Abstract
Purpose
The multitargeted tyrosine kinase inhibitor sorafenib is used for the treatment of advanced-stage renal cell carcinoma. However, the safety and efficacy of this agent have yet to be evaluated in the preoperative period, where there may be potential advantages including tumor downstaging. This prospective trial evaluates the safety and feasibility of sorafenib in the preoperative setting.
Patients and Methods
Thirty patients with clinical stage II or higher renal masses, selected based on their candidacy for nephrectomy, underwent preoperative treatment with sorafenib. Toxicities, surgical complications, and tumor responses were monitored.
Results
Of the thirty patients enrolled, 17 patients had localized disease and 13 had metastatic disease. After a course of sorafenib therapy (median duration, 33 days), a decrease in primary tumor size (median, 9.6%) and radiographic evidence of loss of intratumoral enhancement, quantified using a methodology similar to Choi criteria (median, 13%), was also observed. According to Response Evaluation Criteria in Solid Tumors, of the 28 patients evaluable for response, two patients had a partial response and 26 had stable disease, with no patients progressing on therapy. Toxicities from sorafenib were similar to that expected with this class of medication. All patients were able to proceed with nephrectomy and no surgical complications related to sorafenib administration were observed.
Conclusion
The administration of preoperative sorafenib therapy can impact the size and density of the primary tumor and appears safe and feasible. Further studies are required to determine if preoperative systemic therapy improves outcomes in patients undergoing nephrectomy for renal cell carcinoma.
INTRODUCTION
The advent of molecularly targeted therapeutics in renal cell carcinoma (RCC) has ushered in a change in the standard of care for patients with advanced disease. Among these novel agents are the antiangiogenic tyrosine kinase inhibitors, sorafenib and sunitinib, the vascular endothelial growth factor (VEGF) antibody, bevacizumab, and the mammalian target of rapamycin inhibitors, temsirolimus and everolimus. All of these drugs have gained US Food and Drug Administration approval for use in the metastatic setting based on pivotal phase III studies.1–5 The establishment of efficacy in advanced disease raises critical questions regarding the potential for application of these agents in the preoperative period. As these drugs—particularly those affecting angiogenesis—have potential for disease volume reduction, there has been interest in their application in the preoperative setting in hopes of improving surgical and clinical outcomes.
Importantly, the nature of these agents' effects provides several hypothetical positive impacts on immediate term clinical outcomes. Patients with localized T3 and T4, or node-positive tumors have a very high risk for disease recurrence, and can present complicated surgical resections. The incidence of such presentations is uncommon, but not rare.6 The potential to downsize tumors may afford greater ease and safety of surgical resection, and downstaging may alter the natural history of the disease. Furthermore, first-line therapy may afford an opportunity for immediate systemic therapy in metastatic patients that are marginal surgical candidates at the time of diagnosis, in whom the option of nephrectomy might be reconsidered at a future time point. A somewhat different scenario is that of the unresectable tumor. In a recent study, sunitinib was administered for an extended period of time for such patients with metastatic disease, demonstrating that conversion to a surgically operable primary tumor is possible.7
Cytoreductive nephrectomy—surgical removal of the primary renal tumor in the presence of metastatic disease—has been associated with improved survival in prospective randomized trials when paired with interferon, compared with immunotherapy alone.8,9 A critical unanswered question in contemporary practice is whether the survival benefit associated with cytoreductive nephrectomy extends to the era of targeted therapeutics. Immune-based treatments historically had little effect on the primary tumor, leading to a lack of preoperative experience in this disease.10–13 This has left the RCC field with little precedent for any purposeful role of systemic treatment in the presurgical setting.
Several safety concerns with the perioperative use of antiangiogenic drugs have been raised, including the potential for delayed wound healing, bleeding, and cardiovascular complications. A recent report implementing bevacizumab in the preoperative setting demonstrated the potential for tumor response, but at the expense of impaired wound healing.14 Currently, more objective information regarding the use of targeted therapy in the perioperative setting is necessary. This study is a prospective evaluation of preoperative sorafenib for patients at high risk for recurrence or undergoing cytoreductive nephrectomy, with the primary objective of determining safety and feasibility of adjunctive systemic treatment before intended surgery. Secondary objectives included evaluating the primary tumor response to sorafenib.
