Abstract
Six new psychoactive substances were identified together with two other substances (compounds 1–8) in illegal products by our ongoing survey in Japan between January and July 2014. A new synthetic cannabinoid, FDU-NNEI [1-(4-fluorobenzyl)-N-(naphthalen-1-yl)-1H-indole-3-carboxamide, 2], was detected with the newly distributed synthetic cannabinoid FDU-PB-22 (1). Two 2H-indazole isomers of synthetic cannabinoids, AB-CHMINACA 2H-indazole analog (3) and NNEI 2H-indazole analog (4), were newly identified with 1H-indazoles [AB-CHMINACA and NNEI indazole analog (MN-18)]. In addition, 2-methylpropyl N-(naphthalen-1-yl) carbamate (5) and isobutyl 1-pentyl-1H-indazole-3-carboxylate (6) were detected in illegal products. Compound 6 is considered to be a by-product of the preparation of NNEI indazole analog from compound 5 and 1-pentyl-1H-indazole. A phenethylamine derivative, N–OH-EDMA [N-hydroxy-3,4-ethylenedioxy-N-methylamphetamine, 7], and a cathinone derivative, dimethoxy-α-PHP (dimethoxy-α-pyrrolidinohexanophenone, 8), were newly identified in illegal products. Among them, compounds 1 and 8 have been controlled as designated substances (Shitei-Yakubutsu) under the Pharmaceutical Affairs Law in Japan since August and November 2014, respectively.
Keywords: 2H-indazole isomer, Synthetic cannabinoid, FDU-NNEI, N–OH-EDMA, Dimethoxy-α-PHP, Cathinone derivative
Introduction
The consumption of new psychoactive substances (NPSs) including synthetic cannabinoids and cathinone derivatives has become widespread despite regulatory control measures [1–7]. The EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) reported that 81 NPSs were identified by the EU early warning system in 2013, with 37 NPSs reported from January to May 2014 [2]. Ninety-seven NPSs were reported to the UNODC (United Nations Office on Drugs and Crime) in 2013 alone [3].
We previously reported the appearance of 45 newly distributed substances in Japan between January 2013 and May 2014, by our ongoing survey of NPSs in the illegal drug market in Japan [8–10]. The detected compounds were 18 synthetic cannabinoids, 13 cathinone derivatives, five phenethylamines, and nine other substances, including the N-methyl-d-aspartate (NMDA) channel blocker diphenidine [8–10]. In the present study, we describe the identification of eight newly distributed compounds: four synthetic cannabinoids (1–4), two other substances (5 and 6), a phenethylamine derivative (7), and a cathinone derivative (8) in illegal products purchased from January to July 2014 (Fig. 1a).
Fig. 1.
Structures of the newly detected compounds (1–8, a), detected, but known compounds (b), and related compounds (c)
Materials and methods
Samples for analyses
The analyzed samples were purchased via the Internet between January and July 2014 as 241 chemical-type or herbal-type products being sold in Japan. Among them, we show the analysis data of five products (A–E) for describing the identification of compounds 1–8 in this paper. Each of the herbal-type products (A–D) contained approximately 3 g of mixed dried plants. The single powder-type product called ‘‘fragrance powder’’ consisted of 400 mg of a brown powder (E).
Chemicals and reagents
FDU-PB-22 (1), FUB-PB-22, AB-CHMINACA, 5-fluoro-AMB, AM-2201 indazole analog (THJ-2201), NNEI indazole analog (MN-18), 4-methylbuphedrone, DL-4662, and 3,4-EDMA were purchased from Cayman Chemical (Ann Arbor, MI, USA); 2-methylpropyl N-(naphthalen-1-yl) carbamate (5) and diphenidine from Otava Ltd. (Toronto, Canada) and Tocris Bioscience (Bristol, UK), respectively. All other common chemicals and solvents were of analytical reagent grade or high-performance liquid chromatography (HPLC) grade. As solvents for nuclear magnetic resonance (NMR) analysis, chloroform-d3 (99.96 %), methanol-d4 (99.96 %), methanol-d3 (99 %), pyridine-d5 (99.96 %), and dimethyl sulfoxide (DMSO)-d6 (99.96 %) were purchased from the ISOTEC division of Sigma-Aldrich (St. Louis, MO, USA).
Preparation of sample solutions
For the qualitative analyses, 10 mg of each herbal-type product was crushed into powder and extracted with 1 ml of methanol under ultrasonication for 10 min. A 2-mg portion of each powder-type product was extracted with 1 ml of methanol under ultrasonication for 10 min. After centrifugation (3,000 rpm, 5 min) of each extract, the supernatant solution was passed through a centrifugal filter (Ultrafree-MC, 0.45-µm filter unit; Millipore, Bedford, MA, USA) to serve as the sample solution for the analyses. If necessary, the solution was diluted with methanol to a suitable concentration before instrumental analyses.
Analytical conditions
Each sample solution was analyzed by ultra-performance liquid chromatography-electrospray ionization–mass spectrometry (UPLC-ESI–MS) and by gas chromatography–mass spectrometry (GC–MS) in the electron ionization (EI) mode according to our previous report [11]. Two elution programs were used in the LC–MS analysis. Each analysis was carried out with a binary mobile phase consisting of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in acetonitrile). The elution program (1) used for analysis of cannabinoids was as follows: 35 % B (4-min hold), 65 % B to 75 % B (4–16 min), and up to 90 % B (16–17 min, 6-min hold) at a flow rate of 0.3 ml/min. The elution program (2) used for the analysis of cathinone derivatives and other compounds was as follows: 5 % B to 20 % B (0–20 min), and up to 80 % B (20–30 min, 10-min hold). In this study, products A, B, and D were analyzed using program (1), and products C and E analyzed using program (2). GC–EI–MS was performed on an Agilent 6890N GC with a 5975 mass selective detector (Agilent Technologies, Santa Clara, CA, USA) using a capillary column (HP-1MS capillary, 30 m × 0.25 mm i.d., 0.25-μm film thickness; Agilent Technologies) with helium gas as a carrier at 0.7 ml/min. The conditions were: electron energy, 70 eV; injector temperature, 220 °C; injection, splitless mode for 1.0 min; oven temperature program, 80 °C (1-min hold) and increase at a rate of 5 °C/min to 190 °C (15-min hold) followed by increase at 10 °C/min up to 310 °C (15-min hold); transfer line temperature, 280 °C; scan range, m/z 40–550.
The obtained GC mass spectra were compared to those of an EI-MS library (Mass Spectra of Designer Drugs 2013; WILEY–VCH, Weinheim, Germany). We also used our in-house EI-MS library of designer drugs obtained by our ongoing survey of illegal products and commercially available reagents for the structural elucidation.
We measured the accurate mass numbers of the target compounds by liquid chromatography–quadrupole-time-of-flight–mass spectrometry (LC–QTOF–MS) in the ESI mode according to our previous report [12].
For the isolation of each compound, we performed two preparative methods. One was recycling preparative HPLC on a JAI (Japan Analytical Industry, Tokyo, Japan) LC-9201 instrument with gel permeation JAIGEL-1H columns (JAI) and chloroform as an eluent. The other was silica gel or ODS column chromatography (CC) on a Biotage (Stockholm, Sweden) Isorela One instrument with a SNAP KP-sil column (particle size: 50 μm), SNAP Ultra column (particle size: 25 μm), or SNAP KP-C18-HS column (particle size: 50 μm) (Biotage).
