Abstract
During the latter part of 2014, we experienced an autopsy case in which 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, was identified and quantitated in solid tissues and in three herbal blend products [Forensic Toxicol (2015) 33:112–121]. At that time, although we suspected that there may be some drug(s) other than 5-fluoro-ADB in the herbal products, all trials to find it/them were unsuccessful. Subsequently, we carefully re-examined the presence of other synthetic cannabinoid(s) in the above herbal blend products using accurate mass spectrometry and found two new compounds, 5-fluoro-ADB-PINACA and MAB-CHMINACA (Forensic Toxicol. doi: 10.1007/s 11419-015-0264-y). In the present communication, we report the distribution of MAB-CHMINACA in body fluids and solid tissue specimens collected from the same deceased individual (kept frozen at −80 °C) as described above for demonstration of 5-fluoro-ADB. Unexpectedly, unchanged MAB-CHMINACA could be identified and quantitated in whole blood and in pericardial fluid specimens, but it was below the detection limit (0.1 ng/ml) in the urine specimen. A higher concentration of MAB-CHMINACA could be found in all of the nine solid tissues; the highest concentration of MAB-CHMINACA was found in the liver (156 ng/g), followed by the kidney, pancreas and so on. The compounds were detected in all nine solid tissues; their levels were generally higher than those in the whole blood and pericardial fluid. Contrary to expectations, the concentration of MAB-CHMINACA in the adipose tissue was relatively low. Our results show that the victim smoked one of the three herbal blend products containing both MAB-CHMINACA and 5-fluoro-ADB, resulting in the coexistence of both compounds. It should be concluded that 5-fluoro-ADB and MAB-CHMINACA synergically exerted their toxicities, leading to death after a short interval. The differences in the distribution of 5-fluoro-ADB and MAB-CHMINACA among the cadaver specimens were also discussed in view of the structures of both compounds. To our knowledge, this is the first report to demonstrate MAB-CHMINACA in biological/human specimens.
Keywords: MAB-CHMINACA, Synthetic cannabinoid, Postmortem distribution, Solid tissue, LC–MS–MS, 5-Fluoro-ADB
Introduction
In recent years, various types of synthetic cannabinoids [1–4] and cathinones [5–8] have become widely distributed, and are now causing social problems throughout many parts of the world. Nowadays, the newly emerging drugs seem to have become more potent than the previous ones, and sometimes cause deaths [9–12].
During the latter part of 2014, three herbal blend products, all of which were open, were brought to our laboratory for analysis, together with a cadaver. At the time of autopsy, the primary cause of death was considered to be 5-fluoro-ADB poisoning, as this compound was detected in the stomach contents and in five solid tissues; 5-fluoro-ADB was detected at a concentration of 49.2 ± 2.46 mg/g in the “GM sapphire” product, one of the three herbal blends [13]. At that time, however, we noticed unknown peaks other than 5-fluoro-ADB in the gas chromatography-mass spectrometry (GC-MS) chromatograms for extracts of the three herbal blend products. We consulted the Cayman Spectral Library [14], but it did not suggest any compounds. Shortly after the autopsy, we re-examined the herbal blend extracts for the presence of other drug(s) using matrix-assisted laser desorption ionization (MALDI) tandem quadrupole time-of flight (QTOF) mass spectrometry. The MALDI tandem QTOF mass spectra strongly suggested the presence of 5-fluoro-ADB-PINACA and MAB-CHMINACA without their reference standards. After obtaining the reference standards, we compared the mass spectra of the extracts of the herbal blend products with those of the reference standards using both GC–MS and liquid chromatography–tandem mass spectrometry (LC–MS–MS). The mass spectra of the herbal blend extracts coincided with those of the reference standards, disclosing the presence of 5-fluoro-ADB-PINACA in “AL 37” and “AP 31” and MAB-CHMINACA in “GM sapphire” [15]; we then quantitated the concentrations of both compounds in the herbal blend products using the standard addition method. The concentrations of 5-fluoro-ADB-PINACA were 19.4 ± 0.55 and 19.0 ± 0.47 mg/g (mean ± standard deviation of triplicate determinations) for the herbal product brands “AL 37” and “AP 31,” respectively; that of MAB-CHMINACA was 133 ± 4.5 mg/g for the “GM sapphire” blend product [15].
