Abstract
Objective:
The present study aimed to assess the patient preference and tolerability of oral dipeptidyl peptidase-4 inhibitor (vildagliptin) versus injectable glucagon-like peptide-1 analog (liraglutide) in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy.
Methods:
This 24-week, randomized, multicenter, crossover study, patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy with hemoglobin A1c (HbA1c) ⩾6.5% and ⩽9.0% were randomized in a crossover manner to receive either vildagliptin/metformin single-pill combination (SPC) 50/1000 mg twice daily (n = 32) or 1.2 mg liraglutide as an add-on to metformin (0.6 mg [weeks 0–1] followed by 1.2 mg [weeks 2–12] once daily/1000 mg twice daily) (n = 30) for the first 12 weeks.
Results:
Patient preference at week 24 was similar, with 51.7% (n = 31) patients preferring vildagliptin/metformin SPC compared with 48.3% (n = 29) preferring liraglutide as an add-on to metformin therapy (p = 0.449). Post hoc analyses showed that more elderly patients (⩾65 years) preferred vildagliptin (65%; n = 13) over liraglutide (35%; n = 7) therapy. Liraglutide was associated with better improvement in fasting plasma glucose (–21.5 mg/dl versus –3.4 mg/dl) and HbA1c (–0.5% versus –0.3%) levels. Fewer adverse events were reported with vildagliptin/metformin SPC (n = 16) compared with liraglutide as add-on to metformin treatment (n = 46).
Conclusions:
In this pilot study, although both vildagliptin and liraglutide therapies were preferred similarly by the patients and showed effective control of glycemia over 12 weeks, vildagliptin was associated with fewer adverse events and was preferred more by elderly patients.
Keywords: dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 analog, liraglutide, medication preference, type 2 diabetes mellitus, vildagliptin
Introduction
Incretin-based therapies such as dipeptidyl peptidase-4 (DPP-4) inhibitors or glucagon-like peptide-1 (GLP-1) analogs increase plasma concentrations of active GLP-1 and exert antihyperglycemic effects in a glucose-dependent manner [Scheen, 2013]. Although these therapies are safe and effective in lowering hemoglobin A1c (HbA1c) without the risk of hypoglycemia and weight gain, they vary in administration route, efficacy, and side effects.
Adherence to type 2 diabetes mellitus therapy is vital for effective blood glucose management. Oral antihyperglycemic drugs such as DPP-4 inhibitors are being increasingly used in the management of type 2 diabetes mellitus because of their efficacy and patient preference perceived by the physician [Inzucchi, 2002]. Previous studies have shown that patients with type 2 diabetes mellitus prefer additional oral antihyperglycemic drugs over injectables [Hayes et al. 2006; Khan et al. 2009]. However, there are contradictory findings for patient preference between the oral DPP-4 inhibitor sitagliptin and injectable GLP-1 analog liraglutide in other studies [Dibonaventura et al. 2010; Pratley et al. 2010].
Vildagliptin, a DPP-4 inhibitor, improves glycemic control with a low risk of hypoglycemia and is well tolerated in a wide patient population with type 2 diabetes mellitus [Keating, 2014]. Liraglutide, a GLP-1 analog, is indicated subcutaneously once daily as an add-on to metformin or sulfonylurea in the management of type 2 diabetes mellitus [Novo Nordisk, 2013]. The present crossover study aimed to assess whether patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy preferred treatment with an oral, single-pill combination (SPC) of vildagliptin/metformin versus injectable liraglutide as an add-on to metformin after 12 weeks of treatment with each therapy, for a total duration of 24 weeks.