The nuclear magnetic resonance (NMR) spectra were obtained on ECA-800 and 600 spectrometers (JEOL, Tokyo, Japan). Assignments were made via 1H NMR, 13C NMR, heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple-bond correlation (HMBC), 15N HMBC, HH correlation spectroscopy (HH COSY), nuclear Overhauser effect (NOE), and incredible natural abundance double-quantum transfer experiment (INADEQUATE) spectra.
Isolation of compound 2
A 3.0-g sample of mixed dried plants (product B) was extracted with 250 ml of chloroform by ultrasonication for 30 min. The extraction was repeated three times, and the supernatant fractions were combined and evaporated to dryness. The extract was separated by silica gel CC (SNAP KP-sil, 25 g; Biotage) [hexane/ethyl acetate (100:0–75:25)] and ODS CC (SNAP KP-C18-HS, 12 g; Biotage) [water/methanol (30:70–0:100)]. The extract was then recrystallized in ethyl acetate/hexane, which gave compound 2 (28 mg) as a pale yellow solid.
Isolation of compound 3
A 2.8-g sample of mixed dried plants (product C) was extracted by the same method as that used for compound 2. The separation of extract by silica gel CC (SNAP KP-sil, 50 g; Biotage) [hexane/ethyl acetate (50:50)] and ODS CC (SNAP KP-C18-HS, 12 g; Biotage) [water/methanol (35:65)] resulted in the isolation of compound 3 (8 mg) as a white solid.
Isolation of compounds 4 and 6
A 5.6-g sample of mixed dried plants (product D) was extracted by the same method described above. Separation of extract by silica gel CC (SNAP Ultra, 25 g; Biotage) [hexane/ethyl acetate (87:13–0:100)] gave fractions 1 and 2. Fraction 1 was further separated by silica gel CC (SNAP KP-sil, 50 g; Biotage) [hexane/ethyl acetate (90:10)] to obtain compound 4 (5 mg) as a white solid. Fraction 2 was dissolved in chloroform and purified by recycling preparative HPLC (eluent: chloroform) to give compound 6 (1 mg) as a white solid.
Isolation of compounds 7 and 8
A 600-mg sample of powder (product E) was dissolved in 2.5 % triethylamine (TEA) in hexane/ethyl acetate (50:50) and then loaded onto a silica gel CC (SNAP KP-sil, 50 g; Biotage), which was then eluted with a stepwise gradient of 2.5 % TEA in hexane/2.5 % TEA in ethyl acetate (90:10–0:100) to give compound 7 (73 mg) and compound 8 (302 mg) as a colorless oil, respectively.
Results and discussion
Identification of an unknown peak 1
An unknown peak 1 was detected with an NMDA receptor channel blocker, diphenidine (Fig. 1b) [13] in the LC–MS and GC–MS for product A (Fig. 2a, b, e). Based on the LC–MS and GC–MS data, peak 1 was finally identified as a synthetic cannabinoid FDU-PB-22 (Figs. 1a, 2c, f) by direct comparison of the data to those of the purchased authentic compound (Fig. 2d, g). Compound 1 was thus detected as a newly distributed NPS in Japan. In addition, FDU-PB-22 (1) has been controlled as a designated substance (Shitei-Yakubutsu) in Japan since August 2014.
Fig. 2.
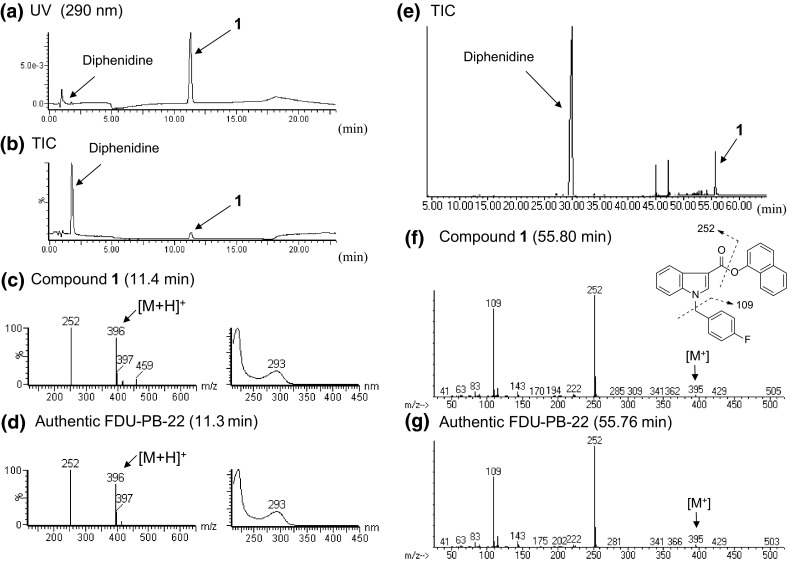
Liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry (GC–MS) analyses of product A. The liquid chromatography–ultraviolet-photodiode array (LC–UV-PDA) chromatogram (a), total ion chromatogram (TIC) (b), and electrospray ionization (ESI) mass and ultraviolet (UV) spectra of peaks 1 (c) and the authentic FDU-PB-22 (d) are shown. TIC (e), electron ionization (EI) mass spectra of peaks 1 (f) and the authentic FDU-PB-22 (g) obtained by GC–MS are also shown
Identification of an unknown peak 2
In the LC–MS and GC–MS analyses, an unknown peak 2 was detected with a synthetic cannabinoid (FUB-PB-22), its synthetic component 8-quinolinol, and a cathinone derivative (4-methylbuphedrone) in product B (Figs. 1b, 3a, b, d, f). By GC–MS analysis, peak 2 showed a molecular ion at m/z 394 (Fig. 3g). The major fragment ions of peak 2 (m/z 109 and 252) are the same as those of FDU-PB-22 (1) except for the molecular ion of compound 1 at m/z 395 (Fig. 2g). In the LC–MS analysis, peak 2 showed the protonated molecular ion at m/z 395 ([M + H]+) (Fig. 3c, e). However, the major fragment ion at m/z 252 of FDU-PB-22 (1) given by cleavage of an ester group (Fig. 2d) was not observed in the mass spectrum of peak 2 (Fig. 3e). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion peak at m/z 395.1565, suggesting that the protonated molecular formula of compound 2 was C26H20N2OF (calcd. 395.1560).
Fig. 3.