PATIENTS AND METHODS
Eligibility Criteria
This study was designed as a nonrandomized, open label prospective pilot trial evaluating the use of sorafenib in the preoperative setting in patients with clinical stage II or greater RCC. Study subjects were screened for eligibility from patients that were identified at the University of North Carolina Multidisciplinary Urologic Oncology Clinic or Rex Hospital affiliate practices in Raleigh, NC. Inclusion criteria required a minimum age of 18 years, radiologically suspicious or histologically proven clinical stage II RCC (greater than 7 cm on radiographic imaging), Eastern Cooperative Oncology Group performance status of 0 or 1, and planned nephrectomy of ≥ 4 weeks from enrollment. This study was designed to allow patient participation during a presurgical window of opportunity in order to avoid introducing a surgical delay. A minimum of 4 weeks before nephrectomy date was required to ensure adequate time for sorafenib administration. Patients who required more urgent surgical accommodation due to tumor-associated symptoms (pain or bleeding) were ineligible. This study was approved by the institutional review board and the University of North Carolina at Chapel Hill Committee on the Protection of the Rights of Human Subjects.
Normal renal, liver, bone marrow, and clotting function were required. Patients were deemed ineligible if they had received any prior therapy for RCC, had an active second malignancy, were pregnant, or had bleeding diathesis, brain metastasis, thromboembolism, congestive heart failure, or uncontrolled hypertension despite optimal management.
Statistical Considerations
Frequency tables, tabulating all toxicities attributed as more than likely to be due to treatment have been reported. The proportion (reported as a percentage) of patients completing sorafenib therapy with a dose delay or reduction has been reported, along with exact 95% CIs. Medians, along with their distribution-free 95% CIs, have been calculated for each of the covariates representing the following: days of treatment before surgery, duration of treatment, net days of treatment, days held, time of surgery, estimated blood loss, and hospital length of stay. Difference scores have been calculated for the paired differences between pre- and post-treatment measurements for both longest diameter and intratumoral density. Both absolute difference scores (pretreatment − post-treatment diameter) for comparison on the additive scale, and natural log difference scores (log pretreatment – log post-treatment diameter) for a proportional or percentage comparison on the multiplicative scale have been calculated. The Wilcoxon signed-rank test was used to test if these difference scores were significantly different from zero. All reported P values are nominal.
Statistical analyses were performed with SAS statistical software, version 9.2 (SAS Institute Inc, Cary, NC).
Sorafenib Administration and Evaluation of Adverse Events
Sorafenib was supplied by Bayer-Onyx and administered according to US Food and Drug Administration recommendations with a dose of 400 mg orally on a twice daily schedule. Dose reductions were allowed for grade 3 toxicities or toxicities intolerable to the patient. Dose level −1 reduction to 200 mg twice daily was allowed. Toxicities to sorafenib therapy were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0. Patients were advised to discontinue sorafenib between 24 to 48 hours before the planned surgery.
Nephrectomy Procedures
All patients underwent screening by a urologic oncologist before enrolling in the trial to ensure their surgical candidacy and the appropriateness of nephrectomy. Data regarding the time on treatment, interval between cessation of sorafenib and operative procedure, operative time for nephrectomy procedure, toxicities to sorafenib, and surgical complications were recorded.
Evaluation of Response
All patients had chest and abdominal imaging (computed tomography or magnetic resonance imaging) before the initiation of sorafenib therapy. Imaging was repeated before nephrectomy to evaluate radiographic response within the primary tumor by using RECIST (Response Evaluation Criteria in Solid Tumors).15 Densitometric measurement of intratumoral enhancement using a modification of the Choi criteria16 was performed to evaluate the effect of treatment on tumor viability.17 Briefly, using axial source computed tomography images (1.5 mm thickness) a three-dimensional image of the kidney was reconstructed with an imaging workstation (Terra Recon, San Mateo, CA). The largest dimensions of the primary renal tumor were outlined and a density measurement in Hounsfield units (HU) were obtained. The percent change in HU after treatment were recorded.