Methods
Study design
This 24-week, randomized, open-label, crossover, multicenter study was conducted across eight centers in Germany in patients with type 2 diabetes mellitus inadequately controlled with metformin therapy (2000 mg for ⩾12 weeks). After a screening period of 1 week, eligible patients were randomized to receive either vildagliptin/metformin SPC (50/1000 mg twice daily) or liraglutide/metformin (0.6 mg [weeks 0–1] followed by 1.2 mg [weeks 2–12] subcutaneously once daily/1000 mg orally twice daily) for 12 weeks (period I). After period I, the patients were crossed over to receive either liraglutide or vildagliptin while continuing metformin treatment for the next 12 weeks (period II; Figure 1). Eligible patients were randomized by third party using an interactive voice response (IVR) system that automates the random assignment of treatment sequences in 1:1 ratio. As patients were followed only telephonically after randomization, at week 0 they were being instructed by their study investigator to take the prescribed study drug correctly and emphasized the importance of compliance for patients’ safety and study validity. The patients were asked to contact their investigator if they were unable to take the prescribed study drug for any reason. All dosages prescribed and dispensed to the patient and all dose changes during the study were recorded on the Dosage Administration Record Case Report Form. Moreover, dose adjustments (except for liraglutide titration) and/or interruptions were not allowed in the study.
Figure 1.
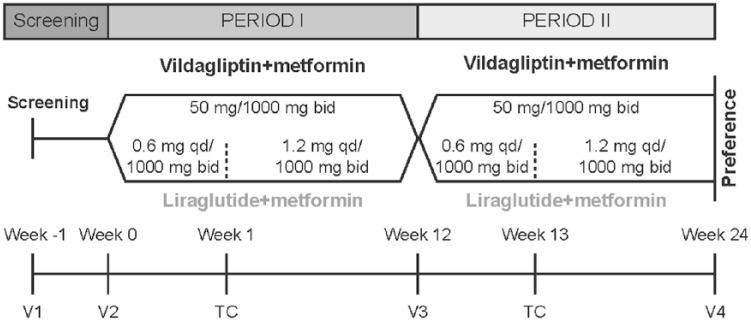
Study design.
TC, telephone contact; V, visit; bid, twice daily; qd, once daily.
Study population
Patients aged 18–80 years with type 2 diabetes mellitus inadequately controlled with metformin (2000 mg) for ⩾12 weeks before randomization, HbA1c ⩾6.5% and ⩽9.0%, and body mass index 19–35 kg/m2 were eligible to participate in this study. The key exclusion criteria included fasting plasma glucose (FPG) levels ⩾15 mmol/l; previous treatment with any antihyperglycemic drug (except metformin) within 3 months before study entry; history of type 1 or secondary forms of diabetes; congestive heart failure (New York Heart Association Class III or IV); myocardial infarction, stroke, or transient ischemic attacks within 6 months before study entry; or unstable angina within 3 months before study entry. Patients with any of the following laboratory abnormalities were excluded from the study: alanine transaminase or aspartate transaminase >3 times the upper limit of normal, total bilirubin >2 times the upper limit of normal, or glomerular filtration rate <60 ml/min/1.73 m2 (measured by Modification of Diet in Renal Disease [MDRD] formula).
Study end points and assessments
The primary endpoint was the proportion of patients preferring oral vildagliptin compared with injectable liraglutide after 24 weeks of treatment. Patients were asked the following two-choice question: ‘Based on your personal experience with both, [vildagliptin] and [liraglutide] plus metformin in the study, which form of medication would you prefer to take in the future?’. Patients were also asked to specify the reasons for their preference and required to select them from a list of reasons provided in the questionnaire [Treatment Satisfaction Questionnaire for Medication (TSQM)-9 questionnaire (official German Translation)] [Bharmal et al. 2009]. Physicians’ preference between oral vildagliptin and injectable liraglutide was also recorded at week 24. The secondary efficacy endpoints, FPG and HbA1c, were recorded at baseline and weeks 12 and 24. Safety assessments including body weight, adverse events (AEs), and serious AEs were monitored and recorded throughout the study.
Sample size and statistical analysis
A sample of 50 patients provided a power of >90% to exclude a preference rate ⩽50% for those preferring vildagliptin/metformin SPC, if the true preference rate was 75%. Demographic characteristics were presented using summary statistics on the safety set. Primary efficacy and patient and physician preferences were analyzed in all randomized patients who completed the preference questionnaire at the end of the study (efficacy set). Safety analyses were performed on all randomized patients who had received at least one dose of the study medication during at least one study period and had at least one safety assessment after baseline (safety set). The primary endpoint was tested using the exact Clopper–Pearson method at 95% confidence intervals (CIs). The secondary efficacy endpoints, FPG and HbA1c, were analyzed using descriptive statistics on the safety set. Treatments were compared using the analysis of variance model, with treatment, period, and patient as factors. Post hoc analysis for patient preference was also conducted in elderly patients (⩾65 years) in the safety set by using descriptive statistics.