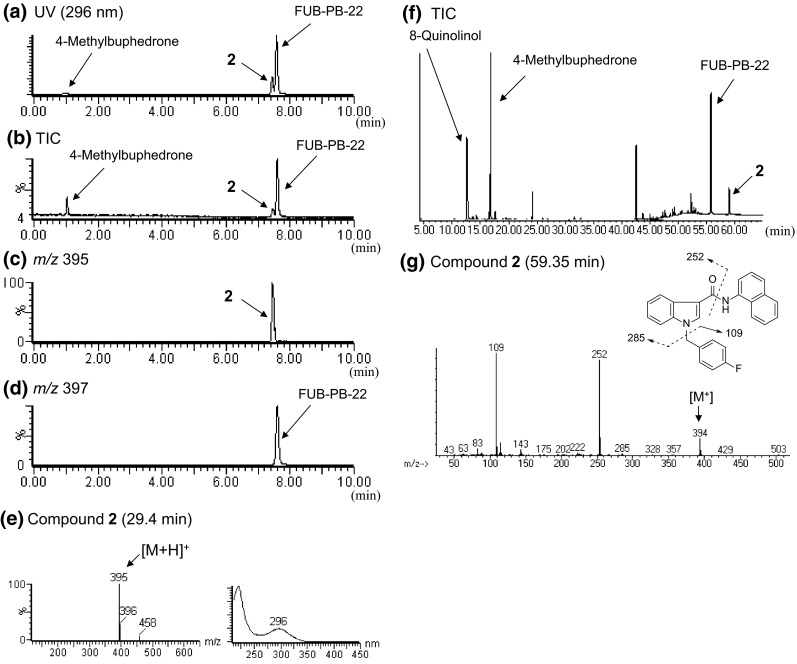
LC-MS and GC–MS analyses of product B. LC–UV-PDA chromatogram (a), TIC (b), extracted-ion chromatograms at m/z 395 (c) and 397 (d), and ESI mass and UV spectra of peak 2 (e) are shown. TIC (f) and EI mass spectrum of peak 2 (g) obtained by GC–MS are also presented
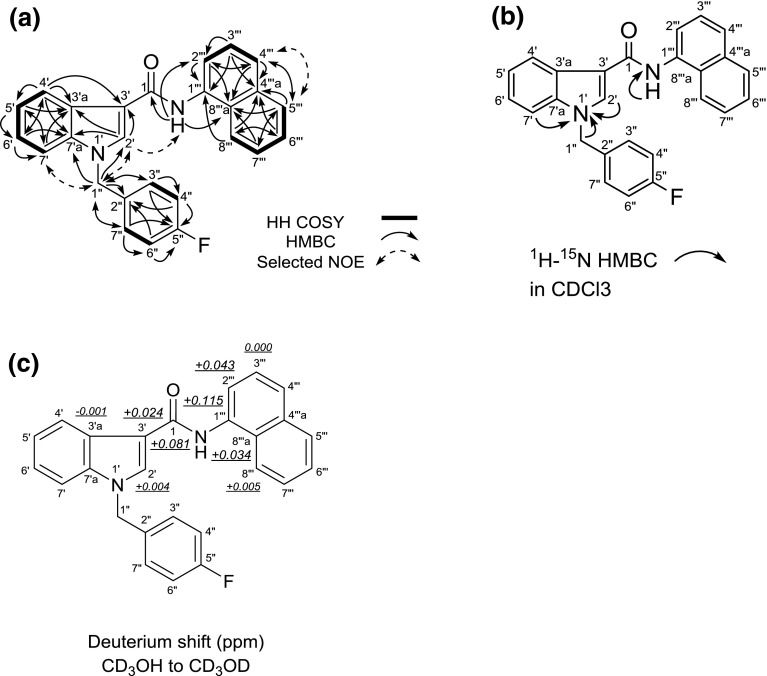
The structure of compound 2 was elucidated by NMR analysis (Fig. 4; Table 1). The analyses by 1H and 13C NMR, HH COSY, HMQC, HMBC, 15N HMBC and 1D-NOE spectra of compound 2 revealed the presence of a 1-(4-fluorobenzyl)-1H-indole (positions 1′ to 7′a and positions 1″ to 7″) and a N-(naphthalen-1-yl)-carboxamide moieties (position 1 and positions 1″′ to 8″′a) as shown in Fig. 4a, b. However, no HBMC correlation between the two moieties was observed. We, therefore, measured the deuterium isotope effect on the NH amide proton on the 13C chemical shift to determine the connection between the two moieties.
Fig. 4.
Results of HH correlation spectroscopy (HH COSY), heteronuclear multiple-bond correlation (HMBC), selected nuclear Overhauser effect (NOE) correlations (a), 1H-15N HMBC correlations (b) and deuterium-induced isotope shift of NH protons for the 13C nuclear magnetic resonance (NMR) signals of compound 2 (FDU-NNEI) in CD3OD (c)
Table 1.
NMR data of compound 2
| No. | 13C | 1H |
|---|---|---|
| 1 | 167.5 | – |
| 2′ | 133.0 | 8.25, 1H, brs |
| 3′ | 111.5 | – |
| 3′a | 128.6 | – |
| 4′ | 122.7 | 8.22, 1H, d, J = 7.8 Hz |
| 5′ | 122.7 | 7.20, 1H, t, J = 7.8 Hz |
| 6′ | 124.0 | 7.24, 1H, t, J = 7.8 Hz |
| 7′ | 111.6 | 7.45, 1H, d, J = 7.8 Hz |
| 7′a | 138.2 | – |
| 1″ | 50.7 | 5.48, 2H, s |
| 2″ | 134.5, d, J = 2.8 Hz | – |
| 3″/7″ | 130.3, d, J = 7.7 Hz | 7.30 and 7.29, each 1H, d, J = 8.7 Hz, overlapped |
| 4″/6″ | 116.6, d, J = 22.2 Hz | 7.08 and 7.07, each 1H, d, J = 8.7 Hz, overlapped |
| 5″ | 163.8, d, J = 244.7 Hz | – |
| 1″′ | 134.9 | – |
| 2″′ | 125.2 | 7.61, 1H, d, J = 6.9 Hz |
| 3″′ | 126.6 | 7.53, 1H, m, overlapped |
| 4″′ | 127.8 | 7.82, 1H, d, J = 8.3 Hz |
| 4″′a | 135.9 | – |
| 5″′ | 129.3 | 7.91, 1H, m |
| 6″′ | 127.1 | 7.51, 1H, m, overlapped |
| 7″′ | 127.2 | 7.51, 1H, m, overlapped |
| 8″′ | 124.1 | 8.06, 1H, m |
| 8″′a | 131.3 | – |
| NH | – | 9.96, 1H, s |
Recorded in CD3OH at 800 MHz (1H) and 200 MHz (13C), respectively; data in δ ppm (J in Hz)
We compared the 13C NMR spectrum of compound 2, measured in CD3OH, with that in CD3OD. The isotope shift values for the 13C NMR signals of this compound are shown in Fig. 4c. The first- to fourth-largest deuterium shifts (0.115, 0.081, 0.043, 0.034 ppm) were observed at the positions of C-1″′, C-1, C-2″′, and C-8″′a of the N-(naphthalen-1-yl)carboxamide moiety. The fifth-largest deuterium shift of 0.024 ppm was attributed to the three-bond deuterium isotope effect of the NH amide proton on the indole carbon (C-3′). These results strongly suggested that the 1-(4-fluorobenzyl)-1H-indole moiety is connected at the 3′-position of the indole to the carboxamide (1-CONH).
On the basis of the above data, compound 2 was finally identified as 1-(4-fluorobenzyl)-N-(naphthalen-1-yl)-1H-indole-3-carboxamide (Fig. 1a). Compound 2 is a novel compound, and therefore, we named it FDU-NNEI because the structure of compound 2 consists of a [1-(4-fluorobenzyl)-1H-indolyl]carbonyl moiety and a (naphthalen-1-yl)amino moiety, which are characteristic parts of two synthetic cannabinoids, FDU-PB-22 (1) and NNEI, respectively (Fig. 1a–c) [10].
Identification of an unknown peak 3
We detected an unknown peak 3 together with seven peaks of five known synthetic cannabinoids (AB-CHMINACA, 5-fluoro-AMB, FUB-PB-22, AM-2201 indazole analog and NNEI indazole analog), a known cathinone derivative DL-4662, and 8-quinolinol in the LC–MS and GC–MS chromatograms for product C (Fig. 5a, b, e). In the LC–MS and GC–MS analysis, the unknown peak 3 showed a protonated molecular ion signal at m/z 357 [M + H+] (Fig. 5c) and a molecular ion signal at m/z 356 [M+] (Fig. 5f). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion signal at m/z 357.2282, suggesting that the protonated molecular formula of compound 3 was C20H29N4O2 (calcd. 357.2291). The presumed molecular formula of compound 3 (C20H28N4O2: 356) was thus the same as that of AB-CHMINACA (Fig. 1b). However, the LC–MS and GC–MS spectra patterns, in addition to each retention time, were different (Fig. 5c, d, f, g).
