Abstract
A number of N-alkyl indole or indazole-3-carbonyl analogs, with modified chemical structures, are distributed throughout the world as synthetic cannabinoids. Like synthetic cannabinoids, cathinone analogs are also abused and cause serious problems worldwide. Acute deaths caused by overdoses of these drugs have been reported. Various analytical methods that can cope with the rapid changes in chemical structures are required for routine analysis and screening of these drugs in seized and biological materials for forensic and clinical purposes. Although many chromatographic methods to analyze each drug have been published, there are only a few articles summarizing these analytical methods. This review presents the various colorimetric detections, immunochemical assays, gas chromatographic–mass spectrometric methods, and liquid chromatographic–mass spectrometric methods proposed for the analysis of synthetic cannabinoids and cathinones.
Keywords: Synthetic cannabinoids, Cannabimimetics, Cathinones, GC–MS-MS, LC–MS-MS, Analytical methods
Introduction
Currently, many illegal drugs are abused worldwide, with serious social problems arising as a consequence. Although various stimulants and narcotics have been in use to date, new drugs targeting cannabinoid receptors have been abused since their existence in herbal mixtures was disclosed in 2008 [1]. HU-210, a synthetic classical cannabinoid, and cyclohexylphenols were commonly used as recreational drugs, but mainstream use has since changed to N-alkyl indole-3-carbonyl derivatives, such as drugs of the JWH and AM series (Fig. 1), because their activities are stronger than those of the conventional cannabinoids. These compounds are called cannabimimetics or synthetic cannabinoids and can be purchased as “spice” or “K2” in the drug market or via the Internet. Cathinones, also known as “bath salts” or “plant food,” are psychoactive drugs and are also abused as recreational drugs. The parent compound, cathinone, is a well-known stimulant, and can be isolated from the khat plant or produced by synthetic means. Cathinone analogs with high selectivity and strong activity for serotonin receptors and monoamine transporters have been distributed in the drug market (Fig. 2). The prevalence of cannabinoid and cathinone abuse in many countries has been reviewed elsewhere [2–7].
Fig. 1.
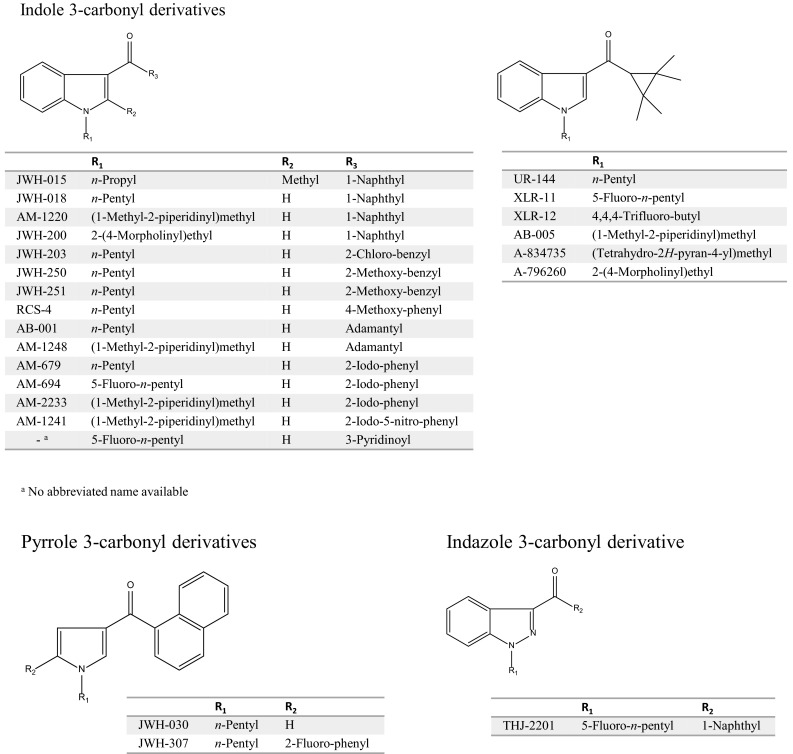
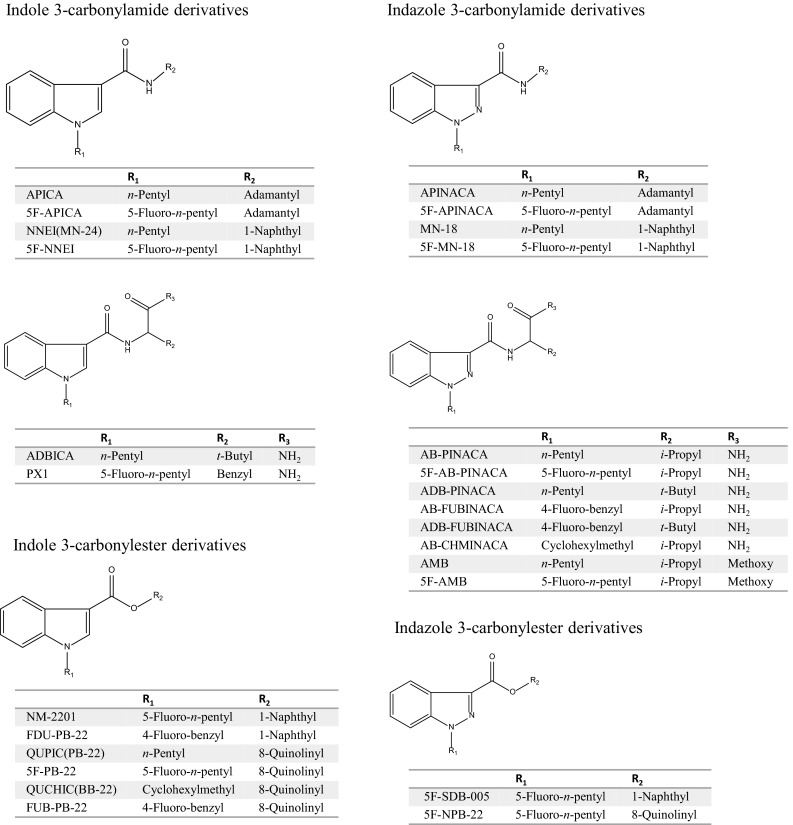
Structures of synthetic cannabinoids
Fig. 2.
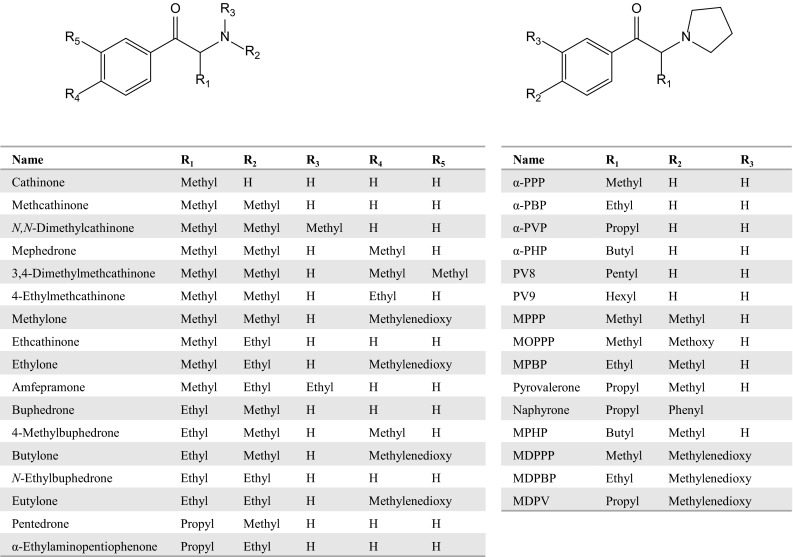
Structures of cathinones
