Abstract
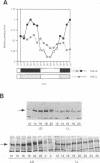
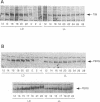
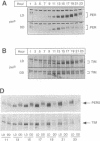
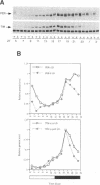
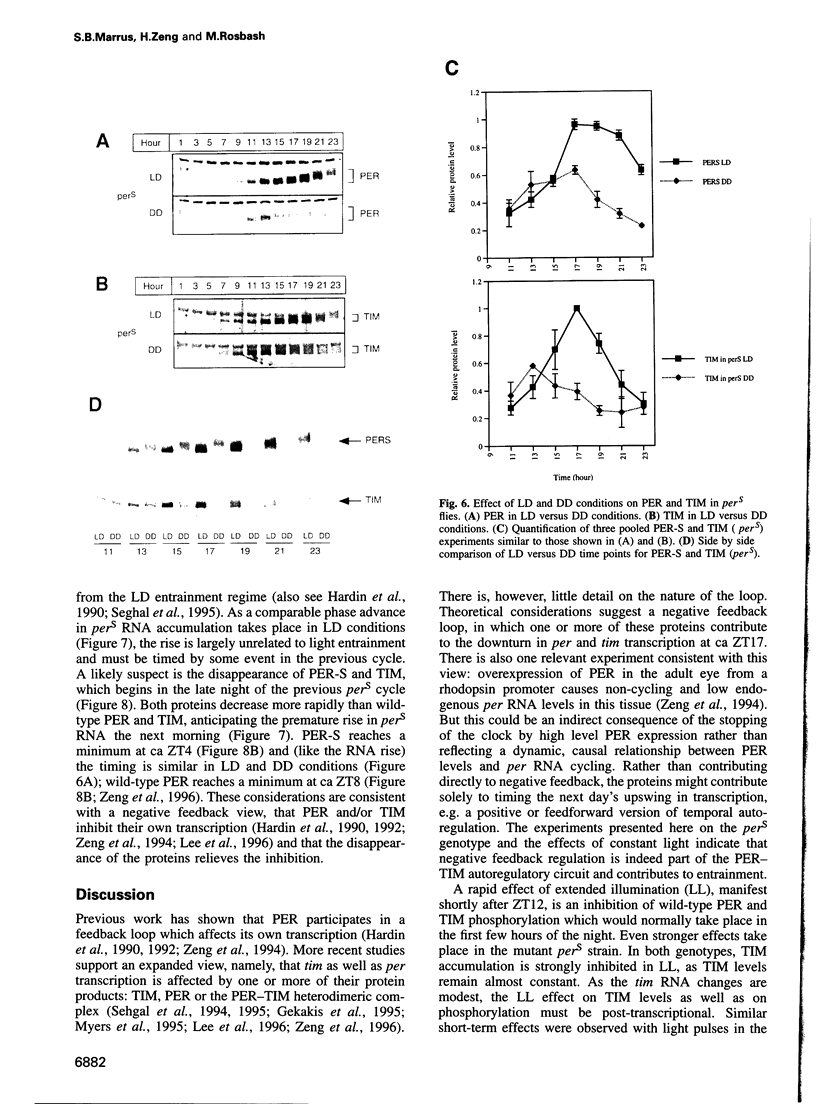
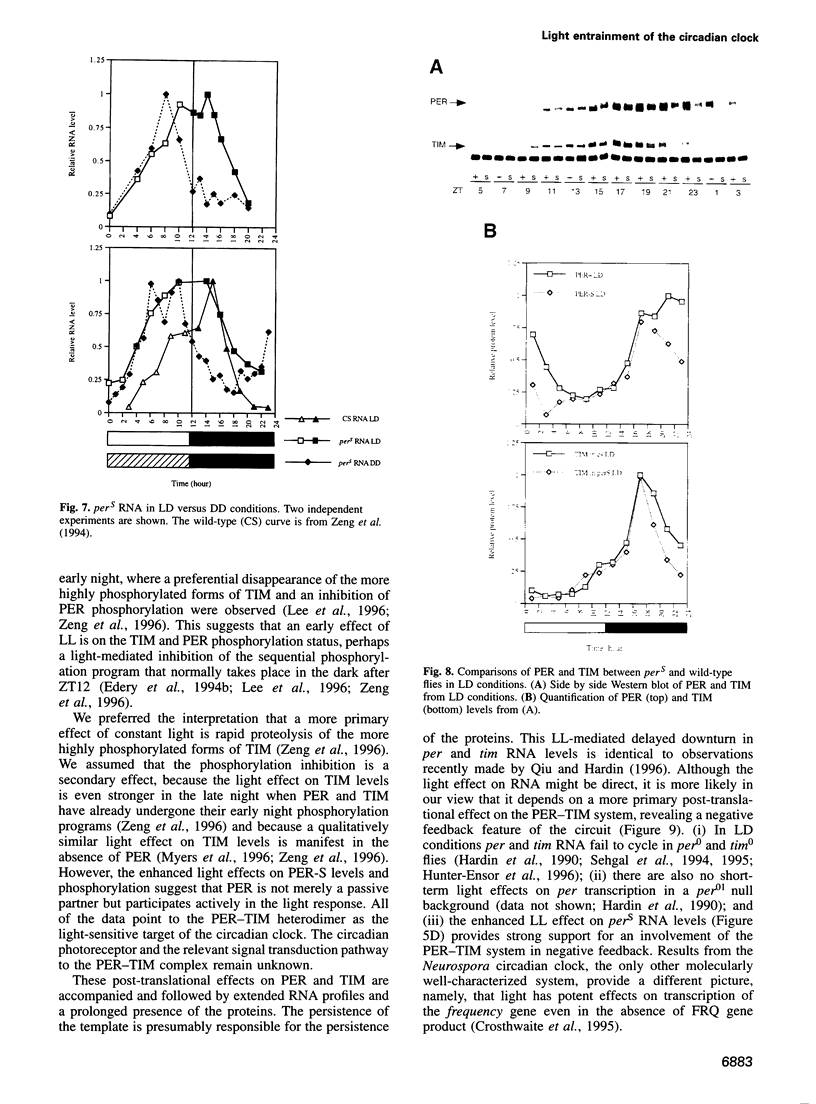
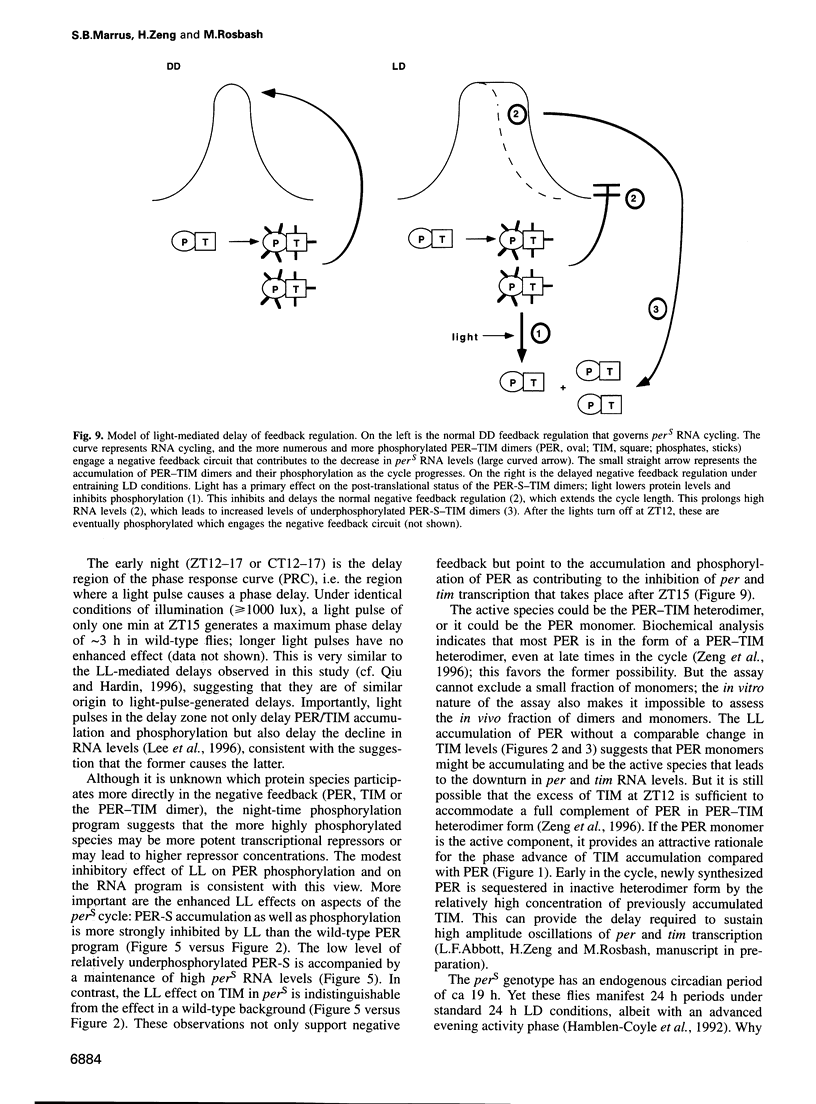
Light is the dominant environmental cue that provides temporal information to circadian pacemakers. In Drosophila melanogaster some period gene mutants have altered free-running circadian periods but entrain to 24 h light-dark cycles. To address the mechanism of light entrainment in Drosophila, we examined the effects of constant light on the period gene (per) and timeless gene (tim) products in wild-type and perS flies. The results indicate that light affects three features of the PER-TIM program: PER and TIM phosphorylation, PER and TIM accumulation, and per and tim RNA cycling. A post-transcriptional effect on the PER-TIM complex is the likely primary clock target, which then delays the subsequent decrease in per and tim RNA levels. This is consistent with a negative feedback loop, in which the PER-TIM complex contributes to the decrease in per and tim RNA levels, presumably at the transcriptional level. There are enhanced constant light effects on the perS mutant, which further support negative feedback as well as support its importance to entrainment of these flies to a 24 h cycle, far from their intrinsic period of 19 h. The perS mutant leads to a truncated protein accumulation phase and a subsequent premature perS RNA increase. A standard 24 h light-dark cycle delays the negative feedback circuit and extends the RNA and protein profiles, compensating for the accelerated RNA increase and restoring the rhythms to wild-type-like periodicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crosthwaite S. K., Loros J. J., Dunlap J. C. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell. 1995 Jun 30;81(7):1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- Curtin K. D., Huang Z. J., Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron. 1995 Feb;14(2):365–372. doi: 10.1016/0896-6273(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Edery I., Rutila J. E., Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science. 1994 Jan 14;263(5144):237–240. doi: 10.1126/science.8284676. [DOI] [PubMed] [Google Scholar]
- Edery I., Zwiebel L. J., Dembinska M. E., Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekakis N., Saez L., Delahaye-Brown A. M., Myers M. P., Sehgal A., Young M. W., Weitz C. J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995 Nov 3;270(5237):811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- Handler A. M., Konopka R. J. Transplantation of a circadian pacemaker in Drosophila. Nature. 1979 May 17;279(5710):236–238. doi: 10.1038/279236a0. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Hunter-Ensor M., Ousley A., Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996 Mar 8;84(5):677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Pittendrigh C., Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989 Sep;6(1):1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- Lee C., Parikh V., Itsukaichi T., Bae K., Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996 Mar 22;271(5256):1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Wager-Smith K., Rothenfluh-Hilfiker A., Young M. W. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996 Mar 22;271(5256):1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Wager-Smith K., Wesley C. S., Young M. W., Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995 Nov 3;270(5237):805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- Petersen G., Hall J. C., Rosbash M. The period gene of Drosophila carries species-specific behavioral instructions. EMBO J. 1988 Dec 1;7(12):3939–3947. doi: 10.1002/j.1460-2075.1988.tb03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. M., Ringo J. M., Dowse H. B. The effects of period mutations and light on the activity rhythms of Drosophila melanogaster. J Biol Rhythms. 1995 Sep;10(3):267–280. doi: 10.1177/074873049501000309. [DOI] [PubMed] [Google Scholar]
- Price J. L., Dembinska M. E., Young M. W., Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995 Aug 15;14(16):4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Hardin P. E. per mRNA cycling is locked to lights-off under photoperiodic conditions that support circadian feedback loop function. Mol Cell Biol. 1996 Aug;16(8):4182–4188. doi: 10.1128/mcb.16.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Price J. L., Man B., Young M. W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994 Mar 18;263(5153):1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Sehgal A., Rothenfluh-Hilfiker A., Hunter-Ensor M., Chen Y., Myers M. P., Young M. W. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science. 1995 Nov 3;270(5237):808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- Winfree A. T. Suppressing drosophila circadian rhythm with dim light. Science. 1974 Mar 8;183(4128):970–972. doi: 10.1126/science.183.4128.970. [DOI] [PubMed] [Google Scholar]
- Zeng H., Hardin P. E., Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994 Aug 1;13(15):3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Qian Z., Myers M. P., Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996 Mar 14;380(6570):129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]