flexiVent, methacholine, bronchoalveolar lavage, cytokines, inflammatory cells
chlorine (Cl2) is an irritant and reactive gas with significant occupational and environmental hazard concerns due to its wide industrial and domestic usage. Spillage of Cl2 in the environment during accidents or terrorist acts may result in significant morbidity and mortality to animals and humans (2, 15, 34, 38). Exposure of animals to Cl2 at concentrations comparable to the vicinity of industrial accidents damages airway epithelia and results in sloughing of airway mucosa, increased alveolar permeability, decreased ability of alveolar epithelial cells to clear fluid, damage to the pulmonary surfactant system (2, 5, 18, 33), coagulation abnormalities (42), and extensive systemic injury (1, 28). Animals surviving the initial exposure show significant structural and functional abnormalities, including the presence of foamy alveolar macrophages, patchy areas of ciliated airway cells (25, 26), the presence of excess amounts of goblet cells, hyperplasia and airway hyperreactivity (11), and inability to clear inhaled fungi (15). These injuries may result from chemical reactions of Cl2 and hypochlorous acid (HOCl), as well as various secondary reaction products, like chloramines (1, 33), chlorinated fatty acid, and low-molecular-weight hyaluronan (HA), a proinflammatory fragment generated by the fractionation of HA (19, 21).
Currently, little is known about the effects of previous existing respiratory infections on the subsequent Cl2-induced lung injury. Herein, we infected BALB/c mice with respiratory syncytial virus (RSV) and then exposed them to a dose of Cl2 (187 ppm for 30 min), which causes mild injury to noninfected mice. Our results demonstrate that RSV accentuates Cl2-induced airway hyperreactivity (AHR) to methacholine, lung inflammation, and upregulation of HA and that inhibition of HA signaling significantly reduces Cl2-induced AHR.
MATERIALS AND METHODS
Animals.
Ten-week-old BALB/c male mice (25 g body wt) were purchased from Charles River Laboratories. All procedures were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC).
Preparation of viral inoculum.
RSV strain A2 was propagated, stocked, and used as reported in our previous studies (8).
Mice RSV infection.
We administered 50-μl viral aliquots of RSV containing either 107 pfu of RSV strain A2 or UV-inactivated RSV to each nostril of anesthetized mice. Mock-infected animals received an equal volume of supernatant from uninfected Hep-2 cells.
Exposure of mice to Cl2 gas.
We exposed all mice to Cl2 gas (187 ppm) for 30 min as previously described (31) at 96 h postinfection. Control mice breathed room air instead of Cl2.