Although the same substances are distributed throughout the world, the times at which they are abused tend to vary depending on whether the substances are controlled by local laws. As shown in the reviews [2–7], new analogs appear in the drug market just after the preceding drug comes under regulation. Although many such substances are controlled in countries throughout the world, the regulations are usually limited by the structures of the drugs. Therefore, when the structure of a side chain or substitution is slightly different from that of the regulated drug, the analog is regarded as being beyond the scope of the regulation. These emerging drugs always show psychoactive actions because their chemical structures are similar to those of the drugs being controlled. However, the detailed pharmacological activities of these analogs are not known, which makes access easy and use of these drugs very dangerous to human health.
Although many researchers have focused on the development of detection methods, only a few analytical reviews that summarize the systematic identification and quantification techniques for these drugs have appeared [8–10]. In this review, we summarize the various techniques for the detection of synthetic cannabinoids and cathinones that have been published up to 2014, including colorimetric, immunochemical, and chromatographic methods.
Synthetic cannabinoids
Colorimetric detection
The Duquenois–Levine color test, which is used to identify classical cannabinoids such as Δ9-tetrahydrocannabinol, is negative for the synthetic cannabimimetics. The van Urk color test, which is used to identify indole-containing drugs of abuse, is also negative for these compounds. The use of 2,4-dinitrophenylhydrazine, which reacts with a keto moiety, is capable of reacting with synthetic cannabimimetics, such as the naphthoylindole, phenylacetylindole, benzoylindole, and cyclopropylindole classes, either in powder form or adsorbed onto plant material, and a positive test solution turns from yellow to orange. Although the LOD concentration was not detailed in the article, the solution tested contained at least 10 mg of cannabimimetic powder suspended in methanol (1 ml) [11]. The Marquis reagent, which reacts with all nitrogen-containing drugs, is positive for cyclohexylphenols and the JWH series. Although Dragendorff reagent is also positive for the JWH series, its LOD concentration is higher than that of Marquis reagent. Fast blue BB reacts with cyclohexylphenols, and the LOD concentration is not lower than that of Marquis reagent [12]. Iodoplatinate is also used as a detection reagent after TLC [13]. Although it is possible to detect synthetic cannabinoids with each reagent in these screening tests, it is difficult to detect small amounts or mixtures of synthetic cannabinoids.