Ethics and good clinical practice
The study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines and was reviewed and approved by the independent ethics committee or institutional review board of the study centers. Written informed consent was obtained from each patient before randomization. The study was registered at EudraCT (no. 2011-003818-16) and ClinicalTrials.gov [ClinicalTrials.gov identifier: NCT01518101].
Results
Patient disposition and baseline demographics
Of 93 patients screened, 62 were randomized (treatment sequence: vildagliptin–liraglutide [n = 32], liraglutide–vildagliptin [n = 30]) and 53 completed the study. The reason for vildagliptin–liraglutide discontinuation was unsatisfactory therapeutic effect (n = 3) whereas the reasons for liraglutide–vildagliptin discontinuation were withdrawn consent (n = 3), therapy no longer required (n = 1), and safety (n = 2) (Table 1).
Table 1.
Patient disposition (safety set).
| Patients, n (%) | Vildagliptin | Liraglutide | Total |
|---|---|---|---|
| Treated* | 60 (100.0) | 62 (100.0) | 62 (100.0) |
| Completed§ | 25 (41.7) | 28 (45.2) | 53 (85.5) |
| Discontinued§ | 3 (5.0) | 6 (9.7) | 9 (14.5) |
| Main cause of discontinuation§ | |||
| Unsatisfactory therapeutic effect | 3 (5.0) | 0 | 3 (4.8) |
| Patient withdrew consent | 0 | 3 (4.8) | 3 (4.8) |
| Adverse event | 0 | 1 (1.6%)§ | 1 (1.6) |
| Abnormal laboratory value | 0 | 1 (1.6%) | 1 (1.6) |
| Therapy no longer required | 0 | 1 (1.6%) | 1 (1.6) |
Refers to the number of patients who received each treatment at least once.
Refers to the last treatment before completion or discontinuation; the patient who discontinued due to an adverse event discontinued only period I but completed period II.
Safety set consisted of all patients who received at least one dose of the study medication during at least one study period and had at least one safety assessment after baseline.
The baseline and demographic characteristics are summarized in Table 2. Overall, the mean patient age was 60.3 years and 22 patients (35.5%) were aged ⩾65 years. The patients were predominantly White (98.4%) and 53.2% were women. The mean body weight was 90.3 kg and the mean HbA1c was ~7.3%. The mean duration of type 2 diabetes mellitus was 7.5 years and that of metformin therapy was 4.4 years.
Table 2.
Patient demographics and baseline characteristics (safety set).
| Parameters | Vildagliptin−Liraglutide (n = 32) | Liraglutide−Vildagliptin (n = 30) |
|---|---|---|
| Age (years) | 60.5 ± 11.23 | 60.1 ± 11.15 |
| ⩾65 (years), n (%) | 12 (37.5) | 10 (33.3) |
| Men, n (%) | 18 (56.3) | 11 (36.7) |
| Women, n (%) | 14 (43.8) | 19 (63.3) |
| Race, n (%) | ||
| White | 31 (96.9) | 30 (100.0) |
| Others | 1 (3.1) | 0 (0.0) |
| Body weight (kg) | 88.7 ± 14.77 | 92.0 ± 14.02 |
| BMI (kg/m2) | 30.5 ± 3.80 | 32.1 ± 3.19 |
| Duration of diabetes (years) | 7.0 ± 4.44 | 8.1 ± 7.21 |
| HbA1c (%) | 7.4 ± 0.58 | 7.3 ± 0.56 |
| FPG (mg/dl) | 159.8 ± 35.29 | 154.3 ± 30.17 |
Data are expressed as mean ± standard deviation, unless otherwise stated.
