Abstract
Diets rich in fruits and vegetables reduce risk of adverse cardiovascular events. However, the constituents responsible for this effect have not been well established. Lately, the attention has been brought to vegetables with high nitrate content with evidence that this might represent a source of vasoprotective nitric oxide. We hypothesized that short-term consumption of spinach, a vegetable having high dietary nitrate content, can affect the arterial waveform indicative of arterial stiffness, as well as central and peripheral blood pressure (BP). Using a placebo-controlled, crossover design, 27 healthy participants were randomly assigned to receive either a high-nitrate (spinach; 845 mg nitrate/day) or low-nitrate soup (asparagus; 0.6 mg nitrate/day) for 7 days with a 1-week washout period. On days 1 and 7, profiles of augmentation index, central, and brachial BP were obtained over 180 min post-consumption in 4 fasted visits. A postprandial reduction in augmentation index was observed at 180 min on high-nitrate compared to low-nitrate intervention (-6.54 ± 9.7% vs. -0.82 ± 8.0%, p = 0.01) on Day 1, and from baseline on Day 7 (-6.93 ± 8.7%, p < 0.001; high vs. low: -2.28 ± 12.5%, p = 0.35), suggesting that the nitrate intervention is not associated with the development of tolerance for at least 7 days of continued supplementation. High vs. low-nitrate intervention also reduced central systolic (-3.39 ± 5.6 mmHg, p = 0.004) and diastolic BP (-2.60 ± 5.8 mmHg, p = 0.028) and brachial systolic BP (-3.48 ± 7.4 mmHg, p = 0.022) at 180 min following 7-day supplementation only. These findings suggest that dietary nitrate from spinach may contribute to beneficial hemodynamic effects of vegetable-rich diets and highlights the potential of developing a targeted dietary approach in the management of elevated BP.
Keywords: Vascular, Augmentation index, Blood pressure, Dietary nitrate, Spinach
Introduction
Hypertension is the most common primary diagnosis affecting 970 million people worldwide [1]. Dietary and lifestyle modifications represent the first line of therapy, with the intent to delay or prevent medication-based control [2]. However, the effect of dietary approaches, their components, or mechanisms of action have yet to receive appreciable attention through well designed clinical trials.
In recent years, considerable evidence has emerged to support dietary nitrate supplementation to increase nitric oxide (NO) mediated vasodilation via the oxygen-independent pathway and thereby affect arterial hemodynamics. Prospective epidemiological cohort studies have shown that dietary patterns rich in vegetables, particularly leafy greens such as those found in the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets, are associated with a decreased risk of cardiovascular disease (CVD), demonstrating reduction in blood pressure (BP) comparable to starting doses of single oral antihypertensive agents [3]. Hord et al. proposed that the BP lowering effects of the DASH diet may be attributed to the high-nitrate vegetables commonly associated with this intervention [4], such as leafy greens and root vegetables. Among the vegetables, spinach has been identified as one of the highest sources of dietary nitrate for humans [4].
While there is emerging clinical and observational data supporting the role of acute nitrate ingestion in peripheral BP, there are limited studies to date evaluating the effect of dietary nitrate on augmentation index (AI), a measure of arterial stiffness and central BP through pulse wave analysis [5,6,7]. Evaluation of parameters of the aortic pressure waveform, including central pressure and arterial stiffness, have been demonstrated to be more closely associated with cardiovascular endpoints compared to the assessment of brachial BP [8]. Therefore, our objectives were to evaluate whether consumption of spinach, a high dietary nitrate source, over 7-days would affect an index of arterial stiffness and lower central and peripheral blood pressures.