Fig. 5.

LC–MS and GC–MS analyses of product C. The LC–UV-PDA chromatogram (a), TIC (b), and ESI mass and UV spectra of peak 3 (c) and the authentic AB-CHMINACA (d) are shown. TIC (e), and EI mass spectra of peaks 3 (f) and the authentic AB-CHMINACA (g) obtained by the GC–MS analysis are also shown
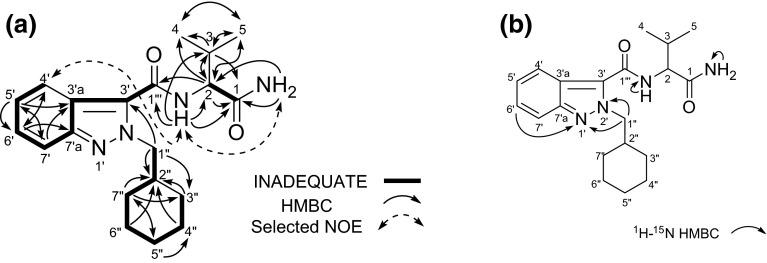
The observed 1H and 13C NMR spectra (Table 2), HH COSY, HMQC, HMBC and 15N HMBC correlations for compound 3 indicated the presence of N-cyclohexylmethyl-indazole and N-(1-amino-3-methyl-1-oxobutan-2-yl)-carboxamide moieties (Fig. 6a, b). Additionally, 2D-INADEQUATE correlation revealed that the cyclohexylmethyl-indazole moiety is connected at the 3′-position of the indazole to the carboxamide (C-1″′). Nevertheless, the 13C NMR chemical shifts of compound 3 were significantly different at the C-3′ (δc 129.1), C-7′ (δc 117.4) and C-7′a (δc 146.4) positions from those of AB-CHMINACA [C-3′ (δc 136.4), C-7′ (δc 110.6) and C-7′a (δc 141.2) as shown in Table 2.
Table 2.
NMR data of compound 3 and AB-CHMINACA
| No. | AB-CHMINACA | Compound 3 | |
|---|---|---|---|
| 13C | 13C | 1H | |
| 1 | 172.6 | 172.6 | – |
| 2 | 56.7 | 58.4 | 4.38, 1H, dd, J = 8.7, 6.9 Hz |
| 3 | 31.2 | 30.1 | 2.15, 1H, m |
| 4 | 19.3 | 19.3 | 0.99, 3H, d, J = 6.4 Hz, overlapped |
| 5 | 17.9 | 18.2 | 0.96, 3H, d, J = 6.9 Hz, overlapped |
| 1′ | – | – | – |
| 2′ | – | – | – |
| 3′ | 136.4 | 129.1 | – |
| 3′a | 121.8 | 120.3 | – |
| 4′ | 121.6 | 120.2 | 7.81, 1H, d, J = 8.3 Hz |
| 5′ | 122.4 | 122.7 | 7.20, 1H, t, J = 7.3 Hz |
| 6′ | 126.6 | 125.7 | 7.31, 1H, t, J = 7.3 Hz |
| 7′ | 110.6 | 117.4 | 7.68, 1H, d, J = 8.7 Hz |
| 7′a | 141.2 | 146.4 | – |
| 1″ | 54.5 | 57.3 | 4.58 and 4.49, each 1H, dd, J = 12.8, 7.3 Hz |
| 2″ | 38.3 | 38.9 | 1.90, 1H, m |
| 3″ | 30.0 | 30.0 | 0.97, 2H, m, overlapped |
| 4″ | 25.0 | 25.1 | 1.11, 2H, m, overlapped |
| 5″ | 25.7 | 25.7 | 1.56, 1H, m 1.09, 1H, m, overlapped |
| 6″ | 25.1 | 25.1 | 1.61, 2H, m |
| 7″ | 29.9 | 29.9 | 1.47, 2H, d, J = 12.4 Hz |
| 1″′-CONH | 161.3 | 159.8 | 8.38, 1H, d, J = 8.7 Hz |
| 1-NH2 | – | – | 7.54 and 7.16, each 1H, brs |
Recorded in DMSO-d 6 at 800 MHz (1H) and 200 MHz (13C), respectively; data in δ ppm (J in Hz)
Fig. 6.
Results of incredible natural abundance double-quantum transfer experiment (INADEQUATE), HMBC, selected NOE correlations (a) and 1H-15N HMBC correlations (b) for compound 3 (AB-CHMINACA 2H-indazole analog)
We next compared the 15N NMR chemical shifts of compound 3 with those of 1H-indazole derivatives (Table 3). The 15N chemical shifts at the N-1′ (δN −85.5) and N-2′ (δN −153.9) of the indazole moiety in compound 3 were different from those of the 1H-indazole moiety in 1-methyl-1H-indazole [N-1′ (δN −202.8) and N-2′ (δN −56.6)] and AB-CHMINACA [N-1′ (δN −191.4) and N-2′ (δN −62.8)], respectively (Table 3; Fig. 1c). However, their shifts of compound 3 were similar to those of 2-methyl-2H-indazole [N-1′ (δN −91.2) and N-2′ (δN −161.0)] (Table 3; Fig. 1c) [14]. Therefore, the structure of compound 3 was determined as AB-CHMINACA 2H-indazole analog [N-(1-amino-3-methyl-1-oxobutan-2-yl)-2-(cyclohexylmethyl)-2H-indazole-3-carboxamide] (Fig. 1a).
Table 3.
15N NMR data of detected and related compounds
| Type | 1H-Indazole | 2H-Indazole | |||||
|---|---|---|---|---|---|---|---|
| Position no. | 1-Methyl-1H-indazolea | AB-CHMINACAb | NNEI indazole analogc | Compd. 6 c | 2-Methyl-2H-indazolea | Compd. 3 b | Compd. 4 c |
| N-1′ | −202.8 | −191.4 | −187.8 | −185.5 | −91.2 | −85.5 | −82.7 |
| N-2′ | −56.6 | −62.8 | −62.0 | −53.0 | −161.0 | −153.9 | −147.4 |
| CONH | – | −273.3 | −262.0 | – | – | −257.2 | −256.5 |
| CONH2 | – | −273.3 | – | – | – | −274.0 | – |
15N NMR chemical shifts, which observed in 1H-15N HMBC spectrum, are referenced to the signal of CH3NO2 (δN 0.0 ppm)
aRef. [11], recorded in DMSO-d 6
bRecorded in DMSO-d 6
cRecorded in CDCl3
It was reported that different forms of tautomerism are very common in nitrogen compounds. For indazoles, in most cases the 1H-tautomer is the most stable; however, sometimes several indazoles of the 2H-tautomer can be more stable than 1H-tautomer [14]. The differences in energy between the 1H- and 2H-tautomers were interpreted in terms of substituent effects [14], and we have assumed that the minor component AB-CHMINACA 2H-indazole analog (3) is generated via tautomerization from AB-CHMINACA (1H-indazole). This is the first report of the identification of 2H-indazole isomers of synthetic cannabinoids in illegal products to our knowledge. The chemical characterization, pharmacological activity and toxicological activity of 2H-indazole isomers of synthetic cannabinoids as NPSs have never been reported before.
