Abstract
Anatase TiO2 nanosheets (NSs) with exposed {001} facets have been widely used because of their high activity and particular surface atomic configuration. However, investigations on their biotoxicity are rare. In this study, bioaccumulation of five different TiO2 (with 10%, 61%, 71%, 74% and 78% exposed {001} facets), as well as copper and enzyme activities in Daphnia magna, are systematically investigated and rationalized. The results indicated that the addition of Cu2+ enhanced agglomeration–sedimentation of TiO2, resulting in the reduction of TiO2 bioaccumulation by 10% to 26%. TiO2 nanoparticles (NPs) increased copper bioaccumulation by 9.8%, whereas the other four TiO2 nanosheets (NSs) decreased it by 43% to 53%, which depended on TiO2 variant adsorption and free Cu2+ concentrations in the supernatant. The levels of superoxide dismutase (SOD) enzyme and Na+/K+-ATPase activities suggested that oxidative stress, instead of membrane damage, was the main toxicity in D. magna. Meanwhile, the SOD enzyme activities increased with decreasing Cu accumulation and increasing Ti accumulation because of the different functions of Cu and Ti in organisms. This research highlighted the important role of the percentage of exposed {001} facets in nanostructured TiO2 on bioaccumulation and biotoxicity of TiO2 and Cu2+ in Daphnia magna.
Nanomaterials are widely applied in various fields because of their unique physical and chemical properties. As one of the most commonly used nanomaterials, nano-sized TiO2 are widely used in photocatalysis, cosmetics, paint, medicine, and others. The estimated worldwide productions of nano-sized TiO2 are 2.5 million metric tons per year by 2025, which become a trillion US-dollar business in the future1,2.
The rapid expansion of nano-sized TiO2 increases the risk of aquatic environment exposure, which draws increasing attention. Lovern and Klaper first reported that nano-sized TiO2 was a hazardous material in aquatic organisms and the lethal concentration was only 10 ppm for Daphnia magna (D.magna) upon 48 h aqueous exposure3. However, the actual concentration of nano-sized TiO2 in natural water is very low (3 ng L−1 to 1.6 mg L−1), and reaching the lethal concentration is difficult4. Due to the special physicochemical characteristics of nano-sized TiO2, the existence of trace nano-sized TiO2 would influence the toxicity of original pollutants in the environment. Nano-sized TiO2 can adsorb other substances in water and influence their biological behaviors and toxicities. The presence of 2 mg L−1 nano-sized TiO2 increased the toxicity of the highly toxic marine antifouling compound tributyltin (TBT) up to 20-fold compared with TBT alone in abalone embryos5. For heavy metals, it was reported that the presence of TiO2 nanoparticles (NPs) as carriers greatly enhanced the accumulation of Cd and As in carp, and Cu in D. magna6,7. On the contrary, Yang’s research showed that nano-sized TiO2 diminished Cd2+ bioavailability and toxicity due to Cd2+ adsorption by TiO2, which decreased its ambient free ion concentrations8.
The biological toxicity of nano-sized TiO2 is closely related to its physicochemical characteristics, such as size, crystal and surface modifications, and radical formation. It was reported that anatase nano-sized TiO2 was more toxic than rutile, and NPs were more toxic than microparticles for cladocerans, algae, rotifers, and plants9. It was reported that biological surface coating of nano-sized TiO2 exerted a negative effect in the molting and development of D. magna10. The influence of TiO2 particle size on cadmium toxicity was confirmed, and Cd2+ with 30 nm TiO2 NPs presented more serious growth inhibition to algal11.
Recent research on nanostructured TiO2 focused on tailoring its shape, size, and exposed facets for enhancing its performance in photocatalysis, solar energy conversion, photochromic devices, and sensors, which was highlighted by the anomalous physicochemical properties of anatase TiO2 nanomaterials with different exposed {001} facets12,13. Theoretical and experimental studies have indicated that the {001} surface of anatase TiO2 is much more reactive than the thermodynamically more stable {101} surface because the average surface energy of the {001} facets of anatase TiO2 (0.90 J m−2) are twice higher than that of the {101} facets (0.44 J m−2)14. TiO2 nanosheets (NSs) with {001} facets exhibit high photocatalytic activity, and their photoactivity exceeds that of P25 by a factor of more than nine times15. However, the effects of TiO2 NSs with different exposed {001} facets on heavy metal accumulation and toxicity remain unexplored.
In this research, TiO2 NPs and NSs with different percentages of exposed {001} facets were prepared and characterized. Bioaccumulation of TiO2 and Cu2+ was investigated under different exposure conditions with D. magna as test organism. The changes in metabolic enzymes, such as superoxide dismutase (SOD) and Na+/K+-ATPase, were also discussed. The results of the present study provide a strong evidence for the environmental risks of TiO2 NPs and NSs.
Results and Discussion
Characterization of prepared TiO2
TiO2 samples with different percentages of exposed {001} facets were synthesized by changing RF and their physical properties are shown in Table 1. The percentage of the exposed {001} facet of TiO2 was calculated using the reported method according to crystal structure16. All the prepared TiO2 was anatase phase, according to X-ray diffraction results (not shown here). The BET surface areas of these TiO2 samples decreased from 156 m2 g−1 to 97 m2 g−1 with increasing {001} facet percentage from 10% to 78%. At the same time, the porosity of TiO2 increased from 55.0% to 67.5%, the pore volumes increased from 0.33 cm3 g−1 to 0.56 cm3 g−1, and the average pore size from 7.4 nm to 20 nm. The existing nanopores (or porosity) were from the aggregation of TiO2 NPs and NSs17.