Materials and Methods
Participants
Healthy participants were recruited based on the following inclusion criteria: 18-50 years of age; normal BP as defined by JNC 7 criteria [9]; BMI < 30 kg/m2; and exclusion criteria: seated brachial systolic/diastolic BP > 140/90 mmHg or diagnosed hypertension; allergy or sensitivity to study materials; presence of gastrointestinal complications or conditions; use of medications or antibiotics within one month of study commencement and self-reported presence of any major condition or illnesses. All participants gave written informed consent prior to the beginning of the study. All study protocols were approved by the St. Michael's Hospital Research Ethics Board and the study was conducted at the Risk Factor Modification Centre at St. Michael's Hospital (Toronto, Canada). The trial was registered on ClinicalTrials.gov identifier NCT01604993.
Interventions
Participants received either a high-nitrate or low-nitrate (control) intervention for 7 days in a crossover fashion. Interventions were administered in the form of a soup meal presented in Table 1. The test meal was the spinach soup containing ~ 845 mg (13.63 mmol) of inorganic nitrate per serving/day, whereas the control, asparagus soup had ~ 0.6 mg (0.01 mmol) of inorganic nitrate. Interventions were matched for energy, carbohydrates, dietary fiber, protein, potassium, sodium content and volume, but differed in nitrate content. The soups comprised of 250 g of spinach or asparagus, 260 g of low-sodium chicken broth, 65 g of onion, 0.5 g of black pepper and were prepared using a single vegetable batch to minimize variability in nitrate concentration. Soup meals were blended upon preparation with the addition of food-grade coloring to match for appearance and texture and were frozen in single meal portions of 500 mL using indistinguishable containers. Level of nitrate was measured in thawed, prepared interventions. Interventions were coded by a study independent blinder who directly administered the soups during clinical visits. Participants were not informed of the soup intervention and were led to believe that both soups may be beneficial. During clinical visits, participants were given 200 mL of water (45 mg/L or 0.73 mmol/L of nitrate) with each intervention.
Table 1. Composition of high-nitrate and low-nitrate interventions.
| Interventions | Control* | High-nitrate |
|---|---|---|
| Ingredients | Asparagus, low-sodium chicken broth, onions, black pepper | Spinach, low-sodium chicken broth, onions, black pepper |
| Composition | ||
| Calories, kcal | 86 | 94 |
| Carbohydrates, g | 16 | 16 |
| Fibre, g | 5 | 6 |
| Protein, g | 7 | 8 |
| Fat, g | 0.4 | 1.1 |
| Nitrate, mg | 0.6 | 845 |
| Nitrite, mg | < 0.1 | 0.1 |
| Sodium, mg | 730 | 730 |
| Serving size, g | 556 | 556 |
| Volume, mL | 500 | 500 |
*Control is low-nitrate intervention.
Study design
The study was conducted in a randomized, controlled, single-blinded, crossover design with two 7-day intervention phases separated by at least 1 week to minimize carry-over effects. Randomization was determined using a random number table. Participants attended the clinic on Day 1 and Day 7 of each intervention phase, following a 10-12 hour overnight fast. Prior to the first clinical visit, participants were instructed to maintain their usual lifestyle and dietary regimens while keeping high-nitrate food intake to < 2 servings per day and to avoid the use of mouthwash for the study duration. Their compliance with these requirements was assessed using a food frequency and lifestyle questionnaire. Anthropometric measurements were taken upon arrival, after which participants remained quietly seated for > 10 minutes to achieve resting heart rate. Baseline BP was performed in a seated position as per JNC 7 recommendations [9]; pulse wave analysis was obtained in a supine position to assess AI and central BP. The intervention was administered subsequently and participants were instructed to finish the intervention within 15 minutes. Baseline (0 minutes) vascular measurements were taken to be the point at which the soup meal commenced. Subsequent vascular measurements were recorded at 60, 120, and 180 minutes post-consumption in the same order. Participants remained seated and did not consume additional food or beverages for the duration of the clinical visit. After a Day 1 visit, participants received 5 frozen soup meals of the corresponding intervention to be consumed at home, 1 soup daily, for Day 2 to 6. On Day 7, participants returned for a clinical visit where a protocol identical to Day 1 was repeated. Following a > 1 week washout period, the same procedure was followed using the alternate intervention.