Immunochemical detection
ELISAs developed in-house could be calibrated at 5 ng/ml with the 5-OH and 4-OH metabolites of JWH-018 and JWH-250, respectively, and evaluated for the detection of synthetic cannabinoids in urine [14]. Recently, some commercially available immunoassay kits, such as DrugCheck K2/Spice Test, DrugSmart Cassette, and RapiCard InstaTest, have been developed for the detection of these drugs in urine. These devices are more useful than the colorimetric methods, because they do not require special reagents or tools, and the results are obtained easily and quickly. The devices also can detect older types of synthetic cannabinoids, such as JWH-018 or JWH-073, but, unfortunately, new designer drugs such as QUPIC and AB-CHMINACA cannot be detected.
GC–MS detection
Typical mass spectra of synthetic cannabinoids are shown in Fig. 3. Molecular (M+) and/or fragment ions observed by full scan data acquisition of GC–MS reflect the structures of the synthetic cannabinoids [13, 15, 16]. As shown in Fig. 4, the fragmentation pathways of naphthoylindoles have been well studied for the identification of synthetic cannabinoids by GC–MS [12, 15]. Therefore, the identification of synthetic cannabinoids is facilitated by comparison of the spectra with commercial and open databases.
Fig. 3.
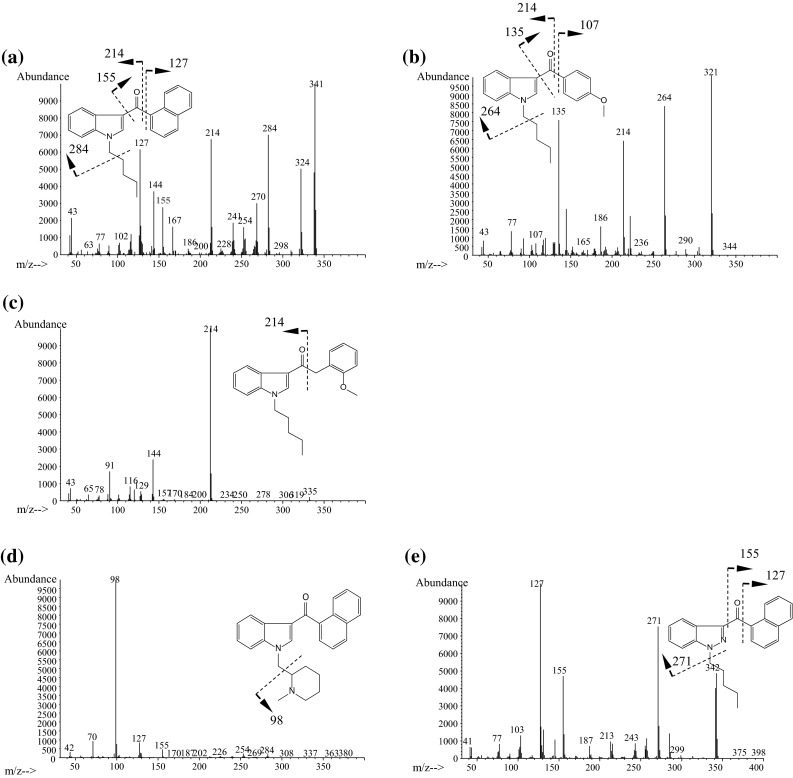
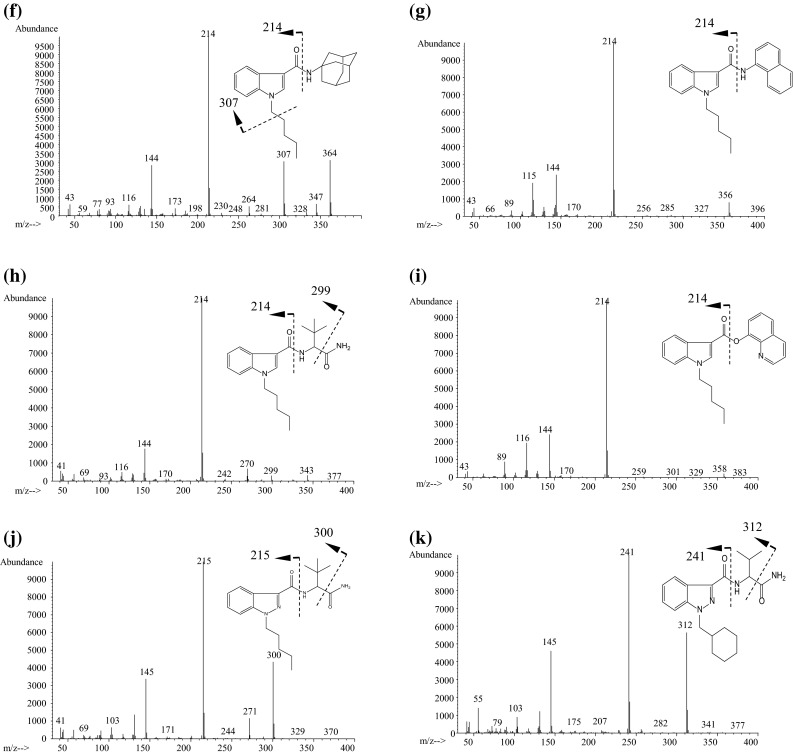
Typical mass spectra of synthetic cannabinoids obtained by GC–MS. a JWH-018, b RCS-4, c JWH-250, d AM-1220, e THJ-018, f APICA, g NNEI, h ADBICA, i QUPIC (PB-22), j ADB-PINACA, k AB-CHMINACA
Fig. 4.