Table 1. Effects of RF on physical properties of TiO2.
| No. | RF | Percentage of {001} | Phase | CS (nm) | SBET(m2/g) | APS (nm) | PV (cm3/g) | Porosity (%) |
|---|---|---|---|---|---|---|---|---|
| NP10 | 0 | 10 | A | 8.9 | 156 | 7.4 | 0.33 | 55.0 |
| NS61 | 0.67 | 61 | A | 12.5 | 128 | 8.8 | 0.35 | 56.5 |
| NS71 | 1 | 71 | A | 13.6 | 114 | 16.0 | 0.52 | 65.8 |
| NS74 | 1.33 | 74 | A | 15.1 | 108 | 19.0 | 0.53 | 66.3 |
| NS78 | 2.67 | 78 | A | 17.9 | 97 | 20.0 | 0.56 | 67.5 |
A, CS, APS, and PV represent anatase, crystalline size, average pore size and pore volume, respectively.
TiO2 NPs (NP10) and NSs (NS78) were characterized by TEM, as shown in Fig. 1. The morphology of TiO2 NSs with 78% {001} facets (Fig. 1b) was different from that of TiO2 NPs with 10% {001} facets (Fig. 1a). According to Table 1, the shape of TiO2 changed from NPs to NSs with increasing RF. TiO2 with 61%, 71%, 74%, and 78% {001} facets were nanosheets, whereas those with 10% {001} facets were nanoparticles. The morphologies of TiO2 NS61, NS71, and NS74 were similar to that of NS78 (TEM pictures not shown).
Figure 1. TEM images of the NP10.
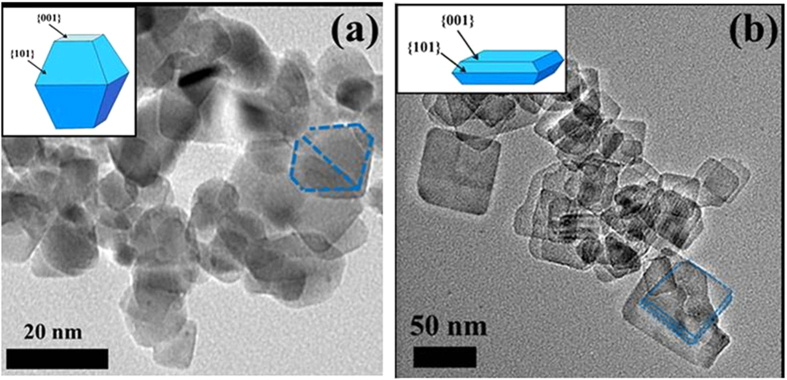
(a) and NS78 (b) samples.
Adsorption of Cu2+ on TiO2
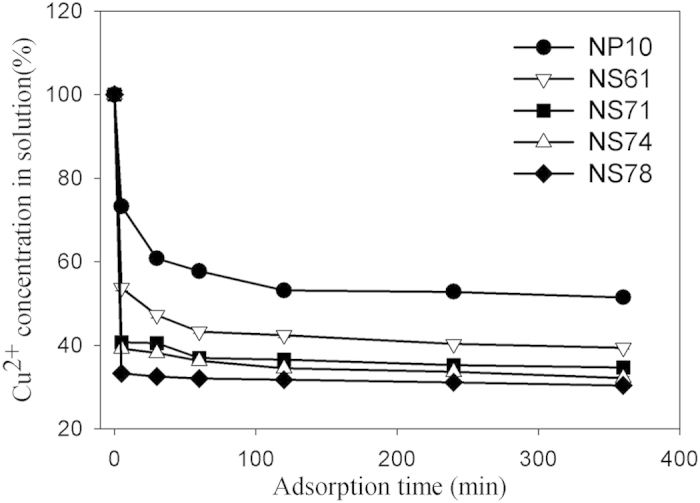
The adsorption of Cu2+ on TiO2 was evaluated from the decrease of Cu2+ concentrations in the supernatant. Figure 2 shows rapid adsorption of Cu2+ on TiO2 with the adsorption equilibrium reaching within the first 60 min. The decrease on Cu2+ concentrations ranges from 50% to 70%, dependent on the percentage of exposed {001} facets in the samples. The TiO2 NSs with higher exposed {001} facets could adsorb more Cu2+ in water than the TiO2 NPs. This finding is related to the surface properties of {001} facets. The high-energy {001} facets of anatase TiO2 have more surface defects such as unsaturated Ti atoms and abundant oxygen holes that are more effective for the dissociative adsorption of H2O molecules than the thermodynamically more stable {101} facets18. As a result, a large number of OH groups are generated on {001} facets. Adsorbates tend to be adsorbed at steps, defects, and domain boundaries because the surface atoms at these sites have fewer coordination numbers19. Consequently, the neutral and unoccupied surface sites of TiO2 {001} facet are Ti-(OH)(OH2) in water20. (These surface OH groups participate in.) These surface OH groups have been proved to produce extra-active centers not only for small organic molecules adsorption21, but also metal ion adsorption on TiO222. The exchange reaction of the metal cations with the -OH on the surface was presumed as presented in the following equation: TiOH3+ + Cu2+ → TiOCu4+ + H+. Therefore, the adsorption capacity of Cu2+ on TiO2 depends on the amount of OH groups on the TiO2 {001} surface. Hence, it is obvious that TiO2 NSs with 78% exposed {001} facets could adsorb the most Cu2+ in water.
Figure 2. Cu2+ Adsorption on the prepared NP10, NS61, NS71, NS74 and NS78 samples in 500 mL of 1 mg/L TiO2 suspension solution. Mean ± standard deviation (n = 2).