Vascular measurements
All vascular measurements were performed in a darkened, quiet, temperature-controlled room, were recorded in triplicates, and the average was taken for subsequent statistical analysis. Brachial BP was recorded following a 10 minutes seated resting period, on the dominant arm using an automatic cuff oscillometric device (OMRON HEM-907, Omron Healthcare, Kyoto, Japan). Central AI and BP were recorded in a supine position at the radial artery obtained noninvasively by applanation tonometry using pulse wave analysis (SphygmoCor Vx, AtCor Medical, Sydney, AU). Measurements were recorded at baseline (0 minutes) and 60, 120, and 180 minutes following the intervention. AI measurements were recorded in a continuous, steady state over a period of 15 seconds and the same position was used for all subsequent measurements. Only values with an operator index > 80% were included in the analysis.
Statistical analyses
Central AI values were adjusted for 75 beats per minute (AI75) and were used in all analyses. AI75, central BP and brachial BP were plotted as change from baseline over 180 minutes on Day 1 and Day 7. All statistical analyses were performed using NCSS 2000 Statistical Software (NCSS, Kaysville, UT USA). The data were checked for normality using the Shapiro-Wilk test. Repeated measures analysis of variance (ANOVA) assessed the interactive and independent effects of interventions on change in AI75 (primary outcome measure), central BP, and brachial BP from baseline measures between high-nitrate and control interventions on Day 1 and Day 7. A value of p < 0.05 was used to define significance. The data presented are expressed as mean ± standard deviation (SD), unless otherwise stated.
A total of thirty participants were to be recruited to detect a 4.5% unit difference in our primary outcome measure, AI75, based on the preliminary data on intra-subject reproducibility response (within subject SD of 5.6%, at a two-sided 0.05 significance level, 80% power and an attrition rate of 10%).
Results
Participants
Twenty seven participants completed the study and were included in the analysis (16 F: 11 M; age: 24.5 ± 11 years; BMI: 22.8 ± 3.7 kg/m2; SBP/DBP: 116/69 ± 14/10 mmHg). Three participants withdrew from the study due to time constraints. All participants were compliant with the study protocols during clinical visits and intervention phase. No changes in weight (data not shown) or baseline BP were observed between study days. Four participants in the high-nitrate intervention and 5 participants in the low-nitrate intervention reported symptoms of mild bloating. The side effects reported between the two interventions were not statistically different relative to each other (p > 0.05). No other adverse events were reported.
Effect on augmentation index
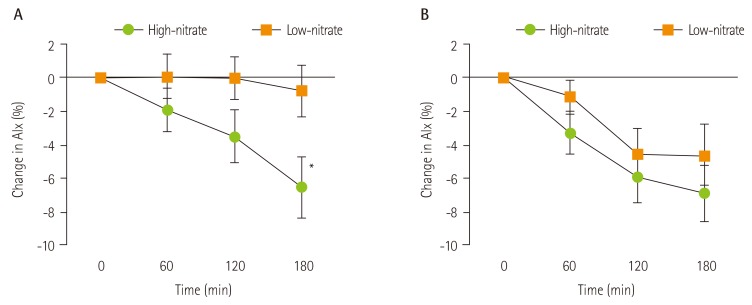
A significant treatment × time interaction was observed on Day 1 for AI75 (p = 0.03, two-way ANOVA). On Day 1, there was a reduction in AI75 following the high-nitrate intervention with a maximum effect of -5.72 ± 10.8% at 180 minutes relative to control (p = 0.01) and -6.54 ± 9.7% reduction relative to baseline (p = 0.002; Figure 1). Following 7 days of intervention, the high-nitrate intervention produced a comparable reduction on AI75 from baseline when compared to Day 1 (6.93 ± 8.7%, p < 0.001). However, on Day 7, the low-nitrate intervention produced a similar effect to the high-nitrate meal, with no differences between interventions (high vs. low-nitrate: -2.28 ± 12.5%, p = 0.35; Figure 1). A significant reduction of AI75 on Day 7 compared to Day 1 was observed in the low-nitrate intervention at 120 minutes (-4.55 ± 10.3%, p = 0.03), but not at 180 minutes (-3.83 ± 10.4%, p = 0.065; Table 2). The within subject coefficient of variation for AI75 was 16.6%, in line with recently reported values [10].