In this communication, we identify and quantitate MAB-CHMINACA in various human specimens collected during autopsy. Unfortunately, however, 5-fluoro-ADB-PINACA was only detected in the stomach contents. To our knowledge, this is the first report to identify and quantitate the new synthetic cannabinoid MAB-CHMINACA from biological specimens.
Case history
Human specimens were collected during the latter part of 2014 from the same deceased individual as described for demonstration of 5-fluoro-ADB [13]; specimens were stored at −80 °C. The deceased was a 30-year-old man found in his room. Three opened, silver-colored herbal blend packages with brand names “AL 37,” “AP 31” and “GM sapphire” were found nearby. Additional details of the case history are described in our previous report [13].
Materials and methods
Materials
The reference standards MAB-CHMINACA [synonym: ADB-CHMINACA, N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide], 5-fluoro-ADB-PINACA, AB-CHMINACA (internal standard, IS), and 5-fluoro-AB-PINACA (IS) were purchased from Cayman Chemical (Ann Arbor, MI, USA). All chemical structures of the above four synthetic cannabinoids are described in our previous report [15]. Other common chemicals used were of the highest purity commercially available. Plastic centrifuge tubes with caps (5-ml capacity, 6 × 1.5 cm external diameter) and stainless beads (5 mm external diameter) for crushing solid tissues were purchased from TAITEC, Saitama, Japan. QuEChERS dispersive-SPE centrifuge tubes with caps (2-ml capacity), each of which contained 25 mg of primary secondary amine (PSA), 25 mg of end-capped octadecylsilane (C18EC), and 150 mg of magnesium sulfate, and Captiva ND Lipids cartridges (3-ml capacity) were purchased from Agilent (Santa Clara, CA, USA).
Whole blood specimens from the right and left atria and femoral vein, pericardial fluid, urine, stomach contents, and nine solid tissue specimens (brain, lung, heart muscle, liver, spleen, kidney, pancreas, skeletal muscle and adipose tissue) were collected from the deceased at autopsy and kept frozen at −80 °C until analysis; the adipose tissue specimen was obtained from the abdominal subcutaneous area.
Extraction procedure for human specimens
For analysis of MAB-CHMINACA, AB-CHMINACA was used as the IS; for that of 5-fluoro-ADB-PINACA, 5-fluoro-AB-PINACA was used. The extraction procedure included crushing each specimen with a bead beater-type homogenizer, QuEChERS dispersive solid-phase extraction, and filtration through a Captiva ND lipids cartridge. Among the solid tissues, some modified steps were added to the regular extraction procedure for the adipose tissue specimen, the details of which are described in our previous report [13].
LC–MS–MS conditions
LC–MS–MS conditions were exactly the same as those described in the previous report [13] except that ion transitions were m/z 371 → 241 for MAB-CHMINACA, m/z 357 → 241 for AB-CHMINACA (IS), m/z 363 → 318 for 5-fluoro-ADB-PINACA, and m/z 349 → 304 for 5-fluoro-AB-PINACA (IS); the fragmentor voltage and collision energy were 120 and 25 V for MAB-CHMINACA, 120 and 21 V for AB-CHMINACA, 120 and 9 V for 5-fluoro-ADB-PINACA, and 120 and 9 V for 5-fluoro-AB-PINACA, respectively.
Standard addition method
The standard addition method is frequently used for analysis by atomic absorption spectroscopy [16]. Because this method does not require a blank matrix and completely overcomes matrix effects, we used it to analyse compound(s) in human specimens with quite different properties [11–13, 17–20]. Method principles and examples of the standard addition calibration curve were discussed in detail in our previous report [21].