Accumulation of TiO2 in D. magna
Ti accumulation in D. magna was determined after exposure to different TiO2 samples at the 1 mg L−1 concentration with and without Cu2+. As shown in Fig. 3, Ti accumulation in D. magna in the presence of Cu2+ was lower than that without Cu2+, suggesting that Cu2+ inhibited the ingestion of TiO2 by D. magna. When TiO2 and Cu2+ coexisted, Ti accumulation in D. magna exposed to the NP10 sample decreased by 26.4%. However, Ti accumulation in D. magna exposed to the other four NS samples (NS61, NS71, NS74, and NS78) decreased by 10%. Moreover, the accumulated Ti in D. magna increased slightly with increasing percentage of {001} facet of TiO2 NSs from 3692 μg g−1 (in NS 61) to 5088 μg g−1 (in NS 78) dry weight in the absence of Cu2+, except for the NS71 sample. Thus, the coexistence of Cu2+ and TiO2 NSs has a negative effect on bioaccumulation of TiO2 in organisms.
Figure 3. Accumulated Ti after 48 h exposure to 1 mg/L of the prepared NP10, NS61, NS71, NS74 and NS78 samples with or without 50 μg/L Cu2+.
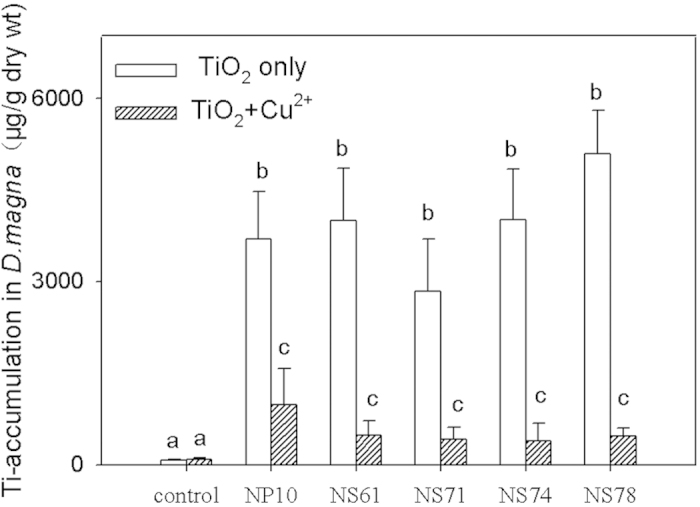
Mean ± standard deviation (n = 3), (P < 0.05, one-way ANOVA).
Free nanomaterials tend to aggregate in aquatic environments because of their large specific surface area. In addition, the less-mobile aggregated nanomaterials can easily combine with filter feeders and sediment-dwelling animals23. The aggregation is influenced by factors such as primary size, pH and ionic strength in aquatic environment24. According to the results of dynamic light scattering, these nano-sized TiO2 aggregated in water were from 1.363 μm to 1.572 μm in size in water with the absence of Cu2+, which further grew to about 2.1 μm in size when Cu2+was adsorbed onto the TiO2 surface. The addition of Cu2+ facilitates the aggregation of TiO2, in agreement of the reported increases the aggregation level when BPA was added into nano-TiO2 dispersions25. Dudev also demonstrated that the hydrodynamic diameter of anatase TiO2 nanoparticles (ANTNPs) increased in the presence of Ca2+, resulting in the aggregation of ANTNPs26. When the aggregated particle size exceeds a certain limit, the settlement behavior would become the key factor. The agglomeration– sedimentation processes resulted in the decreased concentrations of the NPs in the supernatant and then diminished the bioavailability of NPs27. The aggregation of nano-sized TiO2 has an important function in the environmental effects of NPs because the size and shape of NPs will determine the magnitude of any potentially toxic effect. In this experiment, Cu2+ enhanced the aggregation of TiO2 and formed the bigger aggregate in water, retarding the effective uptake of these particles by D. magna. Therefore, the existence of Cu2+ predictably weakened the bioaccumulation of nano-TiO2.
Accumulation of copper in D. magna
Cu accumulation in D. magna at different exposure conditions was investigated in this study, as shown in Fig. 4a. Compared with the control experimental run (treated only with Cu2+), the existence of TiO2 also influenced the bioaccumulation of Cu2+ in D. magna. When D. magna was exposed to water with a mixture of Cu2+ and NP10, Cu2+ accumulation was enhanced by 9.8%. However, Cu2+ accumulation in D. magna was reduced by 43% to 53% when Cu2+ coexisted with TiO2 NSs. Generally, the forms of Cu2+ ingested by D. magna were free Cu2+ and adsorbed Cu2+ on TiO2. When copper coexisted with TiO2, TiO2 could adsorb Cu2+. Thus its free ion concentration decreased in the ambient environment, which diminished a portion of Cu2+ internalization and bioavailability. In contrast, Cu2+ accumulation was enhanced when D. magna swallowed Cu2+-adsorbed TiO2. The factor that dominates in Cu accumulation depends on the unique physicochemical characteristics of TiO2 and exposure condition.
Figure 4.