Figure 1. Mean change from baseline in AI75 (%) of high-nitrate versus low-nitrate intervention on (A) Day 1 and (B) Day 7 in 27 healthy participants. Values are expressed as mean ± SEM. An intervention × time interaction was observed on Day 1 (p = 0.03, repeated measures ANOVA). *Significantly different from low-nitrate intervention as assessed by repeated measures ANOVA, p = 0.01.
Table 2. Mean vascular measurements at baseline and change from baseline in 27 healthy individuals over 180 minutes on Day 1 and Day 7 of a high-nitrate (spinach) intervention or low-nitrate (asparagus) control.
| Outcome measures | Time (min) | Intervention (Day 1) | Intervention (Day 7) | ||||
|---|---|---|---|---|---|---|---|
| Low-nitrate | High-nitrate | p value | Low-nitrate | High-nitrate | p value | ||
| AI75, % | 0 | 5.70 ± 13.4 | 6.81 ± 11.0 | 0.51 | 7.88 ± 11.7 | 8.63 ± 11.9 | 0.67 |
| 60 | 0.08 ± 6.9 | - 1.94 ± 6.6 | 0.17 | - 1.16 ± 5.3 | - 3.33 ± 6.6 | 0.24 | |
| 120 | - 0.04 ± 6.5 | - 3.50 ± 8.3 | 0.07 | - 4.59 ± 7.8 | - 6.06 ± 7.4 | 0.39 | |
| 180 | - 0.82 ± 8.0 | - 6.54 ± 9.7* | 0.01 | - 4.65 ± 9.5* | - 6.93 ± 8.7* | 0.35 | |
| Central SBP, mmHg | 0 | 99.14 ± 11.9 | 98.98 ± 10.9 | 0.89 | 98.67 ± 12.5 | 99.19 ± 9.6 | 0.74 |
| 60 | - 1.40 ± 4.7 | - 0.72 ± 5.6 | 0.65 | - 0.44 ± 4.6 | - 2.45 ± 6.9 | 0.17 | |
| 120 | - 1.91 ± 5.5 | - 2.28 ± 6.9 | 0.82 | - 2.45 ± 6.4 | - 2.91 ± 6.8 | 0.75 | |
| 180 | - 1.92 ± 5.6 | - 4.38 ± 5.9* | 0.12 | - 0.66 ± 4.7 | - 4.05 ± 5.9* | < 0.01 | |
| Central DBP, mmHg | 0 | 69.52 ± 9.0 | 68.59 ± 9.1 | 0.48 | 69.07 ± 8.4 | 69.55 ± 7.4 | 0.71 |
| 60 | - 2.57 ± 5.0 | 0.31 ± 5.3 | 0.05 | - 1.78 ± 5.7 | - 1.17 ± 5.4 | 0.71 | |
| 120 | - 3.15 ± 6.0 | - 1.28 ± 6.8 | 0.28 | - 3.39 ± 4.2 | - 2.08 ± 6.1 | 0.20 | |
| 180 | - 3.13 ± 6.2* | - 2.60 ± 6.5* | 0.75 | - 1.82 ± 5.3 | - 4.43 ± 4.7* | 0.03 | |
| Brachial SBP, mmHg | 0 | 113.04 ± 12.6 | 111.94 ± 11.9 | 0.39 | 112.41 ± 13.4 | 112.67 ± 10.2 | 0.87 |
| 60 | - 2.02 ± 6.2 | - 1.78 ± 8.0 | 0.88 | 0.81 ± 6.6 | - 3.24 ± 7.1 | 0.02 | |