Matrix effects and recovery rates
In our previous report [13] for analysis of 5-fluoro-ADB in human specimens, we used the heart muscle and adipose tissue as example matrices to demonstrate the matrix effects and recovery rates; in the present study, we used femoral vein blood and the liver as example matrices for analysis of MAB-CHMINACA. Other conditions of this experiment and the calculation method were the same as described previously [13].
Results and discussion
Identification trial for 5-fluoro-ADB-PINACA and MAB-CHMINACA
The extracts from all specimens were subjected to detection of peaks of 5-fluoro-ADB-PINACA by LC–MS–MS in various detection modes. The protonated molecular ion at m/z 363 could not be detected in any specimens of the deceased. However, in the selected reaction monitoring (SRM) mode, a very small peak at m/z 318 [above the limit of detection (LOD) and the below the limit of quantitation (LOQ)] could be detected only for the stomach contents; for all other specimens, any peak at m/z 318 was below the LOD in the SRM mode.
In contrast to 5-fluoro-ADB-PINACA in the specimens, the concentrations of MAB-CHMINACA were found at much higher concentrations than those of 5-fluoro-ADB-PINACA. It was possible to obtain product ion mass spectra from femoral vein whole blood and liver tissue specimens. Figure 1 shows an example of product ion mass spectra obtained from the extracts of femoral vein whole blood and liver tissue in comparison with that of the reference standard MAB-CHMINACA. The spectra obtained from the two extracts coincided with that of the reference standard MAB-CHMINACA, with no impurity peaks, confirming the detected compound was MAB-CHMINACA.
Fig. 1.
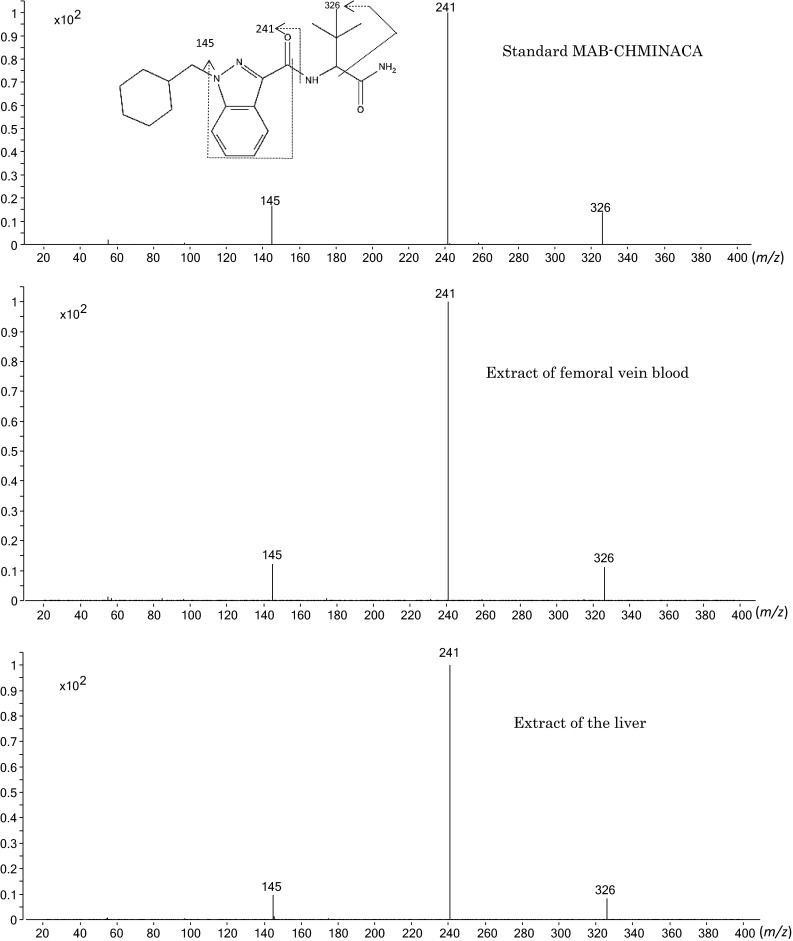