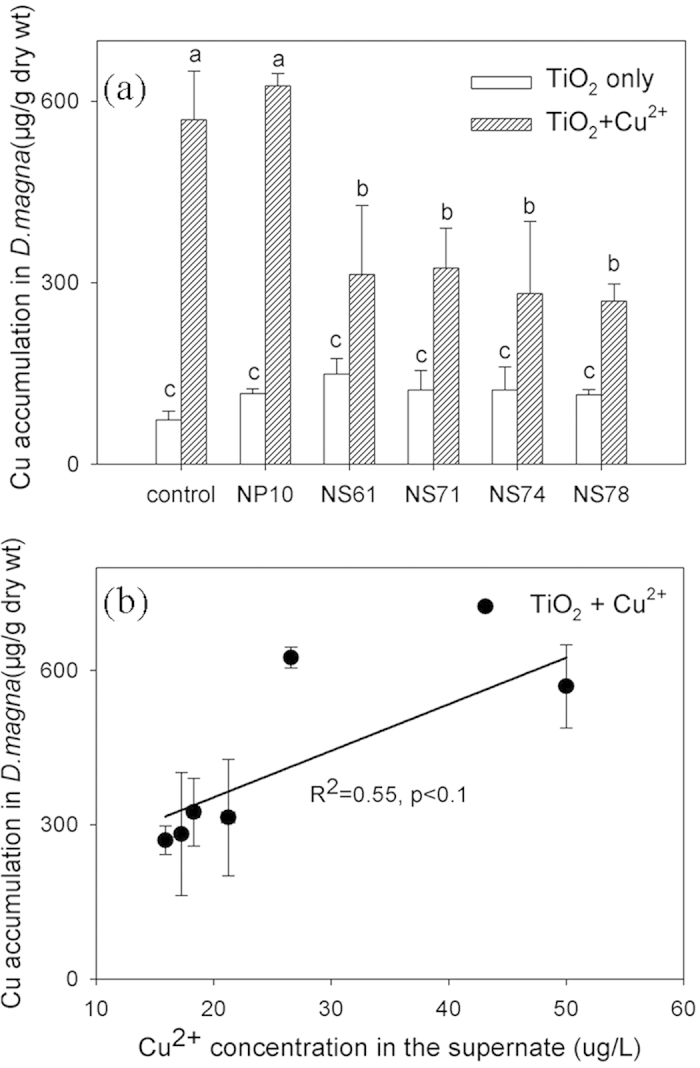
(a) Accumulated copper after 48 h exposure to 50 μg/L Cu2+ with or without 1 mg/L of the prepared NP10, NS61, NS71, NS74 and NS78 samples (P < 0.05, one-way ANOVA). (b) Relationship of copper accumulation in D. magna and Cu2+ concentration in the supernate when Cu2+ and TiO2 coexisted and reached a steady state in water. Mean ± standard deviation (n = 3).
The observed increase in copper accumulation with the presence of TiO2 NP10 is similar to previous report regarding the P2528. The explanation for reduced Cu accumulation with the presence of TiO2 NSs is as follows. Firstly, the Cu2+-adsorption capacities of TiO2 NSs were larger than those of TiO2 NPs, leading to the decrease in Cu2+ concentration. Yang studied Cd2+ toxicity caused by TiO2 NPs, and the results are similar to those of the present study. They suggested that nano-sized TiO2 could reduce free Cd2+ concentration in the media, which further lowers its bioavailability and toxicity to green alga Chlamydomonas reinhardtii8,29. As shown in Fig. 4b, a relative positive relationship exists between Cu accumulation in D. magna and Cu ion concentration in the media. The decrease of free Cu ion concentration was the main factor for the decrease of copper accumulation. Secondly, when Cu2+ coexisted with nano-sized TiO2, nano-sized TiO2 adsorbed Cu2+ and formed big aggregates in water. The large agglomeration–sedimentation of nano-sized TiO2 reduced Ti accumulation in D. magna and weakened the role of Cu as carrier. Thirdly, TiO2 NSs themselves may be toxic because of their insolubility in the gut and could alter Cu2+ toxicity in an antagonistic, synergistic, or additive way.
SOD enzyme and Na+/K+-ATPase activity in D. magna
The SOD enzyme activities in D. magna were investigated because they are antioxidant biomarkers for oxidative stress. As shown in Fig. 5a, when D. magna was exposed only to different nano-sized TiO2, the SOD enzyme activity decreased from 55.5% to 86.6% compared with the control experiment. SOD enzyme activities increased with increasing percentage of {001} facet of TiO2 NSs, although the NS78 sample had the largest Ti accumulation. When D. magna was exposed to different TiO2 and Cu2+, SOD activity decreased by 31.0% to 64.7% compared with the control experiment (only Cu2+). The decrease in SOD activities indicated that both TiO2 and Cu2+ induced a certain degree of oxidative stress and SOD enzyme inactivation30. The nanotoxicity theories were generated by the reactive oxygen species (ROS) and oxidative stress effects31. Nanoparticle stress resulting in ROS generation has already been reported by the Dalai groupand could be related to TiO2 NP cytotoxicity potential32. When D. magna was exposed to two foreign materials, SOD activities in the organisms were further deactivated. In addition, SOD activities in the exposed group were evidently lower than Cu2+ only, implying that Cu and nano-sized TiO2 together are more dangerous than Cu alone in aquatic environments.
Figure 5. SOD enzyme.
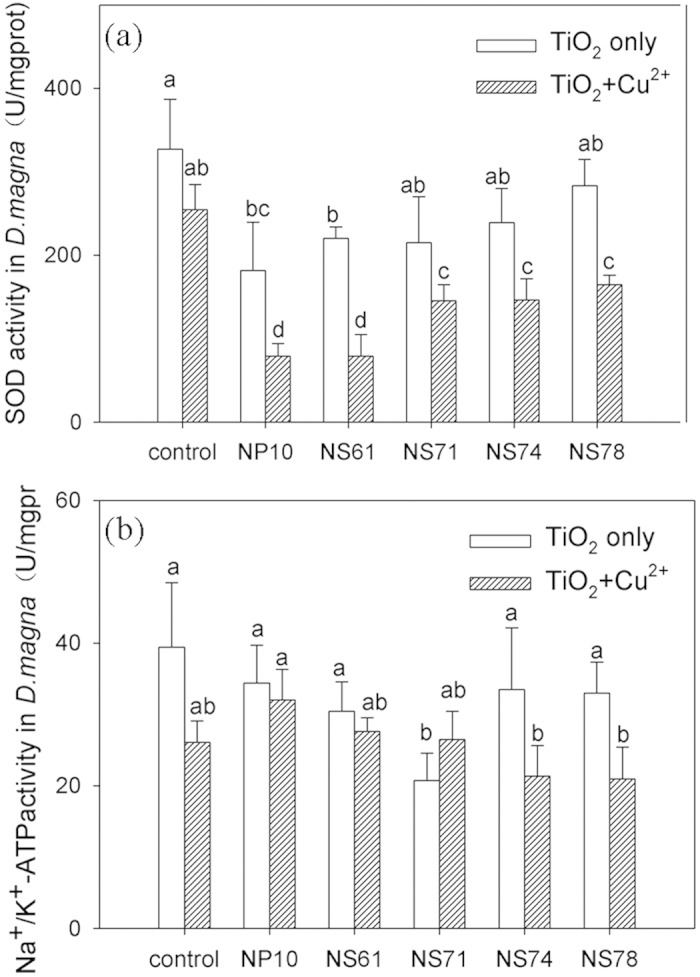
(a) and Na+/K+-ATPase (b) activities in D. magna after 48 h exposure to the prepared NP10, NS61, NS71, NS74 and NS78 samples in the absence and presence of Cu2+. Mean ± standard deviation (n = 3), (P < 0.05, one-way ANOVA).