| 120 | - 0.57 ± 6.0 | - 2.11 ± 8.7 | 0.47 | - 2.30 ± 6.5 | - 3.22 ± 9.6 | 0.66 | |
| 180 | - 0.74 ± 7.3 | - 2.00 ± 6.6 | 0.45 | 1.39 ± 5.5 | - 2.09 ± 6.4 | 0.02 | |
| Brachial DBP, mmHg | 0 | 68.52 ± 8.4 | 67.20 ± 8.6 | 0.36 | 68.48 ± 8.4 | 68.09 ± 6.8 | 0.76 |
| 60 | - 2.07 ± 4.8 | 0.43 ± 4.9 | 0.11 | - 3.06 ± 5.9 | - 1.17 ± 5.6 | 0.28 | |
| 120 | - 2.74 ± 5.9 | - 0.74 ± 5.8 | 0.20 | - 2.89 ± 5.9 | - 2.39 ± 5.5 | 0.71 | |
| 180 | - 3.91 ± 5.5* | - 1.87 ± 6.5 | 0.21 | - 2.26 ± 6.5 | - 4.20 ± 5.3* | 0.13 | |
Values are expressed as mean ± standard deviation. Measured values at 60, 120 and 180 minutes are expressed as mean change from baseline values (0 minutes). p values are expressed as between treatments within the same day assessed by repeated measures ANOVA.
SBP: systolic blood pressure, DBP: diastolic blood pressure, AI75: augmentation index adjusted for 75 beats per minute, SD: standard deviation.
*Significantly different from baseline (p < 0.05).
Effect on central blood pressure
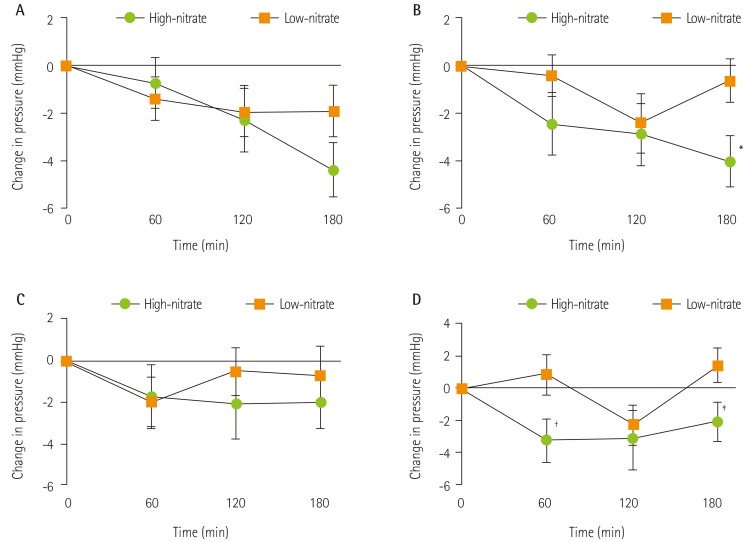
Relative to baseline, high-nitrate intervention significantly reduced central SBP and DBP at 180 minutes on Day 1 (p = 0.001 and p = 0.048, respectively) and Day 7 (p = 0.001 and p < 0.001, respectively; Table 2). High-nitrate intervention significantly reduced end differences on central SBP (-3.39 ± 5.59 mmHg, p = 0.004; Figure 2) and DBP (-2.60 ± 5.81 mmHg, p = 0.028) on Day 7 when compared to the low-nitrate control (Table 2). A significant treatment × time interaction was observed for central DBP on Day 7 (p = 0.024) only.
Figure 2. Mean change from baseline in central systolic blood pressure on (A) Day 1 and (B) Day 7 and in brachial systolic blood pressure on (C) Day 1 and (D) Day 7 after a high-nitrate or low-nitrate intervention in 27 healthy participants. Values are expressed as mean ± SEM. Different symbols denote a significant effect of high-nitrate compared to low-nitrate intervention as assessed by repeated measures ANOVA. *p = 0.004; †p = 0.025; ‡p = 0.022.