Statistical analysis.
Results are reported as group means ± SE. One-way ANOVA was used to determine differences among the group means followed by Tukey's multigroup comparisons. Differences at P < 0.05 were considered significant.
RESULTS
Preexisting RSV infection exacerbates Cl2 exposure-induced inflammation and lung injury.
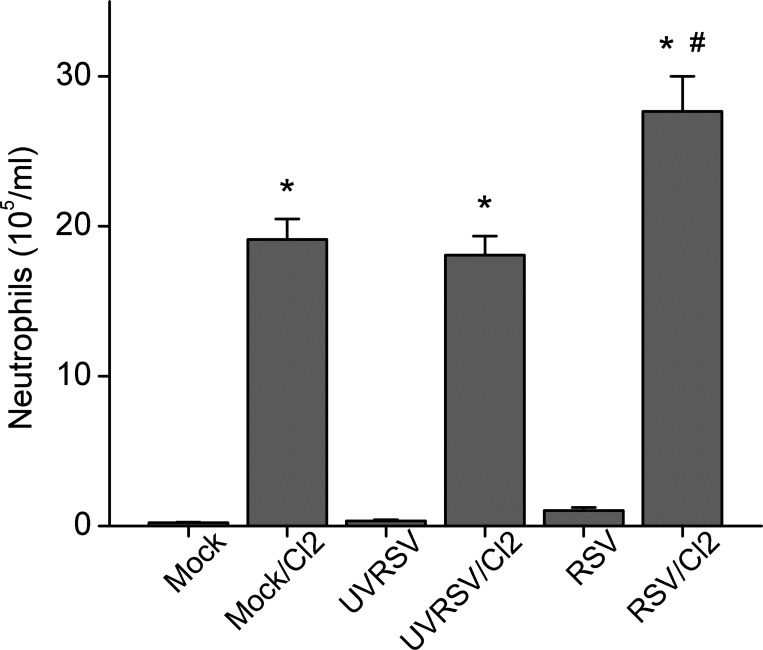
Mice infected with Mock, ultraviolet-inactivated RSV (UVRSV), or RSV for 5 days and exposed to Cl2 (187 ppm for 30 min) or air and returned to air for 24 h maintained the same arterial O2 saturation from pulse oximetry (SpO2) values on the fifth day postinfection as before treatment (94.5%, n = 5 for each group). Mice infected with RSV then exposed to Cl2 4 days later had significantly lower SpO2 at 24 h postexposure compared with their pretreatment values (91.6 ± 0.2 vs. 95.0 ± 0.3%, n = 5; P < 0.05). Both UVRSV and RSV infection, as well as Cl2 exposure, induced a significant exudation of protein in the bronchoalveolar lavage fluid (BALF) compared with mock-infected air control animals; however, no difference was seen between these treatment groups, suggesting no additive effect of RSV and Cl2 in injuring the lung endothelial-epithelial barrier in our present experiment conditions (data not shown). Mice infected with RSV alone had normal levels of neutrophils in their BALF 5 days postinfection; however, the significantly higher neutrophil counts in mice infected with RSV and exposed to Cl2 compared with UVRSV and Cl2 suggests a synergistic upregulation of the inflammation by these two injuries (Fig. 1). Number of alveolar macrophages remained at control levels in all groups (data not shown).
Fig. 1.
Preexisting respiratory syncytial virus (RSV) infection increases number of neutrophils in the bronchoalveolar lavage fluid (BALF) of mice exposed to Cl2. Neutrophil counts in BALF at the 5th day after various treatments (data are means ± 1 SE; *P < 0.05 compared with corresponding non-Cl2-exposed group; #P < 0.05, compared with all other groups; n = 5/group).
Cl2 exposure induces upregulation of cytokines and chemokines in the BALF.
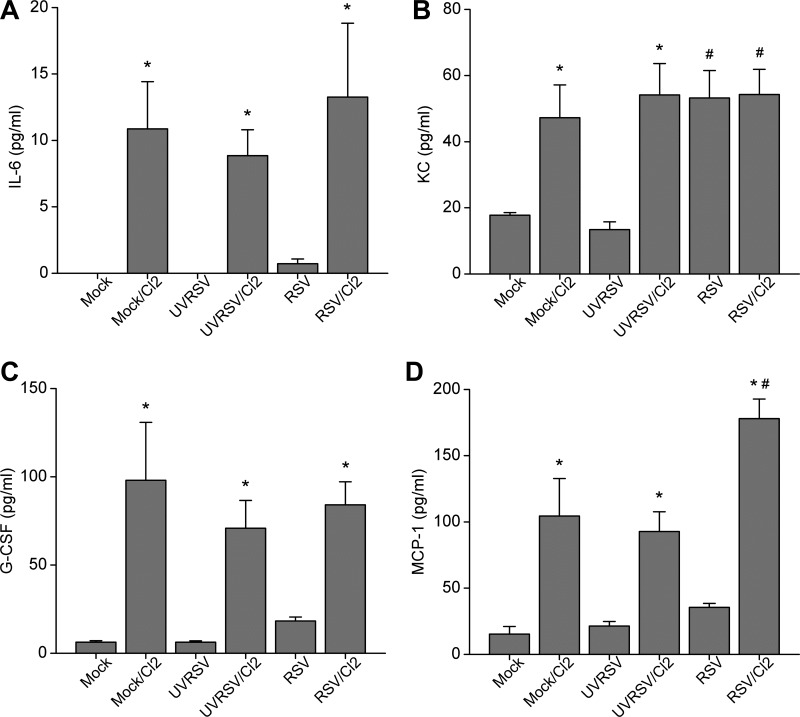
To assess the lung injury induced by RSV infection and Cl2 exposure, we assayed various cytokines and chemokines in the BALF at 24 h postexposure. First, we measured acute-phase cytokines, including IL-1, IL-6, TNF-α, and keratinocyte cytokine (KC, the murine homolog of human IL-8), since they are elevated in response to Cl2 exposure (24). We found that levels of IL-6 and KC were significantly upregulated by Cl2 exposure (Fig. 2, A and B); KC was upregulated by RSV infection as