Product ion mass spectra of the extracts of femoral vein whole blood and liver tissue specimens collected from the deceased in comparison with that of the reference standard MAB-CHMINACA, recorded by liquid chromatography–tandem mass spectrometry (LC–MS–MS) together with the probable fragmentation mode
Validation of the method
Figure 2 shows an example of the SRM chromatograms for the target compound and the IS extracted from femoral vein whole blood and liver tissue. The target compound MAB-CHMINACA and IS AB-CHMINACA appeared at retention times of 8.72 and 7.93 min, respectively. The bottom panel of Fig. 2 shows the absence of AB-CHMINACA in the liver tissue extract, though a very small peak was detected at the same retention time as that of AB-CHMINACA with 100-fold magnification of the vertical axis. It seems reasonable to regard the small peak as a carryover of the AB-CHMINACA spiked into the liver tissue just before the run without the spiking of the IS. It should be noted that the backgrounds were generally very low, and there were no impurity peaks interfering with the target or IS peaks.
Fig. 2.
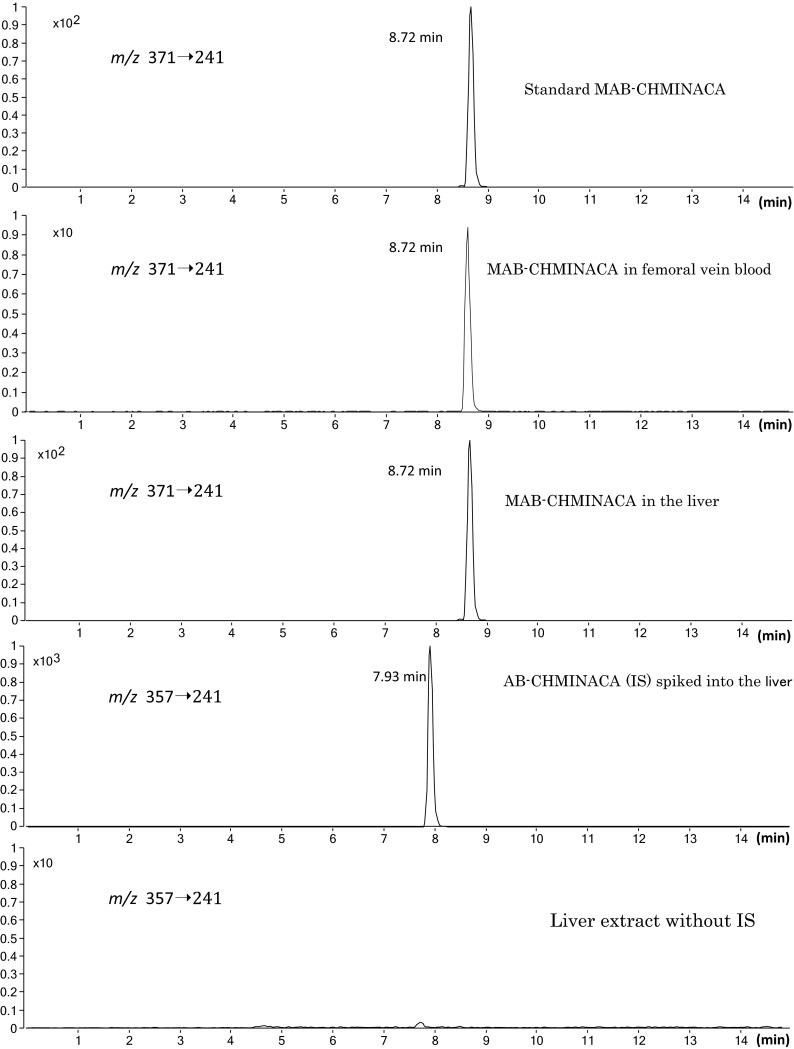
Selected reaction monitoring chromatograms using LC–MS–MS for the reference standard MAB-CHMINACA, the extracts of the femoral vein whole blood and liver tissue specimens, the internal standard (IS) AB-CHMINACA spiked into the liver tissue, and the extract of the liver tissue without addition of the IS
Table 1 shows the standard addition calibration equations for AMB-CHMINACA in 14 specimens, except for urine, in which the target compound was below the LOD. The correlation coefficients for all specimens were greater than 0.999. The LOD (signal-to-noise ratio ≥3) for the compound using the present method was around 0.1 ng/ml or g. The LOQ (signal-to-noise ratio ≥10) was around 0.5 ng/ml or g.
