Abstract
Polysialic acid (PSA) is a specific and highly regulated post-translational modification of the neural cell adhesion molecule NCAM. Synthesis of PSA depends on the activity of a single enzyme, the polysialyltransferase-1 (PST-1), recently cloned from three mammalian species. The present study was carried out to investigate the catalytic mechanism of PST-1. Using a newly developed in vitro assay system, we demonstrate autopolysialylation for PST-1. The synthesis of PSA chains, which involved N-glycosylation sites, occurred immediately after contact with the activated sugar donor CMP-Neu5Ac. In contrast to the polysialylation of NCAM, where terminal sialylation in either the alpha2,3 or alpha2,6 position is required, the autopolysialylation could be started in the asialo-PST-1 isolated from CHO cells of the Lec2 complementation group. Pre-formed PSA chains were not transferred to NCAM. Nevertheless, the autocatalytic step is likely to be a prerequisite for enzymatic activity, since agalacto-PST-1 isolated from Lec8 cells was functionally inactive. Our data describe a novel route of autocatalytic maturation of a glycosyltransferase and thereby provide a new basis for studies aimed at elucidating and influencing the catalytic functions of PST-1.
Full text
PDF
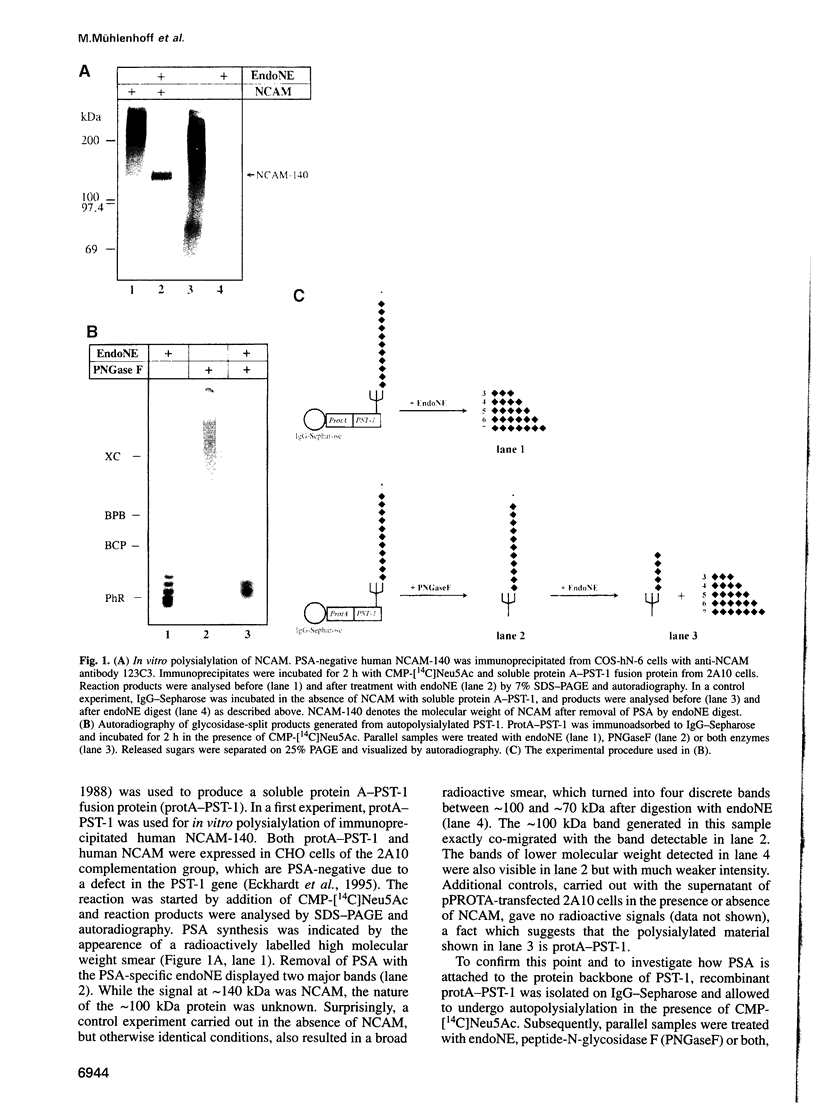
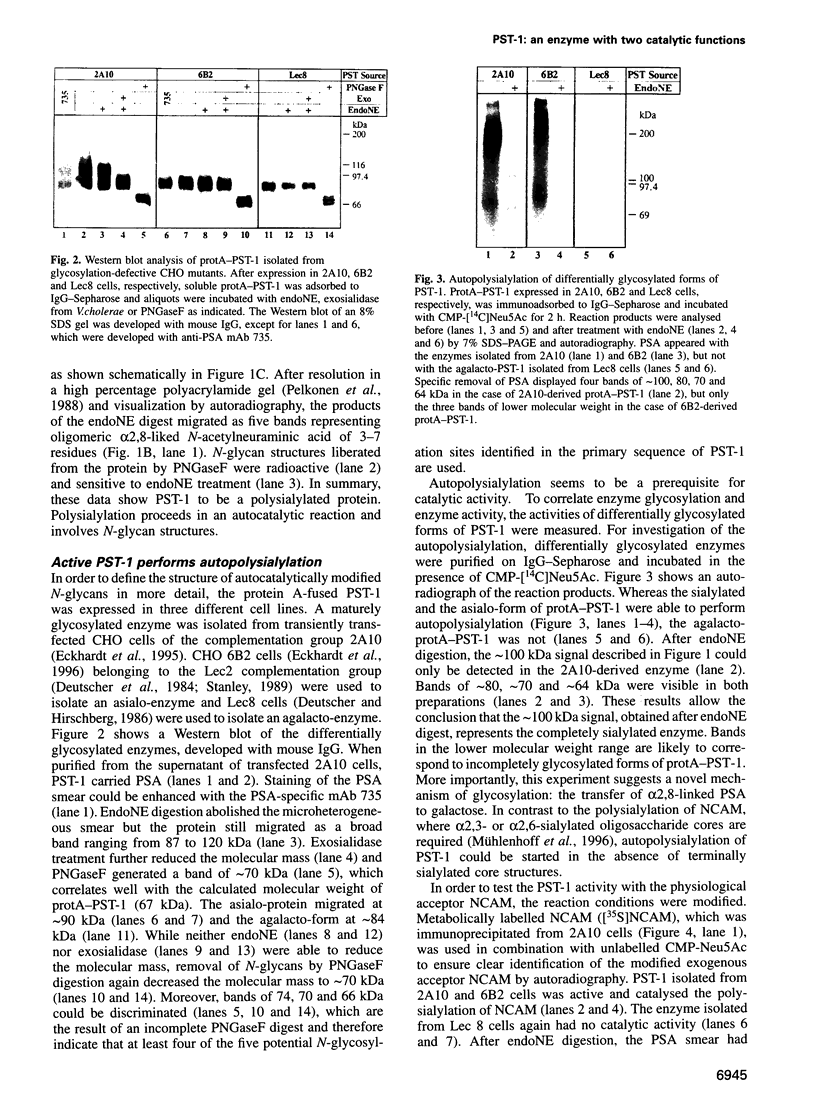




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcaraz G., Goridis C. Biosynthesis and processing of polysialylated NCAM by AtT-20 cells. Eur J Cell Biol. 1991 Jun;55(1):165–173. [PubMed] [Google Scholar]
- Alonso M. D., Lomako J., Lomako W. M., Whelan W. J. A new look at the biogenesis of glycogen. FASEB J. 1995 Sep;9(12):1126–1137. doi: 10.1096/fasebj.9.12.7672505. [DOI] [PubMed] [Google Scholar]
- Barbeau D., Liang J. J., Robitalille Y., Quirion R., Srivastava L. K. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. G., Artola A., Gerardy-Schahn R., Becker T., Welzl H., Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996 Jul 15;45(2):143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994 Feb 3;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Daniloff J. K., Levi G., Grumet M., Rieger F., Edelman G. M. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986 Sep;103(3):929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Doherty P., Cohen J., Walsh F. S. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron. 1990 Aug;5(2):209–219. doi: 10.1016/0896-6273(90)90310-c. [DOI] [PubMed] [Google Scholar]
- Doherty P., Rowett L. H., Moore S. E., Mann D. A., Walsh F. S. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991 Feb;6(2):247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- Doherty P., Walsh F. S. The contrasting roles of N-CAM and N-cadherin as neurite outgrowth-promoting molecules. J Cell Sci Suppl. 1991;15:13–21. doi: 10.1242/jcs.1991.supplement_15.3. [DOI] [PubMed] [Google Scholar]