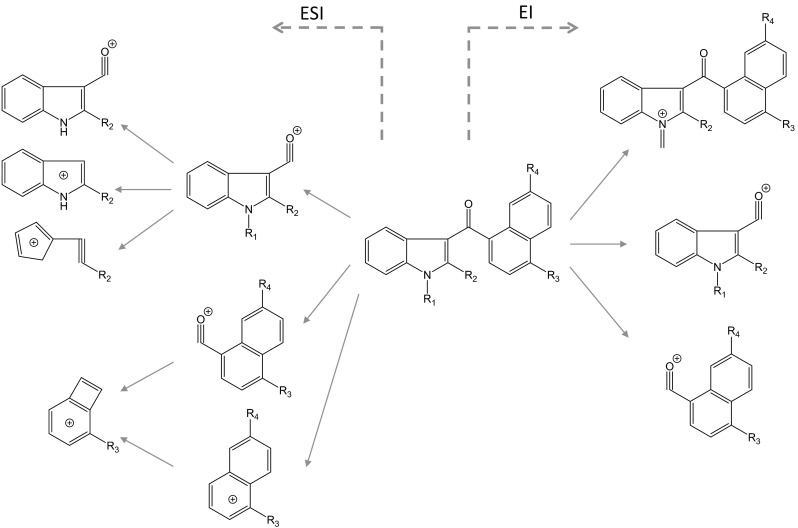
Probable fragmentation pathways of synthetic cannabinoids by electrospray ionization and electron ionization (modified from references [12, 31])
In naphthoylindoles, the carbonyl group fragment ions, which are caused by α-cleavage of the alkylamino group of the indole, are typically observed. In addition, [M−17]+ is certainly observed in naphthoylindoles. For example, fragment ions at m/z 284 and 214 are observed in JWH-018, corresponding to those of the indole moiety caused by α-cleavage of the N-pentyl of indole and naphthoyl. Fragment ions at m/z 127 and 155 are observed in JWH-018, corresponding to the naphthalene group caused by the α-cleavage of the carbonyl group. Moreover, ions at m/z 324 are observed as [M−17]+ (Fig. 3a). Like naphthoylindoles, fragment ions caused by α-cleavage of the alkylamino group of the indole and carbonyl groups are shown, although [M−17]+ is not observed for benzoylindoles. For example, fragment ions at m/z 264 and 214 are observed for RCS-4, caused by α-cleavage of N-pentyl of the indole and 4-methoxybenzoyl. The ions at m/z 127 and 155, which are caused by naphthyl and naphthoyl moieties of naphthoylindoles (Fig. 3a), and the ion at m/z 135 caused by the 4-methoxybenzoyl moiety (Fig. 3b) are useful as precursor ions for identification of these drugs by GC–MS-MS. On the other hand, the methylpiperidine moiety is bound to the nitrogen of the indole, and the ion at m/z 98 is observed as the base peak (Fig. 3d). Unlike naphthoyl and benzoyl indoles, the base peak of the fragment ion caused by the N-alkylindole 3-carbonyl moiety for phenylacetyl (Fig. 3c), cyclopropyl, or adamantyl (Fig. 3f) indoles, is only shown in each full scan spectrum. Analogs, in which the indole skeleton is changed to an indazole, such as THJ-018, have also appeared on the market. In these analogs, molecular and N-dealkylated ions are typically observed in the spectrum (Fig. 3e).