well, although no additive upregulation of KC was seen by RSV and Cl2. On the other hand, the concentrations of IL-1α, IL-1β, and TNF-α were not significantly different among any groups (data not shown), perhaps owing to the relatively low Cl2 concentration in our exposure protocol. Next, we assayed various chemotactic proteins and stimulating factors in the BALF. We found that levels of granulocyte colony-stimulating factor were significantly upregulated by Cl2 exposure but not RSV infection (Fig. 2C). Levels of MCP-1 were also significantly upregulated by Cl2 exposure but not RSV infection; however, there was a synergistic upregulation by preexisting RSV infection and Cl2 exposure (Fig. 2D). Levels of eotaxin, granulocyte macrophage colony-stimulating factor, IL-2, and IL-12 were not significantly different among the groups (data not shown). Type 2 cytokines have been found to be elevated after RSV infection and may play an important role in RSV-induced AHR and augmentation of allergic inflammation after subsequent airway exposure to allergens (29). However, in our experiments, type 2 cytokine levels (IL-4, IL-5, IL-9, and IL-13) were not significantly different among any groups (data not shown), suggesting that type 2 immune response was not responsible for the enhanced Cl2-induced inflammation and AHR by preexisting RSV infection.
Fig. 2.
Cl2 exposure induces upregulation of cytokines and chemokines in the BALF. Cytokines were measured by Bio-Plex multiplex suspension cytokine array (Bio-Rad) at the 5th day after treatment. A: IL-6 (data are means ± 1 SE; *P < 0.05 compared with corresponding non-Cl2-exposed group; n = 5/group). B: keratinocyte cytokine (KC) [data are means ± 1 SE; *P < 0.05 compared with corresponding noninfected group; #P < 0.05 compared with mock or ultraviolet-inactivated RSV (UVRSV)-infected group; n = 5/group]. C: granulocyte colony-stimulating factor (G-CSF) (data are means ± 1 SE; *P < 0.05 compared with corresponding non-Cl2-exposed group; n = 5/group). D: MCP-1 (data are means ± 1 SE; *P < 0.05 compared with corresponding non-Cl2-exposed group; #P < 0.05, compared with all other groups; n = 5/group).
RSV infection and Cl2 exposure synergistically upregulate BALF cytokines and chemokines.
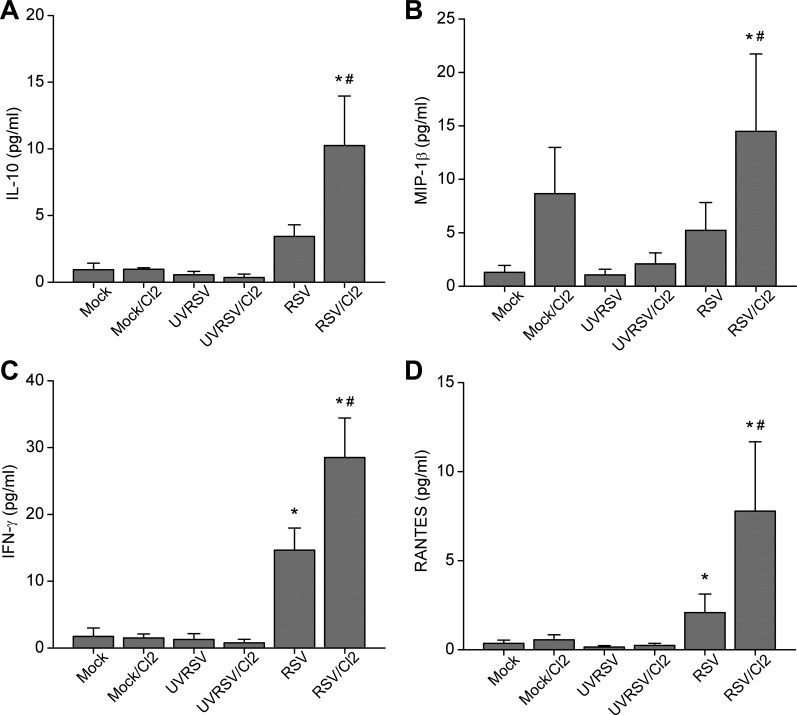
We found evidence of synergism between RSV infection and Cl2 exposure. For example, IL-10 and MIP-1β were only elevated in combined RSV infection and Cl2-exposed groups (Fig. 3, A and B). Moreover, levels of IFN-γ and RANTES were upregulated by RSV infection alone but not Cl2 exposure, but they were further upregulated by combined RSV infection and Cl2 exposure (Fig. 3, C and D).