Identification of unknown peaks 4–6
Three unknown peaks 4, 5, and 6 were detected along with a synthetic cannabinoid NNEI indazole analog in the LC–MS and GC–MS chromatograms for product D (Figs. 7a, b, i, 1b). In the LC–MS and GC–MS analysis, the unknown peak 4 showed a protonated molecular ion signal at m/z 358 [M + H+] (Fig. 7c, d) and a molecular ion signal at m/z 357 [M+] (Fig. 7j). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion signal at m/z 358.1916, suggesting that the protonated molecular formula of compound 4 was C23H24N3O (calcd. 358.1919). Hence, the presumed molecular formula of compound 4 (C23H23N3O: 357) was the same as that of NNEI indazole analog. However, the GC–MS and LC–MS spectra patterns and each retention time of both compounds were different (Fig. 7d, e, j, k).
Fig. 7.
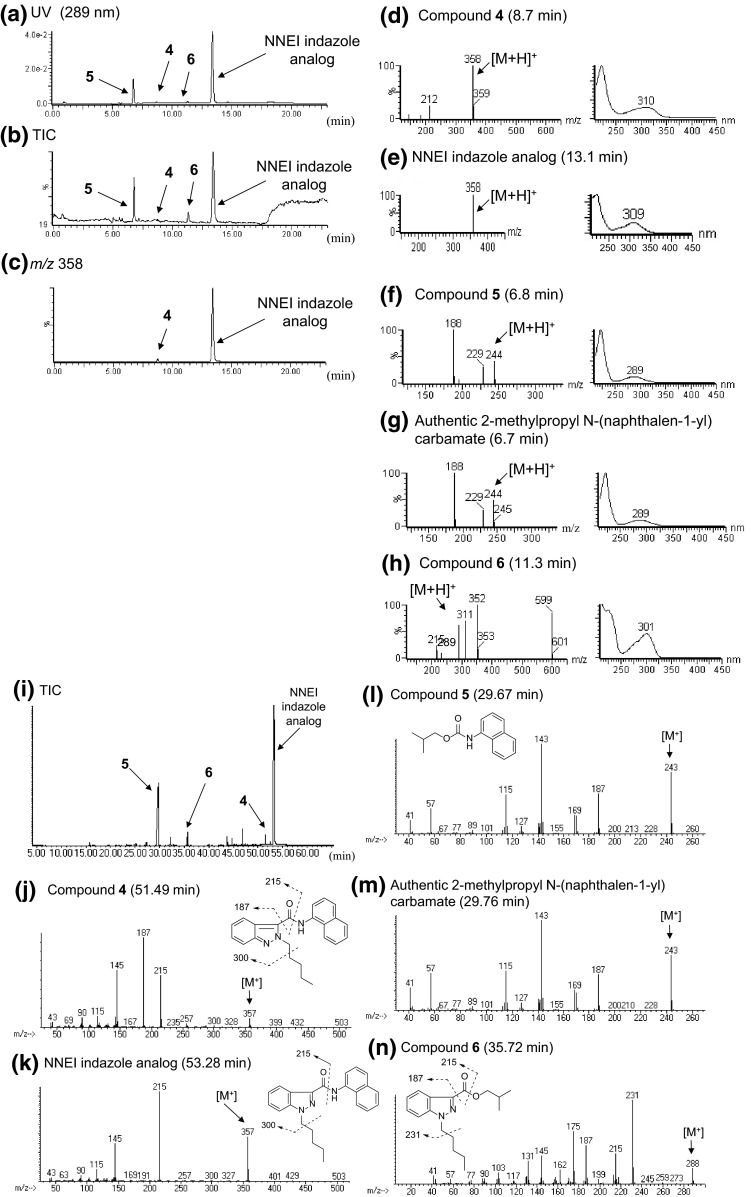
LC–MS and GC–MS analyses of product D. The LC–UV-PDA chromatogram (a), TIC (b) and an extracted-ion chromatogram at m/z 358 (c) are shown, along with the ESI mass and UV spectra of peaks 4 (d), 5 (f), 6 (h), the authentic NNEI indazole analog (e) and the authentic 2-methylpropyl N-(naphthalen-1-yl) carbamate (g). TIC (i) and EI mass spectra of peaks 4 (j), 5 (l), 6 (n), the authentic NNEI indazole analog (k) and the authentic 2-methylpropyl N-(naphthalen-1-yl) carbamate (m) obtained by the GC–MS analysis are also indicated
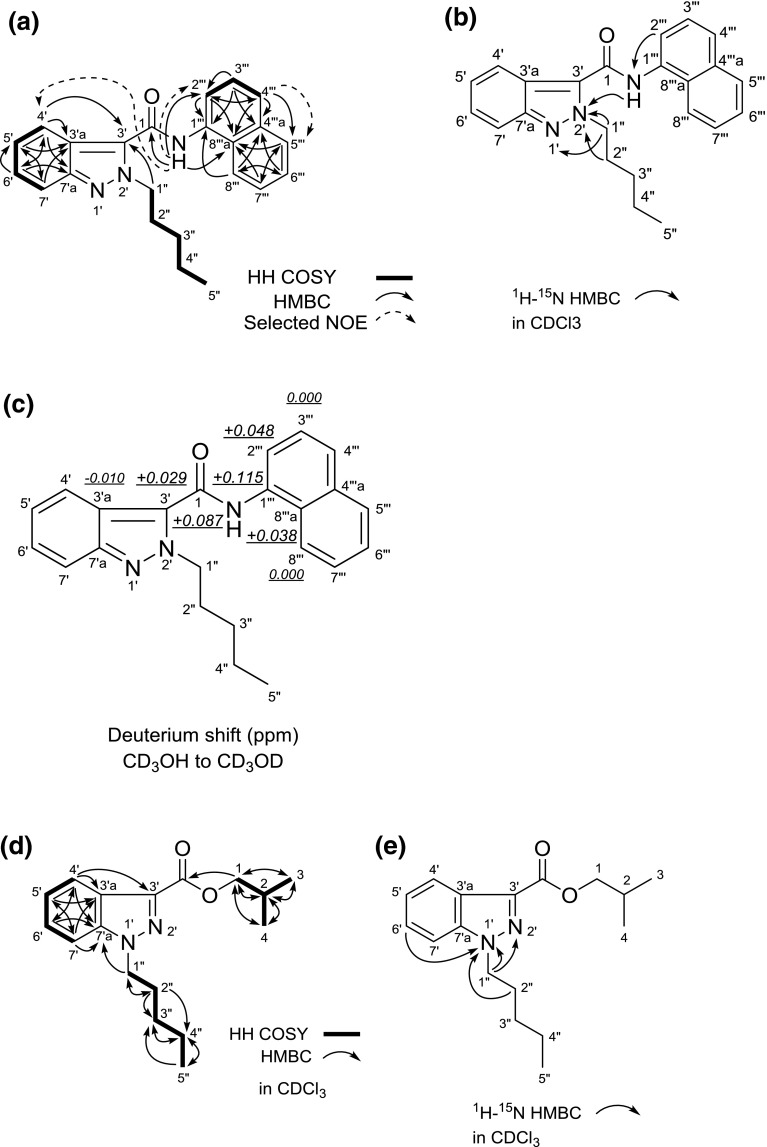
The 1H and 13C NMR spectra (Table 4), HH COSY, HMQC, HMBC and 15N HMBC correlations of compound 4 suggested the presence of N-pentyl-indazole and N-(naphthalen-1-yl)carboxamide moieties (Fig. 8a, b). Therefore, the connectivity of the two moieties (positions 1 and 3′) was not observed. On the other hand, the 13C NMR chemical shifts of compound 4 at the C-3′ (δc 127.1), C-7′ (δc 119.0) and C-7′a (δc 147.3) positions were different from those of NNEI indazole analog [C-3′ (δc 137.3), C-7′ (δc 109.4), and C-7′a (δc 141.1)] (Table 4). Comparing the 15N NMR chemical shifts between the two compounds showed that the 15N chemical shifts at the N-1′ (δN −82.7) and N-2′ (δN −147.4) of the indazole moiety in compound 4 were different from those of the 1H-indazole moiety in NNEI indazole analog [N-1′ (δN −187.8) and N-2′ (δN −62.0)] (Table 3). However, their shifts of compound 4 were similar to those of 2-methyl-2H-indazole [N-1′ (δN −91.2) and N-2′ (δN −161.0)] (Table 3) [14]. Therefore, it was revealed that compound 4 has a 2-pentyl-2H-indazole moiety.