Na+/K+-ATPase indicates the ability of ion transfer in the cell membrane channel. Figure 5b shows the activities of Na+/K+-ATPase enzyme in D. magna under different exposure conditions. Compared with the control group, the Na+/K+-ATP activities exhibited no significant difference after being exposed only to different TiO2. The result is similar to that in C.S. Ramsden’s report, demonstrating that no changes in Na+/K+-ATPase activity were observed in the brain, gill, or liver tissues of the zebrafish after exposure to TiO2 NPs or bulk33. Na+/K+-ATPase enzyme is present at high concentrations in salt-transporting tissues such as intestines and gills, where it maintains the ionic and electrical gradients necessary for transepithelial salt movements. No significant changes in K+, Na+ and Ca2+ concentration were observed in exposure TiO2-only conditions, which resulted in the absence of any treatment-related change in Na+/K+-ATPase activities. When TiO2 co-existed with Cu2+, the Na+/K+-ATPase activities in D. magna were slightly lower than the treatment with TiO2 only. The addition of Cu2+ changed the ionic strength of the solution, and Cu2+ accumulation in the body inhibited Na+ influx and reduced Na+/K+-ATPase activity in organisms34. These results suggest that membrane damage is not the main toxicity to D. magna under this research.
Mechanism of TiO2 NSs effects on Cu2+ biotoxicity
In the coexistence system, Cu2+ affected the stabilities of TiO2 NS suspensions and their ingestion by organisms. The addition of TiO2 NSs changed Cu2+ uptake and biotoxicity in D. magna. As suggested above, the oxidative stress damage instead of membrane damage is the major toxicity. To further investigate the main mechanisms of oxidative stress toxicity, the relationship between superoxide dismutase (SOD) activity and Cu/Ti accumulation was considered when D. magna was exposed to Cu2+ and different TiO2 samples. According to Fig. 6, a linear relationship between SOD activity and Cu/Ti accumulation in D. magna (P < 0.01, one-way ANOVA) appears. SOD activities decreased with increasing copper accumulation and decreasing Ti accumulation in D. magna. These results are related to the physiological effect of Cu and Ti on organisms.
Figure 6. Relationships between SOD activity and accumulated Cu.
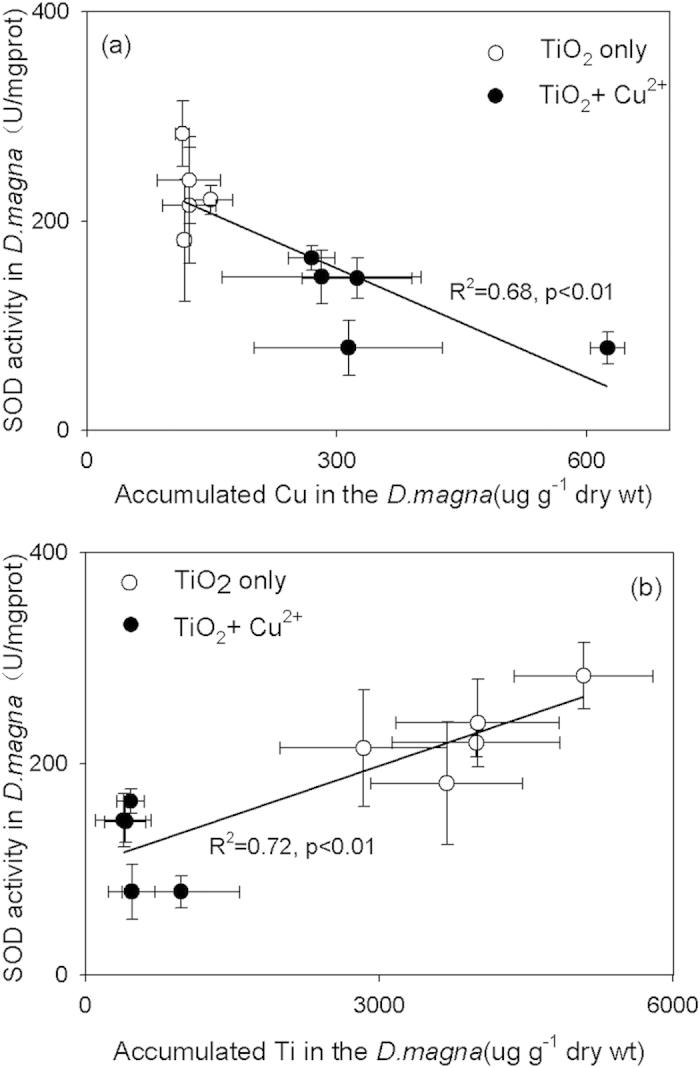
(a) and Ti (b) in D. magna after 48 h exposure to the TiO2 samples prepared with varying RF in the absence and presence of Cu2+. Mean ± standard deviation (n = 3).