Fig. 3.
RSV infection and Cl2 exposure synergistically induced upregulation of cytokines and chemokines in the BALF. A: IL-10. B: MIP-1β (data are means ± 1 SE; *P < 0.05 compared with RSV-infected group; #P < 0.05 compared with all other groups; n = 5/group). C: IFN-γ. D: RANTES (means ± 1 SE; *P < 0.05 compared with mock or UVRSV-infected group; #P < 0.05, compared with all other groups; n = 5/group).
Preexisting RSV infection increases Cl2-induced AHR and HA.
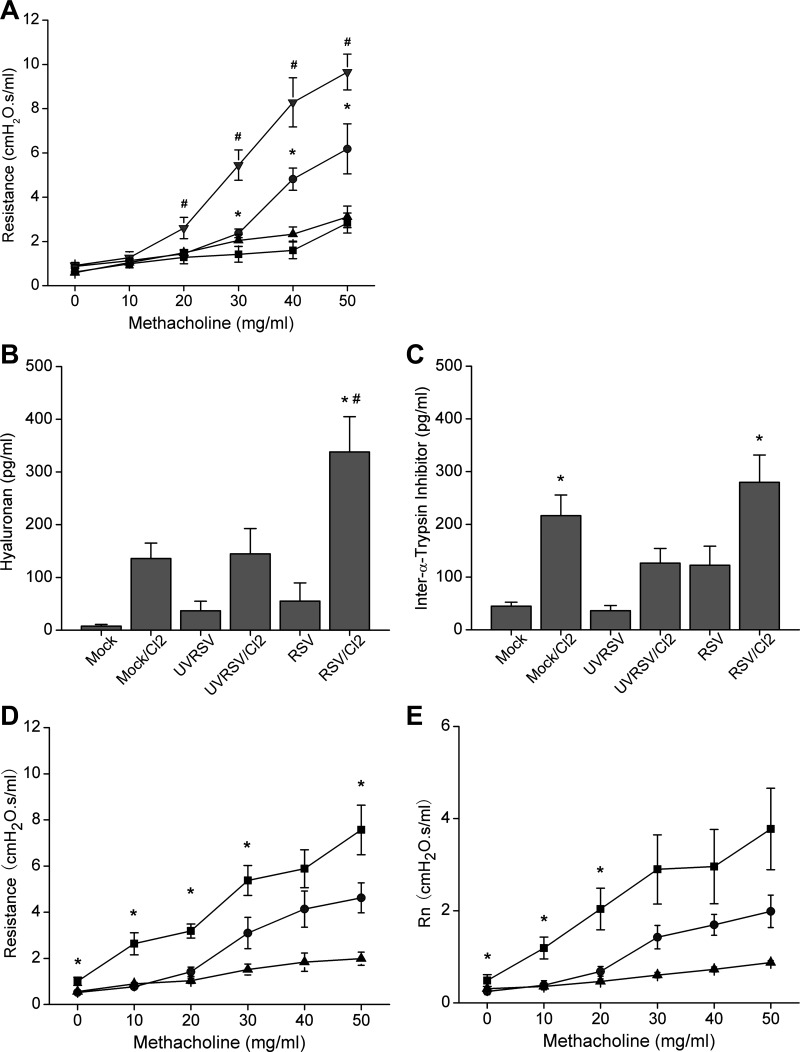
People with preexisting respiratory conditions are at increased risk for reactive airway disease (RADS) after Cl2 exposure (9). We therefore measured airway resistance and reactivity to methacholine at the fifth day after various RSV treatments (i.e., 120 h after mock, UVRSV, or RSV infection; 24 h after Cl2 or air exposure). We found that Cl2 exposure significantly increased airway responsiveness to methacholine challenge in mock-infected mice, consistent with previous reports (Fig. 4A). UVRSV infection alone did not increase airway responsiveness, and UVRSV-infected mice exposed to Cl2 exhibited the same extent of AHR as mock-infected mice exposed to Cl2 (data not shown). RSV infection alone also did not induce AHR compared with mock or UVRSV infection. However, preexisting RSV infection further increased Cl2 exposure-induced AHR (Fig. 4A).