Table 4.
NMR data of compound 4 and NNEI indazole analog
| No. | NNEI 1H-indazole analoga | Compound 4 b | |
|---|---|---|---|
| 13C | 13C | 1H | |
| 1 | 160.9 | 159.0 | – |
| 2′ | – | – | |
| 3′ | 137.3 | 127.1 | – |
| 3′a | 123.0 | 120.6 | – |
| 4′ | 122.9 | 118.2 | 7.94, 1H, d, J = 9.6 Hz, overlapped |
| 5′ | 122.9 | 124.9 | 7.34, 1H, td, J = 7.6, 0.7 Hz |
| 6′ | 126.9 | 126.3 | 7.40, 1H, ddd, J = 7.6, 6.5, 1.0 Hz |
| 7′ | 109.4 | 119.0 | 7.88, 1H, d, J = 8.9 Hz |
| 7′a | 141.1 | 147.3 | – |
| 1″ | 49.6 | 53.4 | 4.89, 2H, t, J = 7.2 Hz |
| 2″ | 29.5 | 31.0 | 2.04, 2H, m |
| 3″ | 29.0 | 28.9 | 1.35, 2H, m, overlapped |
| 4″ | 22.3 | 22.3 | 1.35, 2H, m, overlapped |
| 5″ | 14.0 | 14.0 | 0.87, 3H, t, J = 7.2 Hz |
| 1″′ | 132.4 | 131.8 | – |
| 2″′ | 119.4 | 121.1 | 8.08, 1H, d, J = 7.6 Hz |
| 3″′ | 126.0 | 126.2 | 7.55, 1H, m, overlapped |
| 4″′ | 125.0 | 126.6 | 7.79, 1H, d, J = 8.3 Hz |
| 4″′a | 134.2 | 134.3 | – |
| 5″′ | 128.8 | 129.0 | 7.93, 1H, m, overlapped |
| 6″′ | 125.9 | 126.8 | 7.54, 1H, m, overlapped |
| 7″′ | 126.2 | 125.8 | 7.57, 1H, m, overlapped |
| 8″′ | 120.5 | 120.4 | 7.92, 1H, m, overlapped |
| 8″′a | 126.7 | 127.2 | – |
| NH | – | – | 8.25, 1H, brs |
aRecorded in CDCl3 at 800 MHz (1H) and 200 MHz (13C), respectively; data in δ ppm (J in Hz)
bRecorded in CDCl3 at 600 MHz (1H) and 150 MHz (13C), respectively; data in δ ppm (J in Hz)
Fig. 8.
Results of HH COSY, HMBC, selected NOE correlations (a), 1H-15N HMBC correlations (b) and deuterium-induced isotope shift of NH protons for the 13C NMR signals of compound 4 (NNEI 2H-indazole analog) in CD3OD (c). HH COSY and HMBC correlations (d) and 1H-15N HMBC correlations (e) for compound 6 (isobutyl 1-pentyl-1H-indazole-3-carboxylate), are also shown
We measured the deuterium isotope effect in compound 4 in the same way as that used for compound 2 (Fig. 4c) to determine the connection between the two moieties. The isotope shift values for the 13C NMR signals of this compound are shown in Fig. 8c. The first- to fourth-largest deuterium shifts (0.115, 0.087, 0.048, 0.038 ppm) were observed at the C-1″′, C-1, C-2″′, and C-8″′a positions of the N-(naphthalen-1-yl)-carboxamide moiety. The fifth-largest deuterium shift of 0.029 ppm was attributed to the three-bond deuterium isotope effect of the NH amide proton on the indazole carbon (C-3′). These results suggested that the 2-pentyl-2H-indazole moiety is connected at the 3′-position of the 2H-indazole to the carboxamide (1-CONH). Finally, the structure of compound 4 was determined as NNEI 2H-indazole analog [N-(naphthalen-1-yl)-2-pentyl-2H-indazole-3-carboxamide] (Fig. 1a).
The unknown peak 5 was identified as a 2-methylpropyl N-(naphthalen-1-yl) carbamate (Fig. 7f, l) by direct comparison of the GC–MS and LC–MS data to those of the purchased authentic compound (Fig. 7g, m). This compound has not been reported as any cannabimimetic- or cannabinoid-related substance.
The GC–MS and LC–MS spectra of the unknown peak 6 are shown in Fig. 7h, n. A molecular ion signal of compound 6 was observed at m/z 288 in the GC–MS analysis (Fig. 7n). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion peak at m/z 289.1906, suggesting that the protonated molecular formula of compound 6 was C17H25N2O2 (calcd. 289.1916). One-dimensional (1D)- and 2D-NMR analyses revealed that compound 6 has isobutyl-carboxylate and N-pentyl-indazole moieties, as shown in Fig. 8d and Table 5. The 15N HMBC correlations of N-1′ with H-6′ and H-2″ (Fig. 8e) and the 15N chemical shifts of compound 6 at the N-1′ (δN −185.5) and N-2′ (δN −53.0) of the indazole moiety, which were similar to those of the 1H-indazole moiety in NNEI indazole analog [N-1′ (δN −187.8) and N-2′ (δN −62.0)], suggested the presence of 1H-indazole (Table 3). No HMBC correlation was observed between the two moieties at position-3′ and ester group of compound 6. However, the major GC–MS fragment ion signal at m/z 187 was probably caused by the cleavage of a bond between the 1H-indazole and the ester group (Fig. 7n). In addition, another fragment ion signal at m/z 215 was probably caused by the cleavage of a bond of the ester group (Fig. 7n). Therefore, the structure of compound 6 was elucidated as isobutyl 1-pentyl-1H-indazole-3-carboxylate (Fig. 1a). Compound 6, which is a novel substance, was not reported to have any pharmacological and toxicological activity.
Table 5.