RESULTS
Patient Characteristics
The baseline characteristics of the study patients are displayed in Table 1. Median age was 57 years (range, 22 to 84) and was represented by 23 males (77%). The tumor histology for the group was predominantly clear cell (n = 21; 70%); although both papillary and chromophobe histologies were represented. Two patients with renal masses radiographically consistent with RCC that were identified after nephrectomy as having nonrenal cancers were enrolled, one urothelial carcinoma and one liposarcoma. These two non-RCC patients were included in the safety and toxicity analyses, but excluded from the response analysis. Individual patient and tumor descriptions as well as treatment response and surgical procedures are presented in Table 2. Thirteen patients (43%) had metastatic disease (M1); while the remaining 17 had localized disease. One patient was initially radiographically staged as T4, with local muscle invasion, however, on comparison to the post-treatment image, the primary lesion was retrospectively downstaged to a T1.
Table 1.
Patient Characteristics
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 57 | |
| Range | 22-84 | |
| Sex | ||
| Male | 23 | 77 |
| Female | 7 | 23 |
| Stage | ||
| I | 1 | 3.3 |
| II | 10 | 33.3 |
| III | 4 | 13.3 |
| IV | 15 | 50 |
| Pathology | ||
| Clear cell | 21 | 70 |
| Papillary | 4 | 13 |
| Mixed (clear cell/papillary) | 2 | 7 |
| Chromophobe | 1 | 3 |
| Other | 2 | 7 |
| Median duration of therapy, days | 33 | |
| Range | 8-59 | |
| Median length of time off treatment prior to surgery, days | 3 | |
| Range | 2-14 | |
Table 2.
Individual Patient Characteristics and Primary Tumor Responses
| Patient No. | Sex | Age | Net Days Treated | Days Off Sorafenib Prior to Surgery | Dose Reduced (yes/no) | Surgery | Operative Time (minutes) | TNM Stage | LD Pretreatment (cm) | LD Post-Treatment (cm) | LD Response (%) | Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 57 | 37 | 2 | No | LRN | 137 | T3aN0M0 | 8.6 | 7 | −19 | Clear cell |
| 2 | Male | 59 | 29 | 6 | No | LRN | 132 | T2N0M0 | 14.8 | 14.7 | −1 | Myxoid liposarcoma |
| 3 | Male | 53 | 35 | 2 | No | LRN | 120 | T1bN1M0 | 6.1 | 5.8 | −5 | Mixed |
| 4 | Female | 64 | 33 | 5 | No | LRN | 190 | T3aN0M0 | 7.8 | 6.9 | −12 | Clear cell |
| 5 | Male | 57 | 30 | 2 | No | ORN | 121 | T2N0M0 | 17 | 15.4 | −9 | Clear cell |
| 6 | Male | 72 | 24 | 2 | No | ORN+CT | 390 | T3bN1M1 | 12.1 | 12 | −1 | Papillary |
| 7 | Male | 53 | 39 | 2 | No | LRN+AN | 108 | T2N0M0 | 11.5 | 8.7 | −24 | Clear cell |
| 8 | Male | 73 | 35 | 6 | No | ORN+CT+AN | 321 | T4N0M1 | 8.8 | 8 | −9 | Clear cell |
| 9 | Male | 57 | 21 | 4 | Yes | ORN+CT | 113 | T1bN0M0 | 6.5 | 3.9 | −40 | Clear cell |
| 10 | Male | 59 | 33 | 3 | No | ORN+CT | 484 | T3cN0M1 | 7.8 | 7.8 | 0 | Clear cell |
| 11 | Female | 51 | 8 | 11 | No | LRN+AN | 101 | T2N0M0 | 8.5 | 8.6 | 1 | Chromophobe |
| 12 | Male | 72 | 55 | 2 | No | ORN | 86 | T1bN0M1 | 4.5 | 3.3 | −27 | Clear cell |