Fig. 4.
Preexisting RSV infection exacerbated Cl2 exposure-induced airway hyperresponsiveness (AHR). A: mice were infected with either mock, UVRSV, or RSV and then exposed to 187 ppm Cl2 for 30 min at the 96th hour after infection. Airway resistance and responsiveness to indicated doses of methacholine challenge were measured with flexiVent at 24 h after Cl2 exposure (i.e., 5th day after infection). Control animals for Cl2 exposure were kept in room air only (■, mock/air; ▲, RSV/air; ● mock/Cl2; ▼, RSV/Cl2; data are means ± 1 SE; *P < 0.05 compared with mock/air and RSV/air groups at the same dose of methacholine; #P < 0.05 compared with all other groups; n = 6/group). B and C: RSV infection and Cl2 exposure synergistically upregulated hyaluronan (HA) in BALF. Concentrations of HA (B) and inter-α-trypsin inhibitor (IαI) (C) in the BALF were measured in cell-free BALF as described (19, 20) (data are means ± 1 SE; *P < 0.05 compared with RSV group; #P < 0.05 compared with all other groups; n = 5 for UVRSV and UVRSV/Cl2 groups, n = 10 for all other groups). D and E: IαI antibody reversed RSV/Cl2-induced AHR. RSV-infected and Cl2-exposed mice were treated twice with endotracheal administration of IαI antibody, at 1 h and 23 h after Cl2 exposure. Airway resistance and responsiveness to methacholine challenge were measured at 24 h after Cl2 exposure. Control animals received saline only. D: total resistance. E: Newtonian resistance (Rn) (■, RSV/Cl2/saline; ●, RSV/Cl2/IαI Ab; ▲, air control; data are means ± 1 SE; *P < 0.05 compared with RSV/Cl2/IαI Ab group at the same dose of methacholine; n = 6/group).
To explore the mechanism underlying the increased RSV/Cl2-induced AHR, we measured concentrations of HA and its binding protein inter-α-trypsin inhibitor (IαI) in the BALF at the fifth day after the various RSV treatments, since HA mediates AHR in Cl2-induced lung injury(19). RSV infection or Cl2 exposure alone caused an increase in HA levels, but this was not statistically significant after adjusting for multiple comparisons (Fig. 4B). However, RSV-infected and Cl2-exposed mice showed significantly higher HA levels compared with all other groups, suggesting that preexisting RSV infection and Cl2 exposure synergistically increase HA. IαI concentrations were significantly higher in mock/Cl2 and RSV/Cl2 groups compared with their corresponding non-Cl2-exposed groups, indicating that IαI was mainly upregulated by Cl2 (Fig. 4C). However, RSV infection alone did not induce significant upregulation of IαI.
Since IαI is necessary for optimal binding of HA to its receptor CD44 (17) and the development of Cl2-induced AHR (19), we treated RSV-infected and Cl2-exposed mice twice with endotracheal administration of IαI antibody, at 1 and 23 h after Cl2 exposure. We found that treatment with IαI antibody significantly decreased the Cl2-induced AHR in RSV-infected and Cl2-exposed animals (Fig. 4, D and E), suggesting that HA signaling contributes to increased AHR induced by RSV infection and Cl2 exposure.
DISCUSSION
We show for the first time that preexisting RSV infection increases Cl2-induced inflammation and AHR. RSV infection accounts for 22% of influenza-like illness in all age groups (27), with a particularly high morbidity and mortality in elderly (10) and immunocompromised patients (36), and is the most common cause of lower respiratory tract infection in children (30). Thus it is an ideal model to study the effect of preexisting infection on Cl2-induced lung injury.