Effect on brachial blood pressure
Reductions in brachial SBP at 60 and 180 minutes were observed in the high-nitrate meal relative to low-nitrate intervention on Day 7 only (Figure 2). Compared to baseline, post-prandial brachial DBP at 180 minutes was significantly lower on Day 1 for the low-nitrate intervention (p = 0.001) and on Day 7 for the high-nitrate intervention (p < 0.001). No other differences were observed between and within interventions (Table 2).
Discussion
The present study investigated the effects of a 7-day consumption of spinach as an inorganic nitrate source in a vegetable, on indices of arterial stiffness and hemodynamics in healthy individuals.
The results demonstrated that high-nitrate spinach soup administration, containing ~ 845 mg of nitrate, decreased postprandial arterial stiffness measure by 6.93 ± 8.7% and reduced central SBP by 4.05 ± 5.9 mmHg following 7 days of administration. A consistent reduction in both AI75 and central SBP relative to baseline were observed on the high-nitrate intervention and it appears that the acute responses were maintained following at least one week of continuous supplementation.
Dietary sources of nitrate and their effects on vascular health have received increased attention recently [11,12,13,14]. It is suggested that dietary nitrate may contribute to anaerobic NO production, presumably through the nitrate-nitrite-NO pathway as an alternative to the oxygen-dependent L-arginine-endothelial NO synthase-NO pathway. The alternate NO supply, the anaerobic process, may be especially important in cardiovascular disease pathophysiology, characterized by diminished blood and oxygen supply, where endothelial NO-derived processes decline [15,16].
Spinach represents one of the highest dietary sources of nitrate. To date, proposed associations of dietary inorganic nitrate and NO generation were largely built on interventions utilizing beetroots and beetroot derivatives as a vegetable source of nitrate. The magnitude of brachial BP reductions observed in single-administration trials, when adjusted for nitrate dose, was comparable to Day 1 of spinach administration in the present study. In a recent study, single administration of 220 mg of nitrate from spinach did not acutely affect AI, but significantly altered postprandial systolic BP. However the dose administered was significantly lower than that used in this trial and may account for the observed difference in effect [14].
The quantity of nitrate evaluated in previous trials ranged from 220 to 1488 mg [14,17]. The dose of ~ 845 mg of nitrate administered in the present study was based on quantities that previously demonstrated efficacy and safety, and was selected to be in the range of those estimated in the participants of the DASH diet, of up to approximately 1,222 mg per day [4]. This amount is significantly higher relative to the estimated average nitrate intake of 60-120 mg/day (1-2 mmol/day) in the US and Europe. Nonetheless, if supported by larger trials, increasing daily nitrate load may have viable implications in tailored dietary recommendations.
The most prominent effects on measured hemodynamic indices were observed at 180 minutes, corresponding with the pharmacokinetics of orally administered dietary nitrate. Webb et al. found that after ingestion of a high nitrate product, maximum blood pressure reduction occurred at ~ 2.5-3 hours post consumption, which reflected peak nitrite concentrations [18]. In fact, bioconversion of nitrate to nitrite by the oral microbiota is needed to produce NO, which was found to be ablated with the administration of an antibacterial mouthwash or when saliva swallowing was abstained following a nitrate load [19].
Coincidentally, the AI75 response from the low-nitrate asparagus control on Day 7 was significantly higher compared to that on Day 1 and was similar to the high-nitrate intervention. The surprising effect introduces potential properties of asparagus that have yet to be examined in humans. Asparagus officinalis has been used as a natural diuretic in Chinese cultures [20], but the extent of its effect, mechanism of action and diuretic strength remain unclear in existing literature. Dartsch et al. studied an equal combination of dried Asparagus officinalis roots and parsley and observed dose-dependent stimulation of the metabolism of kidney cells in vitro, yet was unable to directly correlate this finding with increased water excretion [21]. Negi et al. suggested that the 1.7% [22] steroidal saponins found in asparagus appear to be the most biologically active component of the vegetable [23]. Steroidal saponins have been documented in literature to possess vasodilatory properties [24] and may potentially account for the observed effects of asparagus administration after one week, given their potential to modulate gene transcription factors. While asparagus served as a negative control with respect to dietary nitrate levels, it may have had attributes of a positive control, and its potential vascular activity should be evaluated further.