Generally, Cu2+ is a hazardous substance to D. magna and could produce strong oxidative damage. Cu2+-induced cellular toxicity can be explained by the participation of Cu2+ in the formation of ROS. Cu2+ can be reduced to Cu+ in the presence of superoxide (O2−•), and Cu+ is capable of catalyzing the formation of hydroxyl radical (OH•) from hydrogen peroxide (H2O2)35. OH• is a strong oxidizing radical that can practically react with every biological molecule and destroy the antioxidant defense system. SOD enzyme activities in D. magna were deactivated with the accumulation of Cu. On the contrary, Ti is the ninth most abundant element in the earth’s crust, and has a certain stimulating and promoting effect on the growth of plants36. Its beneficial effects on plants have been known since the 1930s37. One mechanism of Ti action is that Ti4+/Ti3+ participates in the metabolism reaction involved in electron transfer in the redox system38. Ti species also involve in activating enzyme activities, such as peroxidase, catalase and nitrate reductase activities in plant tissues39. For these reasons, Ti in daphnids possibly maintains higher SOD enzyme activities to help in the scavenging of generated ROS. However, this supposition needs further studies.
In summary, it was found that bioaccumulation and biotoxicity of nanostructured TiO2 in D. magna was dependent on the percentage of exposed {001} facets. With the co-existence of nanostructured TiO2 and Cu2+, the percentage of exposed {001} facets influenced on the interaction between TiO2 and Cu2+ and therefor played an important role on Cu2+ bioaccumulation and biotoxicity in D. magna. Firstly, Ti bioaccumulation in D. magna increased slightly with increasing percentage of {001} facets, and the addition of Cu2+ reduced Ti bioaccumulation in organisms due to the aggregation of TiO2 induced by adsorbed Cu2+. Secondly, TiO2 NPs enhanced copper accumulation, whereas the other four TiO2 NSs reduced it. Such difference is probably relevant to the different Cu2+ adsorption capacities of TiO2 with different percentage of exposed {001} facets. Thirdly, five types of TiO2 and Cu ingested by D. magna produced relatively strong oxidative stress and inhibited SOD enzyme activity, but the membrane damage was not the main toxicity. Moreover, the SOD activities decreased with increasing copper accumulation because of its oxidative toxicity, whereas SOD increased with increasing Ti accumulation in D. magna probably because of Ti’s positive physiological effect. In sum, it was confirmed that the co-existence of copper and TiO2 is more dangerous than copper alone in aquatic environments. The mechanism of TiO2 NSs on copper biotoxicity requires further exploration.
Methods
Preparation of TiO2 NSs and NPs
TiO2 NSs samples were prepared through solvothermal method using Ti(OC4H9)4 and HF solution as precursors14,15. Briefly, 25 mL of Ti(OC4H9)4 and 3 mL of HF solution (with a concentration of 40 wt.%) were mixed in a dried 100 mL Teflon-lined autoclave, then heated and kept at 180 °C for 24 h. The nominal atomic ratio of F to Ti (RF) was 1. After the solvothermal reaction, the white precipitates were collected after thorough rinse in ethanol and distilled water thrice, and drying in an oven at 80 °C for 6 h. Four TiO2 NSs samples with different percentages of exposed {001} facets were prepared by changing RF (0.67, 1.00, 1.33 and 2.67). Based on the geometric configurations derived from TEM images, the four prepared TiO2 NSs samples appear 61%, 71%, 74%, and 78% exposed {001} facets, which were labeled as NS61, NS71, NS74, and NS78, respectively. TiO2 NPs with 10% exposed {001} facets were hydrothermally prepared in pure water without HF and labeled as NP10. The preparation details of TiO2 NSs and NPs are summarized in Table 1. Finally, TiO2 stock suspensions (1 g L−1) were prepared by dispersing TiO2 NSs or NPs in Milli-Q water using ultrasonic treatment for 30 min (50 W L−1, 40 kHz). The stock solution was stored at room temperature before utilization.
Characterization
Transmission electron microscopy (TEM) analysis was conducted using a JEM-2100F electron microscope (JEOL, Japan) with an accelerating of 200 kV voltage. X-ray diffraction (XRD) (type HZG41B-PC) was used to characterize the crystalline phase and crystallite size of the TiO2 samples. Brunauer–Emmett–Teller (BET) specific surface area (SBET) of the powders was analyzed via nitrogen adsorption in a Micromeritics ASAP 2020 nitrogen adsorption apparatus (USA). All the as-prepared samples were degassed at 180 °C prior to nitrogen adsorption measurements. The BET surface area was determined by a multipoint BET method using adsorption data in the relative pressure (P/P0) range of 0.05 to 0.3. A desorption isotherm was used to determine the pore size distribution via the Barrett–Joyner–Halenda (BJH) method, assuming a cylindrical pore modal.
Adsorption of Cu2+ on TiO2
To study the sorption of Cu2+ on nano-sized TiO2 with different percentages of {001} facets, 1 mg/L nano-sized TiO2 suspensions were prepared using the TiO2 stocks in SM7 medium respectively. Add Cu2+ solution with a known concentration into TiO2 suspension and mix rapidly. Tow replicates were set for each treatment. At 5, 30, 60, 120, 240 and 360 min, 5 mL of the mixture was drawn out. The samples were then centrifuged for 5 min at 12,000 rpm using a versatile compact centrifuge (Himac CF 16RX, Hitachi, Tokyo, Japan) to separate particles from the solution. Cu2+ concentration in the supernatant was determined through ICP-MS (VG PQ2 TURBO). The adsorption amount of Cu2+ on TiO2 was determined by calculating the mass difference between before and after adsorptions.