| 13 | Male | 46 | 40 | 4 | No | LRN+AN | 188 | T2N0M1 | 9 | 8 | −11 | Clear cell |
| 14 | Male | 69 | 20 | 2 | Yes | LRN+AN | 110 | T2N0M0 | 7.9 | 7.9 | 0 | Papillary |
| 15 | Female | 50 | 43 | 2 | No | ORN+CT | 107 | T3aN0M0 | 10.4 | 9.6 | −8 | Clear cell |
| 16 | Male | 53 | 42 | 4 | Yes | LRN | 232 | T2N2M1 | 8.8 | 6.5 | −26 | Clear cell |
| 17 | Male | 69 | 43 | 6 | Yes | LRN | 82 | T1aN2M1 | 4.2 | 2.7 | −36 | Clear cell |
| 18 | Female | 56 | 33 | 3 | No | ORN | 72 | T2N0M1 | 6.2 | 7.2 | 16 | Clear cell |
| 19 | Male | 58 | 31 | 3 | Yes | ORN+CT | 249 | T4N0M1 | 10 | 9.7 | −3 | Urothelial |
| 20 | Female | 84 | 47 | 3 | Yes | ORN+CT | 116 | T3aN2M1 | 12 | 10.8 | −10 | Clear cell |
| 21 | Male | 54 | 36 | 3 | Yes | LRN | 158 | T3N0M0 | 6.3 | 7 | 11 | Clear cell |
| 22 | Male | 48 | 32 | 6 | No | LRN+AN | 251 | T2N0M0 | 11.5 | 11.6 | 1 | Papillary |
| 23 | Male | 59 | 18 | 14 | No | LRN | 95 | T2N0M0 | 7.6 | 6.2 | −18 | Clear cell |
| 24 | Male | 62 | 34 | 3 | Yes | LRN+AN | 163 | T2N0M0 | 13.8 | 13.6 | −1 | Clear cell |
| 25 | Male | 47 | 23 | 4 | Yes | ORN+CT | 347 | T2N0M1 | 6.7 | 5.9 | −12 | Mixed |
| 26 | Female | 22 | 59 | 3 | No | ORN+CT | 362 | T2N2M0 | 12.4 | 12.1 | −2 | Clear cell |
| 27 | Female | 52 | 28 | 4 | Yes | ORN | 116 | T2N0M0 | 14.3 | 13.6 | −5 | Clear cell |
| 28 | Male | 65 | 10 | 21 | No | ORN+CT+LN | 415 | T4N2M0 | 11 | 11.9 | 8 | Papillary |
| 29 | Male | 49 | 50 | 2 | No | LRN+AN | 144 | T1bN0M1 | 5.3 | 4.7 | −11 | Clear cell |
| 30 | Male | 51 | 24 | 2 | No | LRN | 216 | T1bN0M1 | 5.8 | 4.6 | −21 | Clear cell |
Abbreviations: LD, longest dimension; LRN, laparoscopic radical nephrectomy; ORN, open radical nephrectomy; CT, caval thrombectomy; AN, adrenalectomy.
Sorafenib Therapy and Adverse Events
The median number of days on sorafenib before surgery was 33 (range, 8 to 59 days). The duration of therapy varied in this study due to its design as a window of opportunity trial. The adverse event profile was similar to that expected for sorafenib, with the most common adverse effects of any grade being fatigue, nausea, diarrhea, rash, stomatitis, hypertension, and hand-foot skin reaction (Appendix Table A1, online only). There were no grade 4 toxicities attributable to sorafenib therapy. The proportion of patients requiring a dose reduction was 10 (33%; 95% CI, 17% to 53%) of 30. The median time off of sorafenib before surgery was 3 days (range, 2 to 14 days).
Tumor Responses
The median primary tumor shrinkage in response to sorafenib administration was 9.6% (range, +16% to −40%). Most patients did achieve shrinkage of the primary tumor as shown in Figure 1A; five patients demonstrated slight growth. The majority of patients (93%) met RECIST for stable disease. Two patients met criteria for partial response and no patients had either confirmed progressive disease or complete response. Both the absolute and logarithmic differences between the pretreatment and post-treatment tumor diameters were found to be significantly decreased (P < .0001 and P = .0001, respectively).