Research into effective countermeasures for Cl2 exposure has led to new therapies including antioxidants (11, 18, 24, 41), anti-inflammatory agents (4), β2-agonists (31), transient receptor potential channel inhibitors (2), and strategies that improve NO bioavailability (16, 28, 39). However, little is known about the effect of preexisting pulmonary disease on Cl2-induced lung injury. We show that preexisting RSV infection exacerbates Cl2-induced inflammation and AHR. Remarkably, significant injury and AHR developed at 5 days post-RSV infection, a time when most pathological manifestations of RSV infection have returned to normal, and to 187 ppm Cl2 exposure, which only causes mild airway injury and AHR in healthy mice. This clearly demonstrates that preexisting conditions greatly exacerbate the response to even low levels of Cl2 and emphasizes the need for further research on mechanisms and appropriate countermeasures for vulnerable populations.
The lung injury induced by Cl2 exposure in this study is consistent with previous reports (31). We found that RSV infection or Cl2 exposure alone did not induce significant hypoxia, but combined RSV/Cl2 exposure did. This likely resulted from multiple factors including inhibition of epithelial sodium channels (6–8), resulting in decreased alveolar fluid clearance and increase of lung water.
In addition to acute lung injury, AHR is another major complication of Cl2 exposure (37). Interestingly, case reports suggest that host characteristics are crucial for the development of RADS after Cl2 exposure (3, 5, 35). Furthermore, even casual exposure to Cl2 exacerbates pulmonary disease. This underlines the importance of preexisting conditions in determining the effects of Cl2 exposure. The present report is the first to investigate the deleterious effect of a common condition, i.e., preexisting RSV infection, to Cl2-induced lung injury. Importantly, RSV infection has a strong linkage to the development of allergic asthma and may be a common cause of asthma exacerbation (23).
Recently, we showed that Cl2 exposure generates low-molecular-weight HA fragments (L-HA, 100–300 kDa), which activate RhoA and increase intracellular Ca2+ in airway smooth muscle cells, thus causing AHR (19). This led us to hypothesize that L-HA may serve as a novel mediator of the AHR induced by RSV infection and Cl2 exposure. L-HA and its binding partner IαI are increased in a number of lung diseases, such as cystic fibrosis (20). L-HA is both necessary and sufficient for the development of AHR after exposure to oxidant gases like ozone and Cl2 (13, 14, 19). Here, we find for the first time that RSV infection and Cl2 exposure potentiate the upregulation of HA in the BALF. Interestingly, RSV infection induces significant oxidative stress both in vitro and in vivo (32). Oxidative stress has been implicated in the pathogenesis of several acute and chronic airway diseases, like asthma and chronic obstructive pulmonary disease (12), and leads to breakdown of HA (19). Therefore, combined RSV infection and Cl2 exposure may cause additive production of oxidants, resulting in worsening oxidative stress and increased production of L-HA, which in turn mediates exacerbated AHR. Our finding that IαI antibody treatment, which blocks HA binding to CD44, mitigates the combined RSV/Cl2-induced AHR strongly suggests that L-HA signaling mediates exacerbated AHR after combined exposure. Furthermore, this mechanism of exacerbated oxidative stress and increased production of HA provides possible treatment targets of Cl2-induced AHR. Post-Cl2-exposure treatment of human airway smooth muscle cells with an antibody against IαI reverses the L-HA activation of RhoA (22). A previous study has shown increased level of serum urinary trypsin inhibitor, a breakdown product of IαI in patients with RSV and suggested that this may be a marker of the severity of RSV (40). Similarly, we have shown a positive correlation among IαI and number of inflammatory cells in the BALF of children with cystic fibrosis and lower airway disease (20).
In conclusion, our study for the first time showed that preexisting RSV infection exacerbates Cl2-induced inflammation and AHR, providing further evidence that predisposing factors, including preexisting respiratory diseases, play important roles in Cl2-induced lung injury. Our data suggest that the synergistic upregulation of L-HA by RSV and Cl2 contributes to the exacerbated AHR. These data emphasize the necessity of further research into the underlying mechanisms and the development of appropriate treatments for the specific vulnerable populations in the scenario of mass Cl2 exposure.