Recently, amide- or ester-type analogs bonded with an N-alkylindole or N-alkylindazole 3-carbonyl moiety have appeared on the market [17, 18]. In these analogs, the abundance of the molecular ion is low, and the fragment ion caused by the indoyl (or indazoyl) moiety is observed as a base peak (Fig. 3f–k). Although the fragment ion caused by elimination of the terminal CO–NH2 is lower than that of the cleavage of the amide moiety in indole analogs, such as ADBICA (Fig. 3h) [19], the fragment ion caused by elimination of terminal CO–NH2 is as intense as that of the cleavage of the amide moiety in indazole analogs, such as ADB-PINACA and AB-CHMINACA (Fig. 3j, k) [20]. The substitution of the indole skeleton with the indazole moiety, such as in THJ-018 and THJ-2201 [21], has also been observed in these analogs. In these analogs, molecular and N-dealkylated ions are typically observed in the spectrum.
For example, in the simultaneous analysis of synthetic cannabinoid species, 10 mg of ground powder of the dried leaves was extracted with 10 ml of methanol under ultrasonication for 10 min. The extracts were centrifuged for 5 min at 3,000 rpm, and the supernatants were filtered and used for GC–MS analysis. The LODs were 0.5–1.0 mg/l, and linearity was obtained at concentrations up to 100 mg/l [16]. In another article [22], herbal samples (approximately 50 mg) were put into 10-ml headspace vials, and the vials were capped with 20-mm magnetic crimp seal caps with PTFE/silicone septa. The samples were incubated at 200 °C with pulse-agitation at 250 rpm. A StableFlex carboxen/polydimethylsiloxane fiber was inserted into the headspace for 5 min for extraction. The fiber was then injected into the GC inlet for 15 min to desorb the analytes. The LOD of synthetic cannabinoid in the samples was at least 20 μg.
The tentative identification of synthetic cannabinoids appears easy, but similar mass spectra are sometimes obtained by GC–MS because regio- and ring-substituted analogs are still distributed on the market. The misidentification of these analogs arises when using only the information from the mass spectra. When tandem and high-resolution MS are used to identify the conformational isomers or regioisomers, such misidentification does not occur [23–28]. Moreover, identification of cyclopropyl or ester analogs, such as UR-144 or QUPIC, is usually not possible because cyclopropyl analogs are heat-unstable and are easily degraded in the injection port of the GC instrument [29, 30].
LC–MS-MS detection
Many research groups have used LC–MS-MS for determination of synthetic cannabinoids in herbs and biological samples, and some have studied the fragmentation of synthetic cannabinoids in detail [15, 31]. The probable fragmentation pathways are shown in Fig. 4. Because the protonated molecular ion is only observed by LC–MS, and the information acquired by LC–MS is lesser than that for GC–MS, it is necessary to obtain other data that reflect the chemical structures by LC–MS-MS or TOFMS. Fragment ions are observed by product ion scanning when the protonated molecular ion is used as the precursor ion. In naphthoylindole, ions at m/z 127 and 155 are generated by naphthyl and naphthoyl moieties. However, information about the indole moiety tends to be not revealed by LC–MS-MS. On the other hand, the N-alkyl moiety of a synthetic cannabinoid is mainly modified for excretion into urine as a metabolite. Therefore, LC–MS-MS is a useful methodology to search for metabolites of synthetic cannabinoids in urine.
Recently, packages containing mixtures of multiple synthetic cannabinoids have been sold commercially, even though the package ingredients have been largely unknown to both sellers and buyers. In this aspect, the LC–MS-MS screening method is helpful in some estimation of the ingredients. Kneisel and Auwärter [32] demonstrated the simultaneous detection of 30 synthetic cannabinoids in serum; the LODs and LOQs were 0.01–2.0 and 0.1–2.0 ng/ml, respectively. There are many applications for analysis of synthetic cannabinoids in urine, hair, and oral fluids [33–37]. The typical published methods for analysis of synthetic cannabinoids in biological materials are summarized in Table 1 [38–54]. Simple LLE is usually used for the extraction of synthetic cannabinoids from biological materials because of the high hydrophobicity of the drugs. The chromatographic conditions are generally simple and do not require a special technique; octadecyl-type columns were used as analytical columns and analyses were performed in gradient mode.
Table 1.