- Doyle E., Nolan P. M., Bell R., Regan C. M. Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J Neurosci Res. 1992 Mar;31(3):513–523. doi: 10.1002/jnr.490310315. [DOI] [PubMed] [Google Scholar]
- Easton E. W., Schiphorst W. E., Koeleman C. A., Michalides R. J., Van Den Eijnden D. H. CMP-NeuAc:(NeuAc alpha 2-->8)n (colominic acid) sialyltransferase activity in rat brain and in tumour cells that express polysialic acid on neural cell adhesion molecules. Glycoconj J. 1995 Dec;12(6):829–837. doi: 10.1007/BF00731245. [DOI] [PubMed] [Google Scholar]
- Eckhardt M., Mühlenhoff M., Bethe A., Gerardy-Schahn R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7572–7576. doi: 10.1073/pnas.93.15.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M., Mühlenhoff M., Bethe A., Koopman J., Frosch M., Gerardy-Schahn R. Molecular characterization of eukaryotic polysialyltransferase-1. Nature. 1995 Feb 23;373(6516):715–718. doi: 10.1038/373715a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Adhesion and counteradhesion: morphogenetic functions of the cell surface. Prog Brain Res. 1994;101:1–14. doi: 10.1016/s0079-6123(08)61936-6. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardy-Schahn R., Bethe A., Brennecke T., Mühlenhoff M., Eckhardt M., Ziesing S., Lottspeich F., Frosch M. Molecular cloning and functional expression of bacteriophage PK1E-encoded endoneuraminidase Endo NE. Mol Microbiol. 1995 May;16(3):441–450. doi: 10.1111/j.1365-2958.1995.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Gerardy-Schahn R., Eckhardt M., Ledermann J., Kemshead J. T. Topography of NCAM antigenic epitopes recognized by SCLC-cluster-1 antibodies. A consensus view. Int J Cancer Suppl. 1994;8:27–29. doi: 10.1002/ijc.2910570705. [DOI] [PubMed] [Google Scholar]
- Goridis C., Brunet J. F. NCAM: structural diversity, function and regulation of expression. Semin Cell Biol. 1992 Jun;3(3):189–197. doi: 10.1016/s1043-4682(10)80015-7. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Hu H., Tomasiewicz H., Magnuson T., Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996 Apr;16(4):735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- Kern W. F., Spier C. M., Hanneman E. H., Miller T. P., Matzner M., Grogan T. M. Neural cell adhesion molecule-positive peripheral T-cell lymphoma: a rare variant with a propensity for unusual sites of involvement. Blood. 1992 May 1;79(9):2432–2437. [PubMed] [Google Scholar]
- Kitagawa H., Paulson J. C. Differential expression of five sialyltransferase genes in human tissues. J Biol Chem. 1994 Jul 8;269(27):17872–17878. [PubMed] [Google Scholar]