Model organisms
The D. magna used in this study was kept in the laboratory for two years, and were cultured at 23 °C with a light:dark cycle of 16:8 h. The daphnids were cultured in natural water collected from Huo Qi Ying Bridge (116°16’ 732 E, 39°58’ 401 N). The water used was filtered through a 1.2 μm membrane before use. The green alga Chlamydomonas reinhardtii was fed to D. magna at a density of 1 × 105 to 2 × 105 cells mL−1 per day, and the water was replenished every two days. The alga was grown in artificial WC medium40 and was collected at its exponential growth stage by centrifugation.
Acute exposure of D. magna to TiO2 with or without Cu2+
The water used for the exposure experiments was synthetic water, which was simplified Elendt M7 medium (SM7, containing only CaCl2, MgSO4, K2HPO4, KH2PO4, NaHCO3, NaNO3, Na2SiO3, H3BO3, and KCl and without disodium ethylenediaminetetraacetic acid, trace metals, or vitamins). The stocks of five different TiO2 were added to 500 mL SM7 with TiO2 concentration fixed at 1 mg/L. Two groups of samples were set: one contained TiO2 and another contained TiO2 and Cu2+ fixed at 50 μg/L. The control group comprised 500 mL SM7 and Cu2+. Each treatment had three replicates, which contained 50 14-day old D. magna (1 individual/10 mL). The D. magna were not fed during the exposure time. All the samples were treated under the same conditions. All glassware and exposure chambers were previously acid washed and thoroughly rinsed with distilled water.
Determination of Ti and Cu bioaccumulation in D. magna
At the end of exposure, ten D. magna were taken out and rinsed with pure water for three times. They were then placed in a drying oven at 80 °C. These dried D. magna were digested in 68% HNO3 (Aristar grade) and (NH4)2SO4-H2SO4 (98%, Aristar grade) solution at 110 °C41. The digestion solution was transferred into a volumetric flask with 2% HNO3 and diluted for Ti and Cu analysis through ICP-MS. TiO2 and Cu accumulation was calculated based on the dry weight of D. magna (μg/g dry wt).
Determination of SOD and Na+/K+-ATPase activities in D. magna
The other twenty exposed D. magna were weighed after wiping off the water from their surfaces. Tissues of D. magna were homogenized in 0.5 mL sucrose buffer (0.25 M sucrose and 0.1 M Tris-HCl, pH 8.6) by ultrasonication, after which they were centrifuged at a speed of 16000 × g for 20 min. The supernatant fluid was diluted to 1.5 mL using a homogenate. One milliliter of supernatant fluid was used to determine SOD enzyme and Na+/K+-ATPase activities using commercially available kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s protocol.
SOD is a kind of catalytic enzyme that can convert superoxide into oxygen and hydrogen peroxide to protect cells. SOD activity is assayed using a spectrophotometric method based on inhibition of a superoxide-driven NADH oxidation, which consists of a purely chemical reaction sequence which involves EDTA, Mn(II), mercaptoethanol, and molecular oxygen42. Na+/K+-ATPase can keep a high concentration of K+ inside the cell and Na+ outside the cell to maintain the balance of osmotic pressure. Na+/K+-ATPase is assessed based on the amount of inorganic phosphate liberated from hydrolysis of the substrate ATP43.
Additional Information
How to cite this article: Liu, L. et al. Effects of the interaction between TiO2 with different percentages of exposed {001} facets and Cu2+ on biotoxicity in Daphnia magna. Sci. Rep. 5, 11121; doi: 10.1038/srep11121 (2015).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.: 51290283, 51378041), Beijing Natural Science Foundation (8142027), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20131102110035), Major Science and Technology Program for Water Pollution Control and Treatment of China (No. 2012ZX07501001), and China’s National Basic Research Program (No. 2011CB935700).
Footnotes
Author Contributions W.H.F. and L.L.L. designed the experiments; W.X. and H.T.L. prepared materials; W.H.F. and L.L.L. performed the experiments and analyzed the data; W.H.F., L.L.L., H.T.L. and W.X. wrote the paper.
References
- Bernard D. G., Marilyn S. Emerging Challenges-Nanotechnology and the Environment. GEO Year Book. (2007). Available at: http://www.unep.org/yearbook/2007/PDF/7_Emerging_Challenges72dpi.pdf (Accessed: 3rd March 2015) [Google Scholar]
- Robichaud C. O., Uyar A. E., Darby M. R., Zucker L. G. & Wiesner M. R. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 43, 4227–4233 (2009). [DOI] [PubMed] [Google Scholar]
- Lovern S. B. & Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C-60) nanoparticles. Environ. Toxicol. Chem. 25, 1132–1137 (2006). [DOI] [PubMed] [Google Scholar]
- Gottschalk F., Sun T. & Nowack B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 181, 287–300 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhou J. & Cai Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 45, 3753–3758 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 67, 160–166 (2007). [DOI] [PubMed] [Google Scholar]
- Sun H., Zhang X., Zhang Z., Chen Y. & Crittenden J. C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 157, 1165–1170 (2009). [DOI] [PubMed] [Google Scholar]
- Yang W. W., Miao A. J. & Yang L. Y. Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. Plos One. 7, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L., Hurel C. & Marmier N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants - Effects of size and crystalline structure. Chemosphere. 90, 1083–1090 (2013). [DOI] [PubMed] [Google Scholar]