LC–MS or LC–MS-MS conditions for synthetic cannabinoids in biological materials
| Target(s) | Sample(s) | Purification(s) | Column(s) | Mobile phase | LOD (ng/ml) | Linear range (ng/ml) | Reference(s) |
|---|---|---|---|---|---|---|---|
| JWH-018 | Serum | LLE | Luna C18 (2) (150 mm, 2 mm ID, 5 μm) (Phenomenex) | 10 mM ammonium acetate (0.1 % acetic acid, pH 3.2), methanol | 0.07 | 0.21–20 | [38] |
| JWH-018, JWH-073, JWH-019, JWH-250 | Blood | LLE | Acquity UPLC HSS T3 (100 mm, 2.1 mm ID, 1.8 μm) (Waters) | 1 % formic acid, methanol (1 % formic acid) | 0.006–0.016 | 0.1–20 | [39, 40] |
| Aminoalkylindoles, methanandamide | Serum | LLE | Luna phenyl hexyl (50 mm, 2 mm ID, 5 μm) (Phenomenex) | 2 mM ammonium formate (0.2 % formic acid), methanol | 0.1 | 0.1–2, 0.3–2 (methanandamide) | [41] |
| JWH-018, JWH-073, metabolites | Urine | Dilution (hydrolysis) | Zorbax Eclipse XDB-C18 (150 mm, 4.6 mm ID, 5 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | <2.0 | 2–100 | [42] |
| Metabolites of JWH-018 and JWH-073 | Urine | SPE (hydrolysis) | Zorbax Eclipse XDB-C18 (150 mm, 4.6 mm ID, 5 μm) (Agilent) | 0.1 % formic acid/acetonitrile (0.1 % formic acid) (45:55), isocratic | <0.1 | 0.1–100 | [43] |
| Metabolites of 8 synthetic cannabinoids | Urine | LLE (hydrolysis) | AQUASIL C18 (100 mm, 2.1 mm ID, 5 μm) (Thermo Scientific) | 5 mM ammonium acetate, methanol/acetonitrile (1:1, 5 mM ammonium acetate) | 0.1–10 | [44] | |
| Metabolites of JWH-018 and JWH-073 | Urine | LLE (hydrolysis) | Acquity UPLC HSS T3 (100 mm, 2.1 mm ID, 1.8 μm) (Waters) | 0.1 % formic acid (0.1 %), acetonitrile (0.1 % formic acid) | 4–400 | [45] | |
| 30 Synthetic cannabinoids | Serum | LLE | Luna phenyl hexyl (50 mm, 2 mm ID, 5 μm) (Phenomenex) | 0.2 % formic acid (2 mM ammonium formate), methanol | 0.01–2.0 | 0.1–2.0 (2–40 : JWH-387) | [32, 46–48] |
| Metabolites of 7 synthetic cannabinoids | Urine | LLE (hydrolysis) | Luna C18 (150 mm, 2 mm ID, 5 μm) (Phenomenex) | 0.2 % formic acid (2 mM ammonium formate), methanol | [49] | ||
| 22 Synthetic cannabinoids | Hair | Ethanol ext | Luna phenyl hexyl (50 mm, 2 mm ID, 5 μm) (Phenomenex) | 0.2 % formic acid (2 mM ammonium formate), methanol | 0.5 pg/mg | [37] | |
| JWH-018, JWH-073 | Blood | LLE | Acquity UPLC BEH C18 (50 mm, 2.1 mm ID, 1.8 μm) (Waters) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.01 | 0.05–50 | [50] |
| UR-144, metabolites, pyrolysis product | Urine | LLE (hydrolysis) | Zorbax Eclipse XDB-C18 (150 mm, 2.1 mm ID, 3.5 μm) (Agilent) | 20 mM ammonium formate buffer (pH5), acetonitrile | [29] | ||
| UR-144, metabolites | Blood, urine | PP | Kinetex C18 (100 mm, 4.6 mm ID, 2.6 μm) (Phenomenex), Ascentis express C18 (7.5 cm, 2.1 mm ID, 2.7 μm) (Supelco) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.15 (blood) | 0.5–100 (blood) | [51] |
| 9 Synthetic cannabinoids, 20 metabolites | Urine | PP (hydrolysis) | XB-C18 (50 mm, 3.0 mm ID, 2.6 μm) (Kinetex) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.5–10 | [34] | |
| MAM-2201 | Blood | SPE | InertSustain C18 HP (100 mm, 3 mm ID, 3 μm) (GL Sciences) |
0.1 % acetic acid, acetonitrile | 1 | 2.5–100 | [52] |
| 5F-PB-22 | Blood, serum | LLE | Acquity UPLC BEH C18 (100 mm, 2.1 mm ID, 1.7 μm) (Waters) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.1 | 0.5–10 | [53] |
| Indole derivative synthetic cannabinoids | Saliva, urine, blood | PP (blood) LLE (urine, saliva) (hydrolysis) | Ascentis C18 (150 mm, 2.1 mm, 5 μm) (Supelco) | 0.1 % formic acid (5 mM ammonium formate), acetonitrile (0.1 % formic acid) | 0.1–0.5 | [54] |
The identification of an unknown drug in a biological material without information is almost always difficult, even if analysis is carried out with LC–MS-MS. To overcome this situation, high-resolution MS or TOFMS become helpful tools for tentative estimation of parent drugs and their metabolites of synthetic cannabinoids.
To clarify the chemical structure of an unknown drug in a herbal blend product that contains more than several milligrams of the drug, GC–MS, LC–MS-MS, and high-resolution MS (or TOFMS) can be used to estimate the structure. The target compound is then purified by preparative LC or preparative TLC to obtain more than several milligrams of the compound of high purity, which is then analyzed by NMR spectroscopy [17–21]. The detailed chemical structure can be elucidated by the above laborious instrumental analyses.