Fig 1.
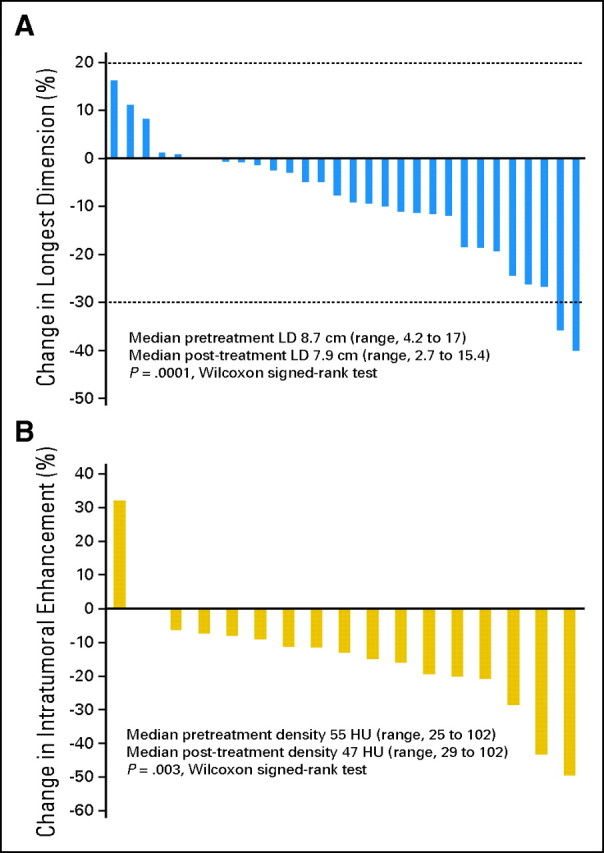
Waterfall plot distributions of primary tumor responses. (A) Waterfall plot of size response. Response Evaluation Criteria in Solid Tumors partial response criteria designated by lower dashed line at −30% and progressive disease criteria designated by upper dashed line at 20%. (B) Waterfall plot of intratumoral enhancement response. Change in contrast enhancement of the primary tumors, 17 patients evaluable (Hounsfield units [HU]). LD, longest dimension.
Radiographic intratumoral changes were also examined. The loss of contrast enhancement centrally within the tumor is consistent with intratumoral necrosis, and represents an alternative radiographic assessment of response to targeted therapy.17 An assessment of intratumoral enhancement was performed on the subset of patients who underwent contrast-enhanced computed tomography imaging before and after sorafenib treatment. Within these 17 evaluable patients, decrease in intratumoral density was observed in 15 patients (Fig 1B), with a median decrease in enhancement of 13%. As seen with dimensional response, the change in intratumoral enhancement was significant for both absolute or percentage measurements (P = .0007 and P = .003, respectively).
Four patients were downstaged from T2 to T1 lesions. Ten patients had T3 or T4 lesions, including cases of renal vein or inferior vena cava involvement present at all levels, including disease extending into the right atrium. None demonstrated disease progression within the vasculature during sorafenib treatment. Two patients with radiographic evidence of renal vein thrombus were pathologically negative for renal vein extension on nephrectomy. Response to therapy did not alter surgical approach (ie, laparoscopic v open) from the plan at the time of study enrollment in any patient in this series.
Nephrectomy Procedures and Surgical Complications
Surgical outcomes after preoperative therapy are presented in Table 3. Sixteen patients (53%) underwent laparoscopic radical nephrectomy; fourteen (47%) had open nephrectomy. Ten patients underwent caval thrombectomy and five had adrenalectomy in addition to their primary surgical procedure. For laparoscopic and open procedures the median operative time was 135 minutes (95% CI, 108 to 190 minutes) and 185 minutes (95% CI, 113 to 390 minutes), respectively; compared to the average institutional duration for laparoscopic and open nephrectomies (years 2006 to 2008) of 182 and 211 minutes, respectively. No complications of delayed wound healing, surgical dehiscence, or excessive bleeding were observed. One patient had a superficial wound breakdown postoperatively (postoperative day 8), which responded to conservative management. One patient suffered a postoperative myocardial infarction on postoperative day 1 in the setting of an extensive surgical resection with caval thrombectomy and adrenalectomy. The median estimated blood loss for patients receiving laparoscopic and open procedures was 150 mL (95% CI, 100 to 200 mL) and 950 mL (95% CI, 200 to 3,000 mL), respectively. The average length of hospital stay for patients having laparoscopic and open nephrectomies was 3.7 days (95% CI, 3 to 4 days) and 7.5 days (95% CI, 5 to 13 days), respectively.