The increased interest in central AI measures is based on evidence from meta-analysis suggesting that it is an independent predictor of cardiovascular events [25]. While our intervention decreased a marker of arterial stiffness, AI75, it is important to distinguish that arterial elasticity is determined by the structural characteristics of the vessel wall, as well as by the vascular smooth muscle tone. Therefore, given the short term nature of the study design, it is plausible that our observations were a factor of functional changes affecting the vascular smooth muscle tone. The effect of our intervention on SBP values support this notion. In addition, arterial stiffness is, in part, determined by mean arterial pressure, thus delineating the effects on AI75 above the effect attributable to blood pressure reduction can be challenging.
With the potential of nitrate-rich dietary interventions affecting hemodynamics and having a role in hypertension management, some literature has raised concerns on the negative connotations in relation to dietary nitrate and increased cancer risk. Dietary nitrate are reduced to nitrite by oral bacteria, which may react with secondary or tertiary amines (i.e. cheese and meat), forming N-nitroso compounds, a class of carcinogenic substances [26]. However, no evidence from chronic animal studies indicates that nitrate consumption is carcinogenic; in fact, nitrate from vegetable foods were associated with a decreased risk of cancer [26]. The World Health Organization Expert Committee on Food Additives maintains that it is inappropriate to compare the exposure of nitrate from vegetables, considering no data supports potential bearing of nitrate within vegetable matrices on the bioavailability of N-nitroso compounds [27].
There are several limitations to the present study. Compliance was estimated using self-reported assessments only and non-disclosure of compliance in order to conform to study criteria is possible. Secondly, plasma nitrate levels were not evaluated in this study; however, dietary nitrate from both cooked and uncooked vegetables has been shown to be absorbed very efficiently, resulting in an absolute nitrate bioavailability of close to 100%. Nonetheless, it would be interesting to observe changes in baseline nitrate/nitrite values over 7 days [28]. Thirdly, this study cannot attribute the significant shift in hemodynamics to a specific bioactive compound and it is possible that other constituents of spinach can also exert an effect. However, except for understanding the underlying mechanism, this is not a limitation in our opinion, as spinach will continue to be consumed as a nutrient dense vegetable and is widely available in the whole form. Thus, understanding the overall health effects of this dietary staple is important. These results need to be confirmed in a longer term feeding trial.
Conclusion
The present preliminary study has demonstrated promising potential for dietary nitrate to improve vascular health, as seen by decreased arterial stiffness and central BP, which in healthy individuals may be more accurate prognostic indicators of cardiovascular health than brachial BP [8]. Overall, this study provides support to the potential use of whole food, un-concentrated dietary nitrate found in natural, commonly consumed vegetables like spinach, as an effective way to aid in maintenance of cardiovascular health. Development of such strategies is needed to reinforce the interest of diet and lifestyle modification as primary means for prevention and management of CVD.
Acknowledgments
This study was supported in part by the internal research donation fund within the Li Ka Shing Knowledge Institute and St. Michael's Hospital held by VV. LB is the recipient of the Undergraduate Research Fund scholarship which in part provided funding for operation of the trial.
Footnotes
Conflict of interest: V. Vuksan holds an American (No. 7,326,404 B2) and Canadian (No. 2,410,556) patent for use of viscous fibre blend in diabetes, metabolic syndrome and cholesterol lowering; V. Vuksan currently holds grant support for ginseng research from the Canadian Diabetes Association and the National Institute of Horticultural & Herbal Science, RDA, Korea. At the time of the study, V. Vuksan was a partial owner of Glycemic Index Laboratories (Toronto, ON., Canada) and has since retired from the organization (April, 2015); A. Jenkins is a VP and partial owner of Glycemic Index Laboratories. No conflict of interest was declared by the other coauthors.