Safety set consisted of all patients who received at least one dose of the study medication during at least one study period and had at least one safety assessment after baseline.
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin.
Treatment preference
Of the efficacy-evaluable population (N = 60), 51.7% (n = 31; 95% CI: 38.4–64.8%) of patients preferred vildagliptin compared with 48.3% (n = 29; 95% CI: 35.2–61.6%) of patients who preferred liraglutide at week 24; this was not statistically significant (p = 0.449; Figure 2).
Figure 2.
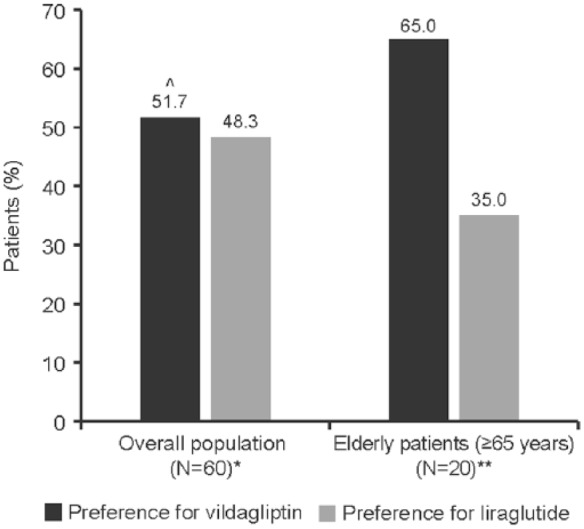
Patient preference for treatment at week 24.
^p = 0.449/
*Efficacy set consisted of all patients who completed the patient preference questionnaire at the end of the study (N = 60).
**Safety set consisted of all patients who received at least one dose of the study medication during at least one study period and had at least one safety assessment after baseline.
The reasons for treatment preferences by patients are presented in Table 3. Among the patients who preferred vildagliptin, 71% (n = 22) ranked the administration route as important/very important compared with 45% (n = 13) among those who preferred liraglutide. Likewise, 67.8% (n = 21) and 41.4% (n = 12) of patients with preference for vildagliptin and liraglutide, respectively, ranked side effects as important/very important reasons for preference. Preferences based on blood glucose lowering and other effects (weight loss, decreased blood pressure) were similar for both the medications.
Table 3.
Reasons for patient preference at week 24 (efficacy set).
| Total (N = 60) |
Patients who preferred vildagliptin (n = 31) |
Patients who preferred liraglutide (n = 29) |
||||
|---|---|---|---|---|---|---|
| N | % | n | % | n | % | |
| How you take the medication (oral or injection) | ||||||
| Very unimportant | 5 | 8.3 | 1 | 3.2 | 4 | 13.8 |
| Unimportant | 15 | 25.0 | 5 | 16.1 | 10 | 34.5 |
| Undecided | 5 | 8.3 | 3 | 9.7 | 2 | 6.9 |
| Important | 22 | 36.7 | 11 | 35.5 | 11 | 37.9 |
| Very important | 13 | 21.7 | 11 | 35.5 | 2 | 6.9 |
| Side effects (nausea, vomiting and diarrhea) | ||||||
| Very unimportant | 4 | 6.7 | 1 | 3.2 | 3 | 10.3 |
| Unimportant | 13 | 21.7 | 5 | 16.1 | 8 | 27.6 |
| Undecided | 10 | 16.7 | 4 | 12.9 | 6 | 20.7 |
| Important | 15 | 25.0 | 7 | 22.6 | 8 | 27.6 |
| Very important | 18 | 30.0 | 14 | 45.2 | 4 | 13.8 |
| Blood sugar lowering | ||||||
| Very unimportant | 3 | 5.0 | 1 | 3.2 | 2 | 6.9 |
| Unimportant | 8 | 13.3 | 3 | 9.7 | 5 | 17.2 |
| Undecided | 3 | 5.0 | 3 | 9.7 | 0 | 0.0 |
| Important | 9 | 15.0 | 7 | 22.6 | 2 | 6.9 |
| Very important | 37 | 61.7 | 17 | 54.8 | 20 | 69.0 |
| Other effects (weight loss and blood pressure decrease) | ||||||
| Very unimportant | 2 | 3.3 | 0 | 0.0 | 2 | 6.9 |
| Unimportant | 10 | 16.7 | 6 | 19.4 | 4 | 13.8 |
| Undecided | 9 | 15.0 | 5 | 16.1 | 4 | 13.8 |
| Important | 18 | 30.0 | 10 | 32.3 | 8 | 27.6 |
| Very important | 21 | 35.0 | 10 | 32.3 | 11 | 37.9 |
Efficacy set consisted of all patients who completed the patient preference questionnaire at the end of the study.