Table 1.
Standard addition calibration equations for MAB-CHMINACA in body fluids and solid tissues of the deceased
| Specimen | Equationa | Correlation coefficient (r) |
|---|---|---|
| Femoral vein blood | y = 0.001353x + 0.008194 | 0.9999 |
| Right heart blood | y = 0.001359x + 0.01447 | 0.9999 |
| Left heart blood | y = 0.001288x + 0.01199 | 0.9999 |
| Pericardial fluid | y = 0.001259x + 0.01882 | 0.9995 |
| Stomach contents | y = 0.001287x + 0.01358 | 0.9998 |
| Brain | y = 0.001017x + 0.01995 | 0.9999 |
| Lung | y = 0.001075x + 0.02416 | 0.9998 |
| Heart muscle | y = 0.001224x + 0.03390 | 0.9999 |
| Liver | y = 0.0009081x + 0.1414 | 0.9999 |
| Spleen | y = 0.00100x + 0.01725 | 0.9998 |
| Kidney | y = 0.0009232x + 0.03637 | 0.9998 |
| Pancreas | y = 0.001081x + 0.03447 | 0.9999 |
| Skeletal muscle | y = 0.001002x + 0.009822 | 0.9999 |
| Adipose tissue | y = 0.000246x + 0.004183 | 0.9998 |
aIf y equals 0, the pre-existing concentration (x) can be calculated as a minus value
Because we employed the standard addition method for quantitation, without the use of blank specimens, it was impossible to present the usual accuracy and precision data. Instead, as shown in Table 2, we repeated intraday and interday determinations of MAB-CHMINACA in the femoral vein whole blood and liver tissue specimens as an example. The repeatability, expressed as relative standard deviations, was not greater than 14.8 %.
Table 2.
Examples of intraday and interday repeatability for determination of MAB-CHMINACA in postmortem femoral vein blood and in the liver of the deceased
| Specimen | Intraday (n = 5) | Interday (n = 5) | ||
|---|---|---|---|---|
| Concentration found (ng/ml)a | Repeatability (%RSD) | Concentration found (ng/ml)a | Repeatability (%RSD) | |
| Femoral vein blood | 6.05 ± 0.185 | 3.05 | 6.48 ± 0.363 | 5.60 |
| Liver | 156 ± 5.83 | 3.74 | 166 ± 24.5 | 14.8 |
RSD relative standard deviation
aData given as mean ± standard deviation (SD)
Although the standard addition method can overcome matrix effects and low recovery rates, we checked them under the present extraction conditions. In this study, we used acetonitrile deproteinization plus QuEChERS dispersive solid-phase extraction plus filtration through a Captiva ND Lipids cartridge coupled to an LC–MS–MS system. The matrix effects for MAB-CHMINACA were 76.9 ± 1.70 and 69.4 ± 2.17 % (n = 3 in each) for the femoral vein whole blood and liver tissue specimens, respectively. The recovery rates of the test compound calculated (for calculation method, see Ref. [13]]) were excellent at 88.8 ± 11.2 and 109 ± 5.82 % (n = 3 in each) for the femoral vein whole blood and liver tissue specimens, respectively.
Postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of the deceased
Table 3 shows the postmortem distribution of MAB-CHMINACA in body fluids, stomach contents, and nine solid tissues. The concentration of the compound in urine was below the LOD (about 0.1 ng/ml), despite detection of the compound in whole blood specimens. This suggests that the interval between inhalation of MAB-CHMINACA and cardiac arrest was so short that the compound could not reach the urinary bladder via the kidney and ureter. In the previous studies, synthetic cannabinoids were reported to accumulate in the adipose tissue at higher concentrations [9, 13, 22]. However, for MAB-CHMINACA in the present case, its concentration in the adipose tissue was not high, but was second from the lowest among the nine solid tissue specimens. This phenomenon may be also due to the shortness of interval between inhalation of the compound and cardiac arrest; it seems likely that it takes some time for MAB-CHMINACA to accumulate up to relatively high levels in the fat of the adipose tissue. The concentration of MAB-CHMINACA was outstandingly highest in the liver, followed by the kidney, pancreas and heart muscle.
Table 3.
Concentrations of MAB-CHMINACA in body fluids and solid tissues of the deceased
| Specimen | Concentration (ng/ml or g) |
|---|---|
| Femoral vein blood | 6.05 ± 0.185 |
| Right heart blood | 10.6 ± 0.667 |
| Left heart blood | 9.30 ± 0.334 |
| Pericardial fluid | 14.9 ± 0.768 |
| Stomach contents | 10.6 ± 0.133 |
| Urine | ND |
| Brain | 19.6 ± 0.572 |
| Lung | 22.5 ± 1.29 |
| Heart muscle | 27.7 ± 0.933 |
| Liver | 156 ± 5.83 |
| Spleen | 17.2 ± 1.00 |
| Kidney | 39.4 ± 1.69 |
| Pancreas | 31.9 ± 0.415 |
| Skeletal muscle | 9.80 ± 0.28 |
| Adipose tissue | 17.0 ± 1.44 |
Data given as mean ± SD obtained by triplicate determinations
ND not detectable [lower than the detection limit (0.1 ng/ml)]
According to our previous study [13], it is evident that this victim smoked the herbal blend of “GM sapphire.” In another study [15], we also disclosed a high concentration of MAB-CHMINACA from the same herbal blend item “GM sapphire,” and here we detected MAB-CHMINACA from body fluid and solid tissue specimens collected from the same cadaver as used in the previous study [13]. These results show that 5-fluoro-ADB and MAB-CHMINACA coexisted in the “GM sapphire” herbal blend that was smoked by the victim. It should be concluded, therefore, that 5-fluoro-ADB and MAB-CHMINACA synergically exerted their toxicities, leading to death after a short interval.
It seems useful to discuss the difference in distribution among the specimens collected from the same deceased for 5-fluoro-ADB and MAB-CHMINACA. In addition, the contents of both compounds in “GM sapphire” are known; they were 49.2 ± 2.46 [13] and 133 ±4.5 mg/g [15], respectively. The first point to be mentioned is the much higher concentrations of MAB-CHMINACA than those of 5-fluoro-ADB in the specimens; the concentrations of 5-fluoro-ADB were only 3.18, 1.90, 1.82, 1.17, 1.61 and 7.95 ng/g in stomach contents, the brain, heart muscle, spleen, pancreas and adipose tissue, respectively, and were below the LOD for body fluid specimens; very small peaks appeared for the lung, liver, kidney, and skeletal muscle, but they were below the LOQ [13]. Even if we take into consideration the fact that the concentration of MAB-CHMINACA is 2.7-fold higher than that of 5-fluoro-ADB in the herbal blend product “GM sapphire," the concentrations of MAB-CHMINACA in various human specimens are much higher than the values expected (Table 3) when compared with those of 5-fluoro-ADB [13]. In addition, the concentrations of MAB-CHMINACA were 6.05–10.6 ng/ml in whole blood specimens, while those of 5-fluoro-ADB in whole blood were below the LOD. These differences in concentrations between the two compounds may be largely due to the stability of the compounds; MAB-CHMINACA has a primary amino group at the terminal part and a cyclohexylmethyl moiety attached to the nitrogen, both of which are much more stable and resistant to chemical decomposition and/or metabolism than the methoxy group and the 5-fluoropentyl moiety present in the 5-fluoro-ADB structure in an alive/dead human body.