GRANTS
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant numbers 5U01ES015676, 3U54ES017218, 5R21 ES024027 02, and 1R21ES025423 01, and the Division of Intramural Research, National Institute of Environmental Health Sciences, NIH (S. Garantziotis). W. Song is the recipient of a FAER research fellowship grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.S., N.A., S.G., and S.M. conception and design of research; W.S., Z.Y., S.F.D., C.S., and S.G. performed experiments; W.S., Z.Y., S.F.D., N.A., C.S., S.G., and S.M. analyzed data; W.S., N.A., C.S., S.G., and S.M. interpreted results of experiments; W.S. and S.G. prepared figures; W.S. and S.M. drafted manuscript; W.S., N.A., S.G., and S.M. edited and revised manuscript; W.S., Z.Y., S.F.D., N.A., C.S., and S.M. approved final version of manuscript.
REFERENCES
- 1.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schoneich C, Reisdorph N, Powell RL, Chandler JD, Day BJ, Veress LA, White CW. SERCA: a critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol 52: 492–502, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balte PP, Clark KA, Mohr LC, Karmaus WJ, Van Sickle D, Svendsen ER. The immediate pulmonary disease pattern following exposure to high concentrations of chlorine gas. Pulm Med 2013: 325869, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Mo Y, Schlueter CF, Hoyle GW. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicol Appl Pharmacol 272: 408–413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark KA, Chanda D, Balte P, Karmaus WJ, Cai B, Vena J, Lawson AB, Mohr LC, Gibson JJ, Svendsen ER. Respiratory symptoms and lung function 8–10 months after community exposure to chlorine gas: a public health intervention and cross-sectional analysis. BMC Public Health 13: 945, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis IC, Lazarowski ER, Chen FP, Hickman-Davis JM, Sullender WM, Matalon S. Post-infection A77-1726 blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am J Respir Cell Mol Biol 37: 379–386, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 173: 673–682, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 286: L112–L120, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Evans RB. Chlorine: state of the art. Lung 183: 151–167, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 639–643, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol 46: 599–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol 429: 251–262, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 14.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol 304: L765–L773, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol 45: 419–425, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev 91: 221–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol 46: 342–354, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazrak A, Creighton JR, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Potts-Kant EN, Garantziotis S, Foster WM, Hollingsworth JW. Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6: e27137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22.Londino JD, Lazrak A, Noah JW, Aggarwal S, Bali V, Woodworth BA, Bebok Z, Matalon S. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J 2015 Mar 20. pii: fj.14–268755. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotz MT, Moore ML, Peebles RS Jr. Respiratory syncytial virus and reactive airway disease. Curr Top Microbiol Immunol 372: 105–118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med 50: 602–608, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo Y, Chen J, Humphrey DM Jr, Fodah RA, Warawa JM, Hoyle GW. Abnormal epithelial structure and chronic lung inflammation after repair of chlorine-induced airway injury. Am J Physiol Lung Cell Mol Physiol 308: L168–L178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L92–L102, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata Y, Falsey AR. Respiratory syncytial virus infection in adults. Antivir Ther 12: 659–670, 2007. [PubMed] [Google Scholar]
- 28.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free Radic Biol Med 53: 1431–1439, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 100: 226–233, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 368: 312–322, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song W, Wei S, Matalon S. Inhibition of epithelial sodium channels by respiratory syncytial virus in vitro and in vivo. Ann NY Acad Sci 1203: 79–84, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 285: 9716–9728, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whimbey E, Ghosh S. Respiratory syncytial virus infections in immunocompromised adults. Curr Clin Top Infect Dis 20: 232–255, 2000. [PubMed] [Google Scholar]
- 37.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7: 257–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 7: 278–283, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasui K, Baba A, Iwasaki Y, Kubo T, Aoyama K, Mori T, Yamazaki T, Kobayashi N, Ishiguro A. Neutrophil-mediated inflammation in respiratory syncytial viral bronchiolitis. Pediatr Int 47: 190–195, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol 45: 386–392, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarogiannis SG, Wagener BM, Basappa S, Doran S, Rodriguez CA, Jurkuvenaite A, Pittet JF, Matalon S. Postexposure aerosolized heparin reduces lung injury in chlorine-exposed mice. Am J Physiol Lung Cell Mol Physiol 307: L347–L354, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]