In contrast, the preference for vildagliptin (n = 13, 65%) was almost double than that of liraglutide (n = 7, 35%) in elderly patients (⩾65 years; Figure 2).
Physicians and patients showed similar preference for oral treatment: 55% physicians (n = 33; 95% CI: 41.6–67.9%) preferred vildagliptin compared with 45% (n = 27; 95% CI: 32.1–58.4%) who preferred liraglutide; however, it was not statistically significant (p = 0.259) (Figure 3).
Figure 3.
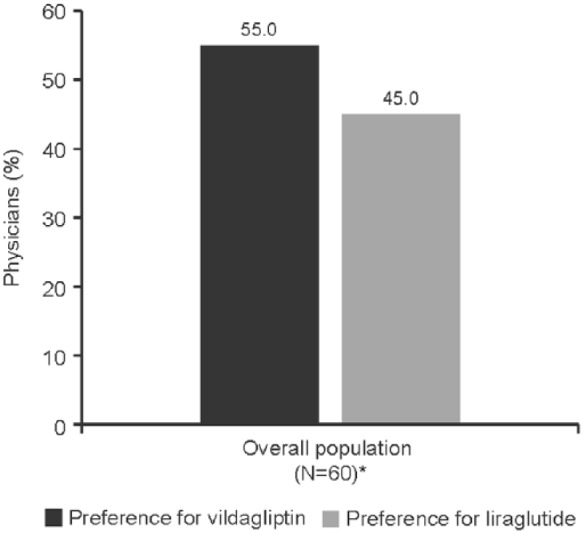
Physician preference for treatment at week 24.
*Efficacy set consisted of all patients who completed the patient preference questionnaire at the end of the study (N = 60).
Secondary efficacy
The lowering of mean [±SD] FPG (−21.5 [± 39.38] versus −3.4 mg/dl [± 31.77]) and HbA1c (−0.5 [± 0.61]% versus −0.3 [± 0.65]%) was higher in liraglutide-treated patients compared with vildagliptin-treated patients over 12 weeks of treatment, with a between-treatment difference of −18.0 [60.36] mg/dl (p<0.001) and −0.2 [1.14]% (p = 0.008), respectively.
Safety
Of 62 AEs reported in 28 patients, 46 were with liraglutide, mainly due to more gastrointestinal and metabolism and nutrition disorders, compared with 16 AEs with vildagliptin (Table 4). All AEs were mild to moderate in severity, except 1 AE (common cold) that was considered severe in a liraglutide-treated patient. Two patients experienced one serious AE while receiving liraglutide (cholecystolithiasis [day 80] and moderate coronary heart disease [day 168]), although neither of these events were considered drug-related. No deaths were reported during the study. Two patients withdrew treatment with liraglutide because of safety reasons (1 patient had abnormal laboratory values and another had moderate diarrhea, which was considered to be drug-related, on day 3). The mean [±SD] change in body weight was −0.1 [2.45] kg with vildagliptin compared with −2.2 [2.64] kg with liraglutide after 12 weeks treatment, with a between-treatment difference of −2.3 [3.90] kg (p < 0.001). There was no change in systolic or diastolic blood pressure or pulse rate between the two treatments after 12 weeks.
Table 4.