Although the outstandingly high concentration of MAB-CHMINACA in the liver is difficult to explain, the second-highest concentration of the compound in the kidney (Table 3) may be due to the ability of this compound to excrete into urine via the kidney, while the concentration of 5-fluoro-ADB in the kidney was below the LOQ [13]. This contrast seems to be due to the hydrophilic nature of the compounds; MAB-CHMINACA is more hydrophilic due to the presence of a primary amino group than is 5-fluoro-ADB with the methoxy group.
Conclusions
In the present study, we identified and quantitated MAB-CHMINACA in human specimens collected from the same victim as used in the previous study on the demonstration of 5-fluoro-ADB. The concentration of MAB-CHMINACA was outstandingly highest in the liver, followed by the kidney, pancreas and heart muscle. The compound was also detected in whole blood and pericardial fluid, but not in urine. To our knowledge, this is the first report to demonstrate MAB-CHMINACA from biological/human specimens.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Footnotes
K. Hasegawa and A. Wurita contributed equally to this work.
References
- 1.Zuba D, Byrska B. Analysis of the prevalence and coexistence of synthetic cannabinoids in “herbal high” products in Poland. Forensic Toxicol. 2013;31:21–30. doi: 10.1007/s11419-012-0159-0. [DOI] [Google Scholar]
- 2.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y. Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol. 2013;31:44–53. doi: 10.1007/s11419-012-0165-2. [DOI] [Google Scholar]
- 3.Chung H, Choi H, Heo S, Kim E, Lee J. Synthetic cannabinoids abused in South Korea : drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol. 2014;32:82–88. doi: 10.1007/s11419-013-0213-6. [DOI] [Google Scholar]
- 4.Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014;32:266–281. doi: 10.1007/s11419-014-0238-5. [DOI] [Google Scholar]
- 5.Shima N, Katagi M, Kamata H, Matsuta S, Nakanishi K, Zaitsu K, Kamata T, Nishioka H, Miki A, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K. Urinary excretion and metabolism of the newly encountered designer drug 3,4-dimethylmethcathinone in humans. Forensic Toxicol. 2013;31:101–112. doi: 10.1007/s11419-012-0172-3. [DOI] [Google Scholar]
- 6.Namera A, Konuma K, Kawamura M, Saito T, Nakamoto A, Yahata M, Ohta S, Miyazaki S, Shiraishi H, Nagao M. Time-course profile of urinary excretion of intravenously administered α-pyrrolidinovalerophenone and α-pyrrolidinobutiophenone in a human. Forensic Toxicol. 2014;32:68–74. doi: 10.1007/s11419-013-0203-8. [DOI] [Google Scholar]
- 7.Zaitsu K, Katagi M, Tsuchihashi H, Ishii A. Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol. 2014;32:1–8. doi: 10.1007/s11419-013-0218-1. [DOI] [Google Scholar]
- 8.Miserez B, Ayrton O, Ramsey J. Analysis of purity and cutting agents in street mephedrone samples from South Wales. Forensic Toxicol. 2014;32:305–310. doi: 10.1007/s11419-014-0232-y. [DOI] [Google Scholar]
- 9.Saito T, Namera A, Miura N, Ohta S, Miyazaki S, Osawa M, Inokuchi S. A fatal case of MAM-2201 poisoning. Forensic Toxicol. 2013;31:333–337. doi: 10.1007/s11419-013-0190-9. [DOI] [Google Scholar]
- 10.Namera A, Urabe S, Saito T, Torikoshi-Hatano A, Shiraishi H, Arima Y, Nagao M. A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of α-pyrrolidinobutiophenone and α-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol. 2013;31:338–343. doi: 10.1007/s11419-013-0192-7. [DOI] [Google Scholar]