References
- 1.World Heart Federation (CH) Hypertension [Internet] Geneva: World Heart Federation; 2012. [cited 2014 September 6]. Available from: http://www.world-heart-federation.org/cardiovascular-health/cardiovascular-disease-risk-factors/hypertension/ [Google Scholar]
- 2.National Heart Lung and Blood Institute (US) How is high blood pressure treated? [Internet] Bethesda (MD): National Heart Lung and Blood Institute; 2012. [cited 2014 September 6]. Available from: http://www.nhlbi.nih.gov/health/healthtopics/topics/hbp/treatment. [Google Scholar]
- 3.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 4.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 6.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63:1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- 7.Jajja A, Sutyarjoko A, Lara J, Rennie K, Brandt K, Qadir O, Siervo M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr Res. 2014;34:868–875. doi: 10.1016/j.nutres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Agabiti-Rosei E, Mancia G, O'Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Dhindsa M, Barnes JN, DeVan AE, Sugawara J, Tanaka H. Comparison of augmentation index derived from multiple devices. Artery Res. 2011;5:112–114. [Google Scholar]
- 11.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 13.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 14.Liu AH, Bondonno CP, Croft KD, Puddey IB, Woodman RJ, Rich L, Ward NC, Vita JA, Hodgson JM. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide. 2013;35:123–130. doi: 10.1016/j.niox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Machha A, Schechter AN. Dietary nitrite and nitrate: a review of potential mechanisms of cardiovascular benefits. Eur J Nutr. 2011;50:293–303. doi: 10.1007/s00394-011-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machha A, Schechter AN. Inorganic nitrate: a major player in the cardiovascular health benefits of vegetables? Nutr Rev. 2012;70:367–372. doi: 10.1111/j.1753-4887.2012.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 18.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Shao Y, Chin CK, Ho CT, Ma W, Garrison SA, Huang MT. Anti-tumor activity of the crude saponins obtained from asparagus. Cancer Lett. 1996;104:31–36. doi: 10.1016/0304-3835(96)04233-4. [DOI] [PubMed] [Google Scholar]
- 21.Dartsch PC. Effect of Asparagus-P on cell metabolism of cultured kidney and inflammation-mediating cells. Phytother Res. 2008;22:1477–1481. doi: 10.1002/ptr.2497. [DOI] [PubMed] [Google Scholar]
- 22.Fang YL. Purification and monosaccharide composition of saponin from Asparagus officianlis L. Sheng Wu Gong Cheng Xue Bao. 2005;21:446–450. [PubMed] [Google Scholar]
- 23.Negi JS, Singh P, Joshi GP, Rawat MS, Bisht VK. Chemical constituents of Asparagus. Pharmacogn Rev. 2010;4:215–220. doi: 10.4103/0973-7847.70921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanovski E, Jenkins A, Dias AG, Peeva V, Sievenpiper J, Arnason JT, Rahelic D, Josse RG, Vuksan V. Effects of Korean red ginseng (Panax ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am J Hypertens. 2010;23:469–472. doi: 10.1038/ajh.2010.5. [DOI] [PubMed] [Google Scholar]
- 25.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Health Canada. Guidelines for Canadian drinking water quality: guideline technical document - nitrate and nitrite [Internet] Ottawa: Health Canada; 2013. [cited 2014 September 8]. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/watereau/nitrate_nitrite/index-eng.php. [Google Scholar]
- 27.Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. 1995;859:1–54. [PubMed] [Google Scholar]
- 28.van Velzen AG, Sips AJ, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol Lett. 2008;181:177–181. doi: 10.1016/j.toxlet.2008.07.019. [DOI] [PubMed] [Google Scholar]