NMR data of compound 6
| No. | 13C | 1H |
|---|---|---|
| COO | 162.9 | – |
| 2′ | – | – |
| 3′ | 134.8 | – |
| 3′a | 123.5 | – |
| 4′ | 122.2 | 8.17, 1H, d, J = 8.3 Hz |
| 5′ | 122.9 | 7.29, 1H, t, J = 8.3 Hz |
| 6′ | 126.6 | 7.41, 1H, t, J = 8.3 Hz |
| 7′ | 109.7 | 7.46, 1H, d, J = 8.3 Hz |
| 7′a | 140.5 | – |
| 1″ | 49.9 | 4.45, 2H, t, J = 7.3 Hz |
| 2″ | 29.6 | 1.94, 2H, m |
| 3″ | 28.9 | 1.29, 2H, m, overlapped |
| 4″ | 22.3 | 1.33, 2H, m, overlapped |
| 5″ | 13.9 | 0.86, 3H, t, J = 7.3 Hz |
| 1 | 71.0 | 4.24, 2H, t, J = 6.4 Hz |
| 2 | 27.9 | 2.19, 1H, m |
| 3/4 | 19.3 | 1.05, 6H, d, J = 6.9 Hz |
Recorded in CDCl3 at 800 MHz (1H) and 200 MHz (13C), respectively; data in δ ppm (J in Hz)
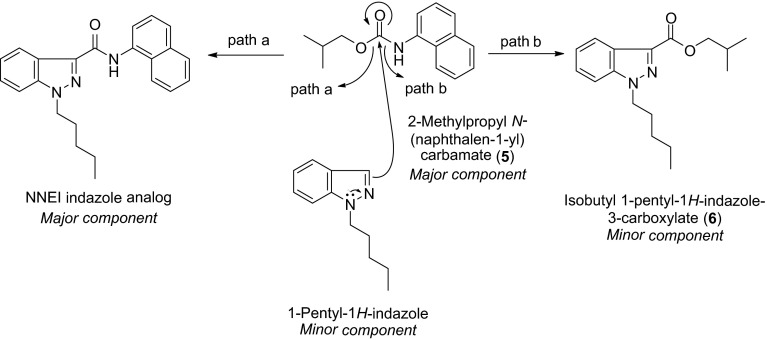
As a result, two major components, i.e., NNEI indazole analog and 2-methylpropyl N-(naphthalene-1-yl) carbamate (5), and three minor components, i.e., isobutyl 1-pentyl-1H-indazole-3-carboxylate (6), NNEI 2H-indazole analog (4) and a presumed 1-pentyl-1H-indazole (elucidated by GC–MS, data not shown) were detected in product D. On the basis of these minor components, we expected the following reaction mechanism for the preparation of the major NNEI indazole analog; compound 5 is likely to react with 1-pentyl-1H-indazole to yield the major component NNEI indazole analog (path a) and the minor component 6 (path b), as shown in Fig. 9. In addition, the 1H- and 2H-indazole tautomerism of the starting material or the reaction product accounted for the existence of the minor 2H-indazole product (4).
Fig. 9.
Possible reaction mechanisms for the preparation of NNEI indazole analog and compound 6
Identification of unknown peaks 7 and 8
Unknown peaks 7 and 8 were detected in the LC–MS chromatogram for product E (Fig. 10a, b, e). In the LC–MS chromatogram, the unknown peak 7 at 12.2 min showed a protonated molecular ion signal at m/z 224 ([M + H]+) (Fig. 10c). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion peak at m/z 224.1280, suggesting that the protonated molecular formula of compound 7 was C12H18NO3 (calcd. 224.1287).
Fig. 10.
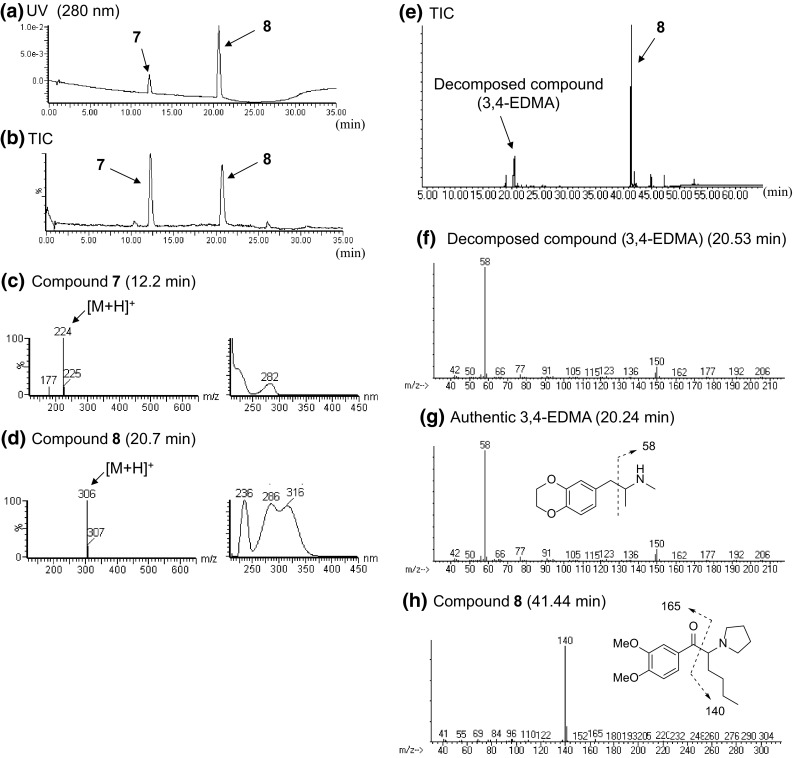
LC–MS and GC–MS analyses of product E. The LC–UV-PDA chromatogram (a), TIC (b), ESI mass and UV spectra of peaks 7 (c) and 8 (d) are shown. TIC (e) and EI mass spectra of peaks of decomposed compound [3,4-ethylenedioxy-N-methylamphetamine (3,4-EDMA)] (f), compound 8 (h) and the authentic 3,4-EDMA (g) obtained by GC–MS analysis are also presented
The observed 1H and 13C NMR (Table 6), HH COSY, HMQC, HMBC and 1D-NOE revealed that the structure of compound 7 was N-hydroxy-3,4-ethylenedioxy-N-methylamphetamine (N-OH-EDMA), as shown in Fig. 11a. In addition, Noggle et al. [15] reported that N-hydroxy-3,4-methylenedioxyamphetamine (N-OH-MDA), an analog of N-OH-EDMA (7), was unstable at high temperatures in a GC–MS analysis, and that N-OH-MDA (Fig. 1c) decomposed to MDA (Fig. 1c) [15]. In the present GC–MS TIC chromatogram, peak 7 (N-OH-EDMA) was not observed (Fig. 10e). However, the peak of its decomposed compound, which was finally identified as 3,4-ethylenedioxy-N-methylamphetamine (3,4-EDMA, Fig. 1b) by direct comparison of the GC–MS data to those of the purchased authentic compound (Fig. 10g), was detected (Fig. 10e, f). No pharmacological information is available for N-OH-EDMA (7); however, 3,4-EDMA has been reported to stimulate the release of serotonin and dopamine from rat brain synaptosomes at 1 μM [16].
Table 6.
NMR data of compound 7
| No. | 13C | 1H |
|---|---|---|
| 1 | 38.3 | 2.84, 1H, dd, J = 13.1, 4.5 Hz |
| 2.26, 1H, dd, J = 13.4, 8.6 Hz | ||
| 2 | 64.2 | 2.67, 1H, m |
| 3 | 14.1 | 0.83, 3H, d, J = 6.5 Hz |
| 1′ | 133.2 | – |
| 2′ | 117.6 | 6.66, 1H, d, J = 1.7 Hz |
| 3′ | 142.9 | – |
| 4′ | 141.4 | – |
| 5′ | 116.5 | 6.71, 1H, d, J = 7.9 Hz |
| 6′ | 121.9 | 6.61, 1H, dd, J = 8.3, 1.7 Hz |
| –O–(CH2)2–O– | 64.0 63.9 |
4.18, 4H, m |
| N–CH3 | 44.2 | 2.46, 3H, s |
| N–OH | – | 7.64, 1H, brs |
Recorded at 600 MHz (1H) and 150 MHz (13C) in DMSO-d 6, respectively; data in δ ppm (J in Hz)
Fig. 11.