Cathinones
Colorimetric detection
The Marquis reagent, which reacts with all nitrogen-containing drugs, is negative for cathinones, such as cathinone and mephedrone, but is positive for cathinone analogs that have a methylenedioxy moiety in each molecule. The cathinone analogs with a methylenedioxy moiety also react with the Chen reagent, which changes to orange in positive tests. Although the LOD concentration is not reported, the described test solution contained cathinone powder (at least 10 mg) suspended in methanol (1 ml) [55–57]. The combination of Marquis, Ehrlich, Simon, Lieberman–Burehand, and Mandelin reagents is useful for the detection of cathinones in samples. Like synthetic cannabinoids, the identification of these compounds, of course, cannot be performed using these methods; moreover, the detection of small amounts or mixtures of cathinones is difficult.
Immunochemical detection
Some researchers have tried to detect cathinones in urine using immunoassay technology [58, 59]. Some articles revealed false-positive results by immunoassays; for example, MDPV was cross-reactive with phencyclidine [60]. Therefore, specific detection of cathinones by a commercial immunoassay is not yet possible.
GC–MS-MS detection
Mass spectral profiles of cathinones are very simple in the positive mode of GC–MS, because only the base peak originating from the immonium ion in each molecule is observed. The probable fragmentation pathways of cathinones are described in previous articles [61–64] and are shown in Fig. 5. However, this phenomenon makes the identification of cathinones difficult. To help identify cathinones, other information, such as tandem mass spectrometric data, are usually used because more structural information about the molecule is obtained.
Fig. 5.
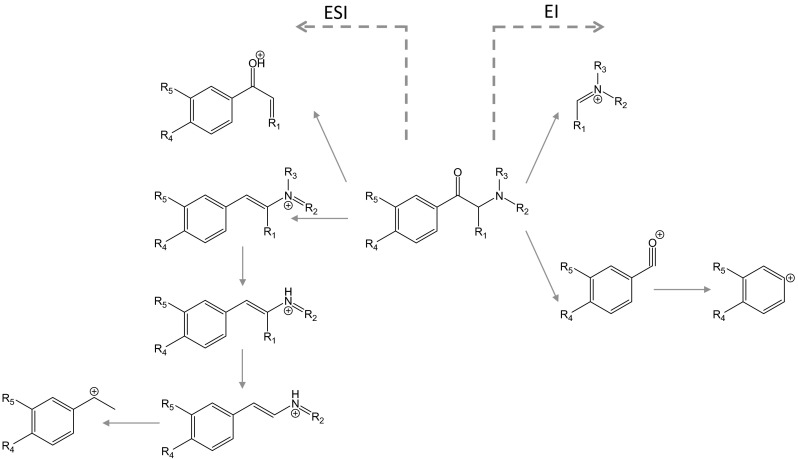
Probable fragmentation pathways of cathinones by electrospray ionization and electron ionization (modified from references [61, 62, 65, 66])
Zuba [61] introduced the systematic identification of cathinones using the mass spectra obtained. First, it should be checked whether the molecular ion is observed. The immonium ion (m/z = 16 + 14 n, n = 1, 2, 3,…) is then checked in the EI spectrum. If the immonium ion is found in the spectrum, the substance could be a straight-chained cathinone. If not, it is checked whether the ion for a pyrrolidine ring is observed (m/z = 70 + 14 n, n = 1, 2, 3,…). If this ion is found in the spectrum, the substance could be a cathinone with a pyrrolidine ring in the molecule [61]. There are various regioisomers in cathinones. To identify the cathinones, it is necessary to assign both the location and length of the bonded alkyl chain. Moreover, the ring-substituted moiety is also needed to be assigned. Zuba [61] demonstrated the following rules: the fragment ions reflecting the ring-substituted moiety are observed at m/z 77 and 105 for a nonsubstituted phenyl ring, at m/z 91 and 119 for a methylphenyl ring, and at m/z 121 and 149 for a methylenedioxyphenyl ring. Matsuta et al. [62] demonstrated the detailed analysis of MS data obtained by GC–EI-MS for identification of cathinones and specified indexing information. However, the ionization rate of the fragments in ring-substituted cathinones is remarkably weaker than that of the immonium ion. Other information obtained by TOFMS or CI-MS is helpful to delineate the molecular structure [63–66]. The identification of the regioisomer of the fluorinated cathinones was demonstrated using CI-MS [67]. However, this phenomenon was suggested to be limited to these analogs.
The published methods for analysis of cathinones in biological materials are summarized in Table 2 [68–79]. Simple LLE is usually used for extraction of cathinones from biological materials. The chromatographic conditions are also simple and do not usually require a special technique.