- 11.Hasegawa K, Suzuki O, Wurita A, Minakata K, Yamagishi I, Nozawa H, Gonmori K, Watanabe K. Postmortem distribution of α-pyrrolidinovalerophenone and its metabolite in body fluids and solid tissues in a fatal poisoning case measured by LC–MS–MS with the standard addition method. Forensic Toxicol. 2014;32:225–234. doi: 10.1007/s11419-014-0227-8. [DOI] [Google Scholar]
- 12.Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in a fatal poisoning case. Forensic Toxicol. 2014;32:243–250. doi: 10.1007/s11419-014-0230-0. [DOI] [Google Scholar]
- 13.Hasegawa K, Wurita A, Minakata K, Gonmori K, Yamagishi I, Nozawa H, Watanabe K, Suzuki O. Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents, and solid tissues of a human cadaver, and in some herbal products. Forensic Toxicol. 2015;33:112–121. doi: 10.1007/s11419-014-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayman Chemical (2014) Cayman spectral library. https://www.caymanchem.com/app/template/SpectralLibrary.vm. Accessed Oct 2014
- 15.Wurita A, Hasegawa K, Minakata K, Gonmori K, Yamagishi I, Nozawa H, Watanabe K, Suzuki O. Identification and quantitation of 5-fluoro-ADB-PINACA and MAB-CHMINACA in dubious herbal products. Forensic Toxicol. 2015 doi: 10.1007/s11419-015-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla E. Flameless atomic absorption spectrophotometric determination of manganese in rat brain and other tissues. Clin Chem. 1978;24:471–474. [PubMed] [Google Scholar]
- 17.Wurita A, Hasegawa K, Minakata K, Watanabe K, Suzuki O. A large amount of new designer drug diphenidine coexisting with a synthetic cannabinoid 5-fluoro-AB-PINACA found in a dubious herbal product. Forensic Toxicol. 2014;32:331–337. doi: 10.1007/s11419-014-0240-y. [DOI] [Google Scholar]
- 18.Wurita A, Suzuki O, Hasegawa K, Gonmori K, Minakata K, Yamagishi I, Nozawa H, Watanabe K. Sensitive determination of ethylene glycol, propylene glycol and diethylene glycol in human whole blood by isotope dilution gas chromatography-mass spectrometry, and the presence of appreciable amounts of the glycols in blood of healthy subjects. Forensic Toxicol. 2013;31:272–280. doi: 10.1007/s11419-013-0188-3. [DOI] [Google Scholar]
- 19.Wurita A, Suzuki O, Hasegawa K, Gonmori K, Minakata K, Yamagishi I, Nozawa H, Watanabe K. Presence of appreciable amounts of ethylene glycol, propylene glycol, and diethylene glycol in human urine of healthy subjects. Forensic Toxicol. 2014;32:39–44. doi: 10.1007/s11419-013-0206-5. [DOI] [Google Scholar]
- 20.Wurita A, Suzuki O, Hasegawa K, Gonmori K, Minakata K, Yamagishi I, Nozawa H, Watanabe K. Occurrence of postmortem production of ethylene glycol and propylene glycol in human specimens. Forensic Toxicol. 2014;32:162–168. doi: 10.1007/s11419-013-0210-9. [DOI] [Google Scholar]
- 21.Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Postmortem distribution of α-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Leg Med. 2014;16:241–246. doi: 10.1016/j.legalmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O. Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of the adipose tissue for detection of the drugs in the unchanged forms. Forensic Toxicol. 2015;33:45–53. doi: 10.1007/s11419-014-0245-6. [DOI] [Google Scholar]