Safety and tolerability (safety set).
| Vildagliptin (n = 60) |
Liraglutide (n = 62) |
|||
|---|---|---|---|---|
| Number of AEs | Number (%) of patients | Number of AEs | Number (%) of patients | |
| All AEs | 16 | 9 (15.0) | 46 | 23 (37.1) |
| All SAEs | 0 | 0 | 2 | 2 (3.2) |
| Severe AEs | 0 | 0 | 1 | 1 (1.6) |
| Common AEs by primary SOC (occurring in ⩾2% of patients in any treatment group) | ||||
| Gastrointestinal disorders | 2 | 2 (3.3) | 20 | 15 (24.2) |
| Infections and infestations | 2 | 2 (3.3) | 5 | 4 (6.5) |
| Metabolism and nutrition disorders | 1 | 1 (1.7) | 9 | 5 (8.1) |
| Nervous system disorders | 2 | 2 (3.3) | 5 | 4 (6.5) |
| Musculoskeletal and connective tissue disorders | 2 | 2 (3.3) | 2 | 2 (3.2) |
| Cardiac disorders | 1 | 1 (1.7) | 2 | 2 (3.2) |
| Skin and subcutaneous tissue disorders | 2 | 2 (3.3) | 0 | 0 |
The columns vildagliptin and liraglutide refer to the last treatment received before the onset of an AE.
Safety set consisted of all patients who received at least one dose of the study medication during at least one study period and had at least one safety assessment after baseline.
AE, adverse event; SAE, serious adverse event; SOC, system organ class.
Discussion
A therapy that improves compliance and adherence is beneficial to achieve treatment goals. Similarly, simple-to-use drugs and patient preference could also affect treatment adherence. In this study, patient preference for vildagliptin and liraglutide was similar. However, 71.0% and 67.8% of patients who preferred vildagliptin ranked administration route and side effects as important and very important, respectively, compared with 45.0% and 41.4% of patients who preferred liraglutide, respectively, suggesting that oral formulations and tolerability are concerning issues for certain patients.
Although both GLP-1 analogs and DPP-4 inhibitors have similar pharmacological approaches, their routes of administration (subcutaneous versus oral) differ considerably [Scheen, 2013]. Furthermore, physicians may guide the pharmacological choice of treatment based on the clinical characteristics of patients with diabetes, drug safety profile, therapeutic goals, and patient preference [Inzucchi, 2002]. The glycemic target range and selection of therapy by physicians for patients with T2DM should be individualized accounting age, disease duration, comorbid conditions and likelihood of treatment compliance, capacity of self-care, propensity for hypoglycemia and overall safety and tolerability [Ismail-Beigi et al. 2011]. These factors are important especially in elderly patients, in whom achieving the glycemic goal is challenging because of comorbidities, cognitive dysfunction, and polypharmacy [Sue et al. 2012]. A published study on individualized treatment targets for elderly patients with type 2 diabetes mellitus using vildagliptin as an add-on or monotherapy (INTERVAL) demonstrated that these patients achieved the glycemic goal without tolerability based on the individualized target [Strain et al. 2013]. In the present study, patients aged ⩾65 years preferred vildagliptin (65%; n = 13) over liraglutide (35%; n = 7), which provides further insights into treatment preferences in this population.
Patients preferred the treatment which is most convenient/flexible, noninjectable, and had fewer physical and emotional side effects [Hayes et al. 2006]. In the present study, patients’ preference for vildagliptin and liraglutide were similar. In contrast, the results of internet survey conducted between 2008 and 2009 in patients from the United States and Europe (N = 3742) reported that most patients preferred a drug with the sitagliptin-like profile over the liraglutide-like profile [Dibonaventura et al. 2010]. In the present study, the most likelihood of patients’ preference for oral therapy is because of the route of administration and side effects, which was comparable with Dibonaventura and colleagues’ study. The likelihood of preference depended on the route of administration and increased significantly with age [Dibonaventura et al. 2010]. Patients with type 2 diabetes mellitus and poor glycemic control with the maximum doses of metformin and sulfonylurea preferred an additional oral therapy rather than injectables [Khan et al. 2009]. Moreover, a 26-week randomized study showed that an oral sitagliptin treatment was a good approach for managing patients with inadequate glycemic control on metformin monotherapy compared with injectable liraglutide [Charbonnel et al. 2013].