Table 2.
GC–MS conditions for cathinones in biological materials
| Target(s) | Sample(s) | Purification | Derivatization(s) | Column(s) | LOD(s) (ng/ml) | Linear range (ng/ml) | Reference |
|---|---|---|---|---|---|---|---|
| MDPV, metabolites | Urine | SPE (hydrolysis) | Methyl, acetyl, trimethylsilyl | HP-1 (12 m, 0.2 mm ID, 0.33 μm) (Agilent) | – | – | [68] |
| MDPV, metabolites | Cellular fraction, urine | LLE (hydrolysis) | Trimethylsilyl | 5 % Phenyl-methylsilicone (17 m, 0.2 mm ID, 0.33 μm) (J and W) | 2 | 10–2,000 | [69] |
| Mephedrone, MDPV | Blood, urine | LLE | DB-1 (30 m, 0.32 mm ID, 0.25 μm) (Agilent) | – | – | [70] | |
| MDPV | Urine | LLE | Heptafluorobutyryl | HP-5MS (12 m, 0.2 mm ID, 0.33 μm) (Agilent), ZB-5MS (12 m, 0.2 mm ID, 0.33 μm) (Phenomenex) | 10 | 20–2,000 | [71] |
| Methylone | Blood | LLE | Heptafluorobutyryl | RTx-5 MS (30 m, 0.25 mm ID, 0.25 μm) (Restek) | 50 | 100–2,000 | [72] |
| α-PVP, pyrovalerone (PV), MDPV | Blood | SPME | InertCap 5 (30 m, 0.25 mm ID, 0.25 μm) (GL Sciences) | 0.5 (PV, PVP), 1.0 (MDPV) | 1–200 | [73] | |
| MDPV, α-PVP, α-PBP | Blood | LLE (Extrelut) | InertCap 5MS/NP (30 m, 0.25 mm ID, 0.25 μm) (GL Sciences) | 1 | 2–2,000 | [74] | |
| MDPV | Blood, tissue, urine | SPE | Zebron Guardian ZB-50 (10 m, 0.18 mm ID, 0.18 μm) (Phenomenex) | – | 10–2,000 | [75] | |
| MDPV | Blood, urine | LLE | Rtx-5 ms (30 m, 0.25 mm ID, 0.25 μm) (Restek) | – | – | [76] | |
| 3,4-Dimethylmethcathinone, metabolites | Urine | LLE | Trifluoroacetyl | DB-5MS (30 m, 0.25 mm ID, 0.25 μm) (Agilent) | – | – | [77] |
| 16 Synthetic cathinones | Urine | LLE | Trifluoroacetyl | CP7684 (10 m, 0.15 mm ID, 0.12 μm) (Agilent) | – | – | [78] |
| α-PVP, metabolites | Urine | LLE | Trimethylsilyl | DB-5MS (30 m, 0.25 mm ID, 0.25 μm) (Agilent) | – | – | [79] |
LC–MS-MS detection
The strategy for the detection of cathinones by LC–MS-MS is almost same as that for synthetic cannabinoids; almost all methods use MRM or SRM mode for sensitive determination. The probable fragmentation pathways are shown in Fig. 5. The [M+H]+ ion is selected as a precursor ion, and three product ions that reflect the chemical structures of the cathinones are selected. Using this method, 30–50 drugs are monitored simultaneously in samples [80–82]. The published methods for analysis of cathinones in biological materials are summarized in Table 3 [68, 69, 74, 75, 77, 79, 82–98]. Simple LLE is usually used for the extraction of cathinones from biological materials. The chromatographic conditions are also simple and do not require a special technique.
Table 3.
LC–MS or LC–MS-MS conditions for cathinones in biological materials
| Target(s) | Sample(s) | Purification(s) | Column(s) | Mobile phase(s) | LOD(s) (ng/ml or g) | Linear range (ng/ml or g) | Reference |
|---|---|---|---|---|---|---|---|
| MDPV, metabolites | Urine | SPE (hydrolysis) | Hypersil Gold column (10 mm, 2.1 mm ID, 1.9 μm) (Thermo Scientific) | 10 mM ammonium formate (0.1 % formic acid), acetonitrile (0.1 % formic acid) | [68] | ||
| MDPV, metabolites | Cellular fraction, urine | LLE (hydrolysis) | Zorbax Eclipse Plus C18 (100 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | [69] | ||
| MDPV | Serum | SPE | Phenyl–hexyl (50 mm, 3.0 mm ID, 3 μm) (Phenomenex) | 10 mM ammonium acetate (0.1 % formic acid), methanol | 3 | 10–500 | [83] |
| Mephedrone | Plasma | LLE | Synergi Fusion (150 mm, 4.6 mm ID)(Phenomenex); Spherisorb (150 mm, 4.6 mm ID) (Waters) | 10 % acetonitrile (25 