Table 3.
Surgical Outcomes After Neoadjuvant Therapy With Sorafenib
| Parameter | Laparoscopic Nephrectomy |
Institutional Average for Laparoscopic Nephrectomy |
Open Nephrectomy |
Institutional Average for Open Nephrectomy |
||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |
| No. | 16 | — | — | 14 | — | — | ||
| % | 53 | 47 | ||||||
| Operative time, minutes | 135 | 108 to 190 | 182 | 185 | 113 to 390 | 211 | ||
| Estimated blood loss, mL | 150 | 100 to 200 | — | — | 950 | 200 to 3,000 | — | — |
| Length of hospital stay, days | 4 | 3 to 4 | — | — | 6 | 5 to 13 | — | — |
Clinical Outcomes
Currently only two of the 15 patients with localized disease have relapsed after surgery (both stage III) after a median follow-up of 217 days. Those patients with metastatic disease at the time of enrollment were routinely restarted on systemic therapy postoperatively, but only after 4 to 6 weeks and when wound closure was secure. These patients were managed in a variety of ways including therapy with high-dose interleukin-2 (n = 1), temsirolimus (n = 1), further tyrosine kinase inhibitor (sorafenib n = 3, sunitinib n = 5), and even a period of monitoring off therapy (n = 2). Decisions regarding subsequent therapy postnephrectomy were determined on a patient-by-patient basis.
DISCUSSION
Great interest has emerged in establishing the value of combining new molecularly targeted therapeutics with the standard practices of cytoreductive or curative nephrectomy. Many questions regarding longer-term clinical outcomes with these novel approaches remain unanswered at the moment; however, this prospective study of sorafenib in the preoperative setting provides important information to address concerns for safety and feasibility. In this study, sorafenib was well tolerated with no unexpected adverse events.
The study was designed to evaluate primary tumor response to therapy synchronously with surgery to avoid any potential complications due to rebound growth with a more prolonged wash-out period before surgery. No adverse surgical complications were observed even though patients discontinued therapy a brief median of 3 days before the operation. Sorafenib is advantageous in this regard due to its shorter half-life (25 to 48 hours) compared to sunitinib (40 to 60 hours; active metabolite, 80 to 110 hours) and bevacizumab (20 days; range, 11 to 50 days). There are benefits and drawbacks to any of the VEGF-targeted agents in this setting, and the optimal therapeutic agent in this setting remains to be clarified as we gain further experience with treatment in the preoperative setting.7,14,18
The optimal duration of preoperative therapy is also uncertain. In the Treatment Approaches in Renal Cancer Global Evaluation Trial study, patients with complete or partial response achieved best response in a median of 80 days.1 However, delaying nephrectomy for an extended period of time may be unacceptable. This study used an interval of 4 to 8 weeks of therapy as a time frame which approximates two cycles of treatment, when repeat imaging is typically obtained.
Biopsy was not mandated in this trial leading to two non-RCC lesions being treated, a liposarcoma and an urothelial carcinoma. These occurrences demonstrate the inability of radiographic imaging to conclusively diagnose renal cell carcinoma and supports the necessity of pretreatment biopsy prior embarking on preoperative systemic therapy.