The FPG and HbA1c reductions were greater with liraglutide than with vildagliptin. However, vildagliptin had a better tolerability profile, particularly with respect to the incidence of gastrointestinal and metabolic disorders. Although the preferences for both medications were similar, the use of a single questionnaire at the end of the study (instead of after each study period) may also have introduced recall bias. In addition, study limitations such as small sample size, short duration, and crossover design without a washout period may not allow clear assessments of the efficacy.
The results from this pilot study indicated similar patient preference for oral therapy, vildagliptin and injectable therapy, liraglutide. Although the preference for vildagliptin was greater in elderly patients, and it was associated with fewer AEs, this data should be interpreted cautiously.
Acknowledgments
The authors would like to thank the patients and staff who participated in this study. All authors participated in the development and writing of the paper and approved the final manuscript for publication. The authors take full responsibility for the content of the paper and thank Anuja Shah, PhD and Madhavi Dokku, PhD (both from Novartis Healthcare Pvt Ltd, Hyderabad, India) for assisting in writing of the manuscript, collating comments from all authors, and editing the final manuscript.
Footnotes
Declaration of Conflicting Interests: JL has received fees for consultancy advisory boards, education talks, and speaker fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly & Co., Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi, outside the submitted work. ED and MD are employees of Novartis Pharma GmBH and as such may be eligible for the company’s stock and stock-options, outside the submitted work.
Funding: The study was funded by Novartis Pharma GmbH, Germany. The study sponsor participated in the study design, data collection, data review, data analysis, and writing of the report, and the decision to submit the article for publication.
Contributor Information
Jörg Lüdemann, Diabetes Medical Center, Poststraße 48, 14612 Falkensee, Germany.
Eva D. Dütting, Novartis Pharma GmbH, Nuernberg, Germany
Markus Dworak, Novartis Pharma GmbH, Nuernberg, Germany.
References
- Bharmal M., Payne K., Atkinson M., Desrosiers M., Morisky D., Gemmen E. (2009) Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnel B., Steinberg H., Eymard E., Xu L., Thakkar P., Prabhu V., et al. (2013) Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia 56: 1503–1511. [DOI] [PubMed] [Google Scholar]
- Dibonaventura M., Wagner J., Girman C., Brodovicz K., Zhang Q., Qiu Y., et al. (2010) Multinational internet-based survey of patient preference for newer oral or injectable type 2 diabetes medication. Patient Prefer Adher 4: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R., Bowman L., Monahan P., Marrero D., McHorney C. (2006) Understanding diabetes medications from the perspective of patients with type 2 diabetes: prerequisite to medication concordance. Diabetes Educ 32: 404–414. [DOI] [PubMed] [Google Scholar]
- Inzucchi S. (2002) Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 287: 360–372. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi I., Moghissi E., Tiktin M., Hirsch I., Inzucchi S., Genuth S. (2011) Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 154: 554–9. [DOI] [PubMed] [Google Scholar]
- Keating G. (2014) Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs 74: 587–610. [DOI] [PubMed] [Google Scholar]
- Khan K., Coyle F., Chowdhury T. (2009) Patients’ preference for subsequent therapy following secondary failure of metformin and sulphonylurea. Pract Diab Int 26: 282–284. [Google Scholar]
- Novo Nordisk (2013) Liraglutide (Victoza®), 0.65 mg subcutaneous injection, prescribing information. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails [accessed 28 March 2015]
- Pratley R., Nauck M., Bailey T., Montanya E., Cuddihy R., Filetti S., et al. (2010) Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Scheen A. (2013) GLP-1 receptor agonists or DPP-4 inhibitors: how to guide the clinician? Ann Endocrinol (Paris) 74: 515–522. [DOI] [PubMed] [Google Scholar]
- Strain W., Lukashevich V., Kothny W., Hoellinger M., Paldánius P. (2013) Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet 382: 409–416. [DOI] [PubMed] [Google Scholar]
- Sue K., Briscoe V., Clark N., Florez H., Haas L., Halter J., et al. (2012) Diabetes in older adults: a consensus report. J Am Geriatr Soc 60: 2342–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]