- Kojima N., Yoshida Y., Kurosawa N., Lee Y. C., Tsuji S. Enzymatic activity of a developmentally regulated member of the sialyltransferase family (STX): evidence for alpha 2,8-sialyltransferase activity toward N-linked oligosaccharides. FEBS Lett. 1995 Feb 20;360(1):1–4. doi: 10.1016/0014-5793(95)00059-i. [DOI] [PubMed] [Google Scholar]
- Kojima N., Yoshida Y., Tsuji S. A developmentally regulated member of the sialyltransferase family (ST8Sia II, STX) is a polysialic acid synthase. FEBS Lett. 1995 Oct 9;373(2):119–122. doi: 10.1016/0014-5793(95)01024-9. [DOI] [PubMed] [Google Scholar]
- Komminoth P., Roth J., Lackie P. M., Bitter-Suermann D., Heitz P. U. Polysialic acid of the neural cell adhesion molecule distinguishes small cell lung carcinoma from carcinoids. Am J Pathol. 1991 Aug;139(2):297–304. [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Poirier G. G., Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995 Oct;20(10):405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Livingston B. D., Paulson J. C. Polymerase chain reaction cloning of a developmentally regulated member of the sialyltransferase gene family. J Biol Chem. 1993 Jun 5;268(16):11504–11507. [PubMed] [Google Scholar]
- Martersteck C. M., Kedersha N. L., Drapp D. A., Tsui T. G., Colley K. J. Unique alpha 2, 8-polysialylated glycoproteins in breast cancer and leukemia cells. Glycobiology. 1996 Apr;6(3):289–301. doi: 10.1093/glycob/6.3.289. [DOI] [PubMed] [Google Scholar]
- Martini R., Schachner M., Brushart T. M. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994 Nov;14(11 Pt 2):7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzman R. A., Warhol M. J., Gee B., Roth J. Polysialic acid as a marker of both immature and mature neural tissue in human teratomas. Mod Pathol. 1991 Jul;4(4):491–497. [PubMed] [Google Scholar]
- Miller P. D., Styren S. D., Lagenaur C. F., DeKosky S. T. Embryonic neural cell adhesion molecule (N-CAM) is elevated in the denervated rat dentate gyrus. J Neurosci. 1994 Jul;14(7):4217–4225. doi: 10.1523/JNEUROSCI.14-07-04217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar C. E., Muller E. J., Schol D. J., Figdor C. G., Bock E., Bitter-Suermann D., Michalides R. J. Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 1990 Feb 15;50(4):1102–1106. [PubMed] [Google Scholar]
- Muller D., Stoppini L., Wang C., Kiss J. Z. A role for polysialylated neural cell adhesion molecule in lesion-induced sprouting in hippocampal organotypic cultures. Neuroscience. 1994 Aug;61(3):441–445. doi: 10.1016/0306-4522(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M., Eckhardt M., Bethe A., Frosch M., Gerardy-Schahn R. Polysialylation of NCAM by a single enzyme. Curr Biol. 1996 Sep 1;6(9):1188–1191. doi: 10.1016/s0960-9822(02)70687-8. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Fukuda M. N., Fredette B., Ranscht B., Fukuda M. Expression cloning of a human polysialyltransferase that forms the polysialylated neural cell adhesion molecule present in embryonic brain. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7031–7035. doi: 10.1073/pnas.92.15.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J., Fukuda M. A human polysialyltransferase directs in vitro synthesis of polysialic acid. J Biol Chem. 1996 Jan 26;271(4):1829–1832. doi: 10.1074/jbc.271.4.1829. [DOI] [PubMed] [Google Scholar]
- Ono K., Tomasiewicz H., Magnuson T., Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994 Sep;13(3):595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan C. M., Fox G. B. Polysialylation as a regulator of neural plasticity in rodent learning and aging. Neurochem Res. 1995 May;20(5):593–598. doi: 10.1007/BF01694541. [DOI] [PubMed] [Google Scholar]
- Rousselot P., Lois C., Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J Comp Neurol. 1995 Jan 2;351(1):51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Scheidegger E. P., Lackie P. M., Papay J., Roth J. In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Lab Invest. 1994 Jan;70(1):95–106. [PubMed] [Google Scholar]
- Scheidegger E. P., Sternberg L. R., Roth J., Lowe J. B. A human STX cDNA confers polysialic acid expression in mammalian cells. J Biol Chem. 1995 Sep 29;270(39):22685–22688. doi: 10.1074/jbc.270.39.22685. [DOI] [PubMed] [Google Scholar]
- Stanley P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol. 1989 Feb;9(2):377–383. doi: 10.1128/mcb.9.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu K., Auerbach B., Gerardy-Schahn R., Eckhardt M., Jaques G., Madry N. Characterization of tumor-associated neural cell adhesion molecule in human serum. Cancer Res. 1994 May 15;54(10):2598–2603. [PubMed] [Google Scholar]
- Tang J., Rutishauser U., Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994 Aug;13(2):405–414. doi: 10.1016/0896-6273(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H., Ono K., Yee D., Thompson C., Goridis C., Rutishauser U., Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993 Dec;11(6):1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Troy F. A., 2nd Polysialylation: from bacteria to brains. Glycobiology. 1992 Feb;2(1):5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- Yang P., Major D., Rutishauser U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem. 1994 Sep 16;269(37):23039–23044. [PubMed] [Google Scholar]
- Yoshida Y., Kojima N., Tsuji S. Molecular cloning and characterization of a third type of N-glycan alpha 2,8-sialyltransferase from mouse lung. J Biochem. 1995 Sep;118(3):658–664. doi: 10.1093/oxfordjournals.jbchem.a124960. [DOI] [PubMed] [Google Scholar]
- Zuber C., Lackie P. M., Catterall W. A., Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992 May 15;267(14):9965–9971. [PubMed] [Google Scholar]