The effect of sorafenib on the primary tumor was previously undocumented, and in this study, we observed a size decrease in the primary tumor in the majority of patients; two had partial responses using standard RECIST. Although not a validated end point for measuring tumor responses to VEGF-targeted therapy, the effect of sorafenib therapy on intratumoral enhancement of the primary tumor was measured, demonstrating a similarly significant effect on the primary tumor. Prior studies have confirmed that this radiographic change represents the accumulation of tumor necrosis,17 thus potentially adding to the evidence in support of a potent effect of targeted therapy on the cells of the primary tumor.
The results of our investigation further substantiate the findings of other recently published retrospective reports which suggest the utility of this multimodality approach to impact primary tumors, and in some cases render unresectable tumors resectable.18–22 Although no objective criteria exist to reproducibly define surgical resectability, the situation in which a tumor is not resectable due to size or local invasion occurs, although infrequently. This scenario represents an ideal setting for preoperative therapy, to the extent that a given agent may yield tumor volume reduction. Two recent series of patients treated preoperatively with intent to improve surgical candidacy have been published. In the first series, 19 patients with inoperable tumors were treated with sunitinib until they were deemed operable.7 Four patients were able to proceed with nephrectomy due to tumor downstaging after 6 months median follow-up, and no surgical complications were reported. In a similar retrospective analysis, three of 10 patients with inoperable tumors were able to be converted to operable candidates with sunitinib preoperative therapy.23
The tumor downstaging effect of targeted therapeutics such as sorafenib thus offers several potential advantages. In extreme cases it may afford the chance to render a difficult resection possible, and downstaging may impact the natural history of the disease with decreased risk of recurrence. Demonstration of clinical response in the cytoreductive setting may serve as a litmus test of response to targeted therapy. Certainly larger studies with longer follow-up are required to demonstrate such benefits, but these factors provide a lens through which we might begin to conceptualize the rationale for neoadjuvant therapy in RCC. In addition to potential direct clinical benefit to the patient, preoperative use of systemic therapy also affords the opportunity for sample collection to explore for predictive or pharmacodynamic correlative biologic markers.
This pilot study is limited by its small sample size, and the mixture of patients with localized and metastatic disease and varied histologies precludes a robust analysis of clinical end points. In addition, duration of therapy in this study varied as it was conceived with a window of opportunity design; continuing therapy to maximum tumor response may also be a reasonable approach. The selection of preoperative therapy should be considered with care. Among the tyrosine kinase inhibitors, sunitinib is more widely used in the first-line setting due to its reported superior response rate and progression-free survival and may warrant consideration in selected cases; however, the surgical findings with neoadjuvant sorafenib cannot be assumed to translate across therapies. Data is emerging from studies with both sunitinib and bevacizumab, as well as sorafenib, to enable clinicians to evaluate the relative risks and benefits of these biologic agents in the preoperative setting.
Based on our findings, sorafenib administration in the preoperative setting is safe and feasible and use of this class of agents should be considered for evaluation in larger prospective neoadjuvant trials designed to evaluate recurrence and survival end points for high-risk localized RCC. Future trials should be designed to address survival end points as combined-modality therapy in both locally advanced and metastatic renal cell carcinoma patients is further explored.
Acknowledgment
We thank the Lineberger Cancer Center for support of the Clinical Protocol Office and the Tissue Procurement Facility; and Stella Nelson for valuable assistance. We owe our deepest gratitude to the courageous patients who were willing to participate in this study to further advance the clinical management of renal cell carcinoma.
Appendix
Table A1.