- Dabrunz A. et al. Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. Plos One. 6, 1–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann N. B. et al. Algal testing of titanium dioxide nanoparticles-Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicol. 269, 190–197 (2010). [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu R. & Liu C. Enhanced photocatalysis on TiO2 nanotube arrays modified with molecularly imprinted TiO2 thin film. J. Hazard. Mater. 182, 912–918 (2010). [DOI] [PubMed] [Google Scholar]
- Yang X., Qin J. & Li Y. Graphene-spindle shaped TiO2 mesocrystal composites: Facile synthesis and enhanced visible light photocatalytic performance. J. Hazard. Mater. 261, 342–350 (2013). [DOI] [PubMed] [Google Scholar]
- Xiang Q. & Yu J. Photocatalytic activity of hierarchical flower-like TiO2 superstructures with dominant {001} facets. Chinese. J. Catal. 32, 525–531 (2011). [Google Scholar]
- Xiang Q., Lv K. & Yu J. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl. Catal. B-Environ. 96, 557–564 (2010). [Google Scholar]
- Liu S., Yu J. & Jaroniec M. Anatase TiO2 with dominant high-energy {001} facets: synthesis, properties, and applications. Chem Mater. 23, 4085–4093 (2011). [Google Scholar]
- Lv K., Xiang Q. & Yu J. Effect of calcination temperature on morphology and photocatalytic activity of anatase TiO2 nanosheets with exposed {001} facets. Appl. Catal. B-Environ. 104, 275–281 (2011). [Google Scholar]
- Li Y. F., Liu Z. P., Liu L. & Gao W. Mechanism and activity of photocatalytic oxygen evolution on titania anatase in aqueous surroundings. J. Am. Chem. Soc. 132, 13008–13015 (2010). [DOI] [PubMed] [Google Scholar]
- Wang Y., Ye Y. & Wu K. Adsorption and assembly of copper phthalocyanine on cross-linked TiO2 (110)-(1×2) and TiO2 (210). J. Phys. Chem. B. 110, 17960–17965 2006. [DOI] [PubMed] [Google Scholar]
- Stone T. A., Torrents A. & Smolen J. Adsorption of organic compounds possessing ligand donor groups at the oxide/water Interface. Environ. Sci. Technol. 27, 895–909 (1993). [Google Scholar]
- Zhou P., Zhu X. F., Yu J. G. & Xiao W. Effects of adsorbed F, OH, and Cl ions on formaldehyde adsorption performance and mechanism of anatase TiO2 nanosheets with exposed {001} facets. ACS Appl. Mater. Interfaces. 5, 8165–8172 (2013). [DOI] [PubMed] [Google Scholar]
- Kim M. S., Hong K. M. & Chung J. G. Removal of Cu(II) from aqueous solutions by adsorption process with anatase-type titanium dioxide. Water. Res. 37, 3524–3529 (2003). [DOI] [PubMed] [Google Scholar]
- Farré M., Gajda-Schrantz K., Kantiani L. & Barceló D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem. 393, 81–95 (2009). [DOI] [PubMed] [Google Scholar]
- Sharma V. K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment-A Review. J. Envirn. Sci. Heal. A. 44, 1485–1495 (2009). [DOI] [PubMed] [Google Scholar]
- Dudev T. & Lim C. Effect of carboxylate-binding mode on metal binding/selectivity and function in proteins. Accounts. Chem. Res. 40, 85–93 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng D., Wang N. & Wang X. Effects of the interaction of TiO2 nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity. J. Hazard. Mater. 199-200, 426–432 (2012). [DOI] [PubMed] [Google Scholar]
- Dalai S. et al. Cytotoxicity of TiO2 nanoparticles and their detoxification in a freshwater system. Aquat. Toxicol. 138, 1–11 (2013). [DOI] [PubMed] [Google Scholar]
- Fan W. H. et al. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut. 159, 729–734 (2011). [DOI] [PubMed] [Google Scholar]
- Yang W. W., Li Y., Miao A. J. & Yang L. Y. Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart. Ecotox. Environ. Safe. 85, 44–51 (2012). [DOI] [PubMed] [Google Scholar]
- Fan W. H., Cui M. M., Shi Z. W., Tan C. & Yang X. P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of nano-TiO2. J. Nanomater. 10.1155/2012/398720 (2012). [DOI] [Google Scholar]
- Marcone G. P. S. Oliveira Á. C. & Almeida G. Ecotoxicity of TiO2 to Daphnia similis under irradiation. J. Hazard. Mater. 211, 436–442 (2012). [DOI] [PubMed] [Google Scholar]
- Dalai S., Pakrashi S., Kumar R. S. S., Chandrasekaran N. & Mukherjee A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 1, 116–130 (2012). [Google Scholar]
- Ramsden C. S., Henry T. B. & Handy R. D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 126, 404–413 (2013). [DOI] [PubMed] [Google Scholar]
- Morth J. P. et al. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell. Bio. 12, 60–70 (2011). [DOI] [PubMed] [Google Scholar]
- Gaetke L. M. & Chow C. K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 189, 147–163 (2003). [DOI] [PubMed] [Google Scholar]
- Hruby M., Cigler P. & Kuzel S. Contribution to understanding the mechanism of titanium action in plant. J. Plant. Nutr. 25, 577–598 (2002). [Google Scholar]
- Konishi K., Tsuge T. Inorganic constituents of green-manure crops. J. Agr. Chem. Soc. 12, 916–930 (1936). [Google Scholar]
- Carvajal M. & Alcaraz C. F. Why titanium is a beneficial element for plants. J. Plant. Nutr. 21, 655–664 (1998). [Google Scholar]
- Pais I. The biological importance of titanium. J. Plant. Nutr. 6, 3–131. (19). [Google Scholar]
- Guillard R. R. L. & Lorenzen C. J. Yellow-green algae with chlorophyllide C. J. Phycol. 8, 10–14 (1972). [Google Scholar]
- Tan C., Fan W. H. & Wang W. X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia Magna. Environ. Sci. Technol. 46, 469–476 (2012). [DOI] [PubMed] [Google Scholar]
- Paolett F., Aldinucci D., Mocali A. & Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 154, 536–541 (1986). [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S. & Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100, 95–97 (1979). [DOI] [PubMed] [Google Scholar]