HH COSY, HMBC, and selected NOE correlations for compound 7 (N-OH-EDMA, a) and for compound 8 (dimethoxy-α-PHP, b)
The proposed fragment pattern and the presumed structure of peak 8 obtained by the GC–MS analysis are shown in Fig. 10h. The LC–MS data revealed that peak 8 gave a protonated ion signal at m/z 306 ([M + H]+) (Fig. 10d). The accurate mass spectrum obtained by LC–QTOF–MS gave an ion peak at m/z 306.2072, suggesting that the protonated molecular formula of compound 8 was C18H28NO3 (calcd. 306.2069).
The 13C NMR spectrum of compound 8 was similar to a combination of two known cathinone derivatives: an α-pyrrolidinohexanone moiety of α-PHP and a 3,4-dimethoxyphenyl moiety of DL-4662 (Table 7) [13]. The observed 1H and 13C NMR (Table 7), HH COSY, HMQC, HMBC, and 1D-NOE correlations (Fig. 11b) suggested that the structure of compound 8 is dimethoxy-α-pyrrolidinohexanophenone (dimethoxy-α-PHP), as shown in Fig. 1a. The fragment ions at m/z 140 and 165 of compound 8 in the GC–MS spectrum corroborated the structure (Fig. 10h). Compound 8 was detected as a newly distributed designer drug, and its chemical and pharmaceutical data have not been reported. Dimethoxy-α-PHP (8) has been controlled as a designated substance (Shitei-Yakubutsu) in Japan since November 2014.
Table 7.
NMR data of compound 8
| No. | 13C | 1H |
|---|---|---|
| 1 | 199.1 | – |
| 2 | 68.7 | 4.08, 1H, dd, J = 9.3, 4.8 Hz |
| 3 | 30.0 | 2.08, 1H, m |
| 1.83, 1H, m | ||
| 4 | 28.9 | 1.32, 2H, m |
| 5 | 23.1 | 1.26, 2H, m |
| 6 | 14.1 | 0.78, 3H, t, J = 7.2 Hz |
| 1′ | 130.6 | – |
| 2′ | 111.9 | 8.03, 1H, d, J = 1.7 Hz |
| 3′ | 149.5 | – |
| 4′ | 154.1 | – |
| 5′ | 111.1 | 7.00, 1H, d, J = 8.6 Hz |
| 6′ | 123.7 | 8.15, 1H, dd, J = 8.6, 1.7 Hz |
| 2″/5″ | 50.7 | 2.75, 2H, m |
| 2.64, 2H, m | ||
| 3″/4″ | 23.9 | 1.63, 4H, m |
| 3′-MeO | 55.9/55.7 | 3.78, 3H, s |
| 4′-MeO | 55.7/55.9 | 3.78, 3H, s |
Recorded at 600 MHz (1H) and 150 MHz (13C) in pyridine-d 5, respectively; data in δ ppm (J in Hz)
Conclusions
Four synthetic cannabinoids: FDU-PB-22 (1), FDU-NNEI (2), two 2H-indazole isomers of synthetic cannabinoids, i.e., AB-CHMINACA 2H-indazole analog (3) and NNEI 2H-indazole analog (4), a phenethylamine derivative N-OH-EDMA (7), and a cathinone derivative dimethoxy-α-PHP (8) were newly identified in illegal products obtained in Japan. In addition, 2-methylpropyl N-(naphthalen-1-yl) carbamate (5) and isobutyl 1-pentyl-1H-indazole-3-carboxylate (6), which is probably a by-product in the synthesis of NNEI indazole analog from 1-pentyl-1H-indazole and compound 5 (Fig. 9), were detected. Among them, compounds 3, 4, and 6 were detected as minor components; their pharmacological and toxicological properties have never been reported. This appears to be the first report to estimate the synthetic pathway of illegal synthetic cannabinoids on the basis of detailed analytical data of illegal products.
Acknowledgments
A portion of this work was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labour, and Welfare, Japan.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
References
- 1.EMCDDA (2014) European drug report 2014: trends and developments, May 2014. http://www.emcdda.europa.eu/attachements.cfm/att_228272_EN_TDAT14001ENN.pdf. Accessed Nov 2014
- 2.EMCDDA–Europol (2013) Annual report on the implementation of Council Decision 2005/387/JHA, July 2014. http://www.emcdda.europa.eu/publications/implementation-reports/2013. Accessed Nov 2014
- 3.UNODC (2014) 2014 Global synthetic drugs assessment. Amphetamine-type stimulants and new psychoactive substances, May 2014. http://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf. Accessed May 2014
- 4.Zuba D, Byrska B. Analysis of the prevalence and coexistence of synthetic cannabinoids in “herbal high” products in Poland. Forensic Toxicol. 2013;31:21–30. doi: 10.1007/s11419-012-0159-0. [DOI] [Google Scholar]
- 5.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y. Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol. 2013;31:44–53. doi: 10.1007/s11419-012-0165-2. [DOI] [Google Scholar]
- 6.Chung H, Choi H, Heo S, Kim E, Lee J. Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol. 2014;32:82–88. doi: 10.1007/s11419-013-0213-6. [DOI] [Google Scholar]
- 7.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y. Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test Anal. 2014;6:832–839. doi: 10.1002/dta.1584. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Identification of two new-type designer drugs, piperazine derivative MT-45 (I-C6) and a synthetic peptide Noopept (GVS-111), with synthetic cannabinoid A-834735, cathinone derivative 4-methoxy-α-PVP and phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol. 2014;32:9–18. doi: 10.1007/s11419-013-0194-5. [DOI] [Google Scholar]
- 9.Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol. 2014;32:105–115. doi: 10.1007/s11419-013-0217-2. [DOI] [Google Scholar]
- 10.Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, Urade Y, Goda Y. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci Int. 2014;243:1–13. doi: 10.1016/j.forsciint.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31:223–240. doi: 10.1007/s11419-013-0182-9. [DOI] [Google Scholar]
- 13.Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014;32:266–281. doi: 10.1007/s11419-014-0238-5. [DOI] [Google Scholar]
- 14.Elguero J, Silva AMS, Tomé AC. Five membered heterocycles: 1,2-azoles. Part 1. Pyrazoles. In: Alvarez-Builla J, Vaquero JJ, Barluenga J, editors. Modern heterocyclic chemistry. Weinheim: WileyVCH Verlag; 2011. pp. 635–725. [Google Scholar]
- 15.Noggle FT, Jr, Clark CR, Valaer AK, DeRuiter J. Liquid chromatographic and mass spectral analysis of N-substituted analogues of 3,4-methylenedioxyamphetamine. J Chromatogr Sci. 1988;26:410–417. doi: 10.1093/chromsci/26.8.410. [DOI] [PubMed] [Google Scholar]
- 16.McKenna DJ, Guan X-M, Shulgin AT. 3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine. Pharmacol Biochem Behav. 1991;38:505–512. doi: 10.1016/0091-3057(91)90005-M. [DOI] [PubMed] [Google Scholar]