Toxicities Attributable to Sorafenib Treatment
| Adverse Event | Grade |
|||
|---|---|---|---|---|
| II |
III |
|||
| No. | % | No. | % | |
| Anemia | 1 | 3 | 0 | |
| Anorexia | 3 | 10 | 0 | |
| Constipation | 1 | 3 | 0 | |
| Diarrhea | 1 | 3 | 0 | |
| Fatigue | 2 | 7 | 1 | 3 |
| Hand-foot syndrome | 5 | 17 | 2 | 7 |
| Headache | 2 | 7 | 1 | 3 |
| Hypertension | 6 | 20 | 1 | 3 |
| Hypophosphatemia | 2 | 7 | 0 | |
| Mucositis | 2 | 7 | 0 | |
| Rash, acneiform | 2 | 6 | 4 | 13 |
| Weight loss | 1 | 3 | 0 | |
Footnotes
Supported by the Doris Duke Charitable Fund (W.K.R.) and Bayer and Onyx Pharmaceuticals.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00405366.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: W. Kimryn Rathmell, Bayer/Onyx (C) Stock Ownership: None Honoraria: W. Kimryn Rathmell, Bayer/Onyx Research Funding: W. Kimryn Rathmell, Bayer/Onyx Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Chirag Amin, Dominic T. Moore, W. Kimryn Rathmell
Administrative support: Gayle Grigson, Cathy Watkins
Provision of study materials or patients: C. Lance Cowey, Chirag Amin, Raj S. Pruthi, Eric M. Wallen, Matthew E. Nielsen, Keith V. Nance, Jeffrey Crane, William Y. Kim, Paul A. Godley, Young E. Whang, Julia R. Fielding, W. Kimryn Rathmell
Collection and assembly of data: C. Lance Cowey, Gayle Grigson, Cathy Watkins, Julia R. Fielding
Data analysis and interpretation: C. Lance Cowey, Dominic T. Moore
Manuscript writing: C. Lance Cowey, Dominic T. Moore, W. Kimryn Rathmell
Final approval of manuscript: C. Lance Cowey, Raj S. Pruthi, Eric M. Wallen, Matthew E. Nielsen, Dominic T. Moore, William Y. Kim, Paul A. Godley, Young E. Whang, Julia R. Fielding, W. Kimryn Rathmell
REFERENCES
- 1.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 6.Frank I, Blute ML, Leibovich BC, et al. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173:1889–1892. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 7.Thomas AA, Rini BI, Lane BR, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181:518–523. doi: 10.1016/j.juro.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 9.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 10.Atzpodien J, Schmitt E, Gertenbach U, et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: Results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) Br J Cancer. 2005;92:843–846. doi: 10.1038/sj.bjc.6602443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JI, Atkins MB, Urba WJ, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: A cytokine working group randomized trial. J Clin Oncol. 2003;21:3133–3140. doi: 10.1200/JCO.2003.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: A multicentric randomized study. J Clin Oncol. 2001;19:425–431. doi: 10.1200/JCO.2001.19.2.425. [DOI] [PubMed] [Google Scholar]
- 13.Messing EM, Manola J, Wilding G, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: An Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21:1214–1222. doi: 10.1200/JCO.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Jonasch E, Wood CG, Matin SF, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–4081. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 17.Cowey CL, Fielding J, Rathmell WK. The loss of radiographic enhancement in primary renal cell carcinoma tumors following multi-targeted receptor tyrosine kinase therapy is an additional indicator of response. Urology. doi: 10.1016/j.urology.2009.06.105. in press. [DOI] [PubMed] [Google Scholar]
- 18.Amin C, Wallen E, Pruthi RS, et al. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology. 2008;72:864–868. doi: 10.1016/j.urology.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 19.Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–98. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Shuch B, Riggs SB, LaRochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: Observations and implications for a new treatment paradigm. BJU Int. 2008;102:692–696. doi: 10.1111/j.1464-410X.2008.07660.x. [DOI] [PubMed] [Google Scholar]
- 21.Karakiewicz PI, Suardi N, Jeldres C, et al. Neoadjuvant sutent induction therapy may effectively down-stage renal cell carcinoma atrial thrombi. Eur Urol. 2008;53:845–848. doi: 10.1016/j.eururo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Robert G, Gabbay G, Bram R, et al. Complete histologic remission after sunitinib neoadjuvant therapy in T3b renal cell carcinoma. Eur Urol. 2009;55:1477–1480. doi: 10.1016/j.eururo.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Bex A, van der Veldt AA, Blank C, et al. Neoadjuvant sunitinib for surgically complex advanced renal cell cancer of doubtful resectability: Initial experience with downsizing to reconsider cytoreductive surgery. World J Urol. 2009;27:533–539. doi: 10.1007/s00345-008-0368-7. [DOI] [PubMed] [Google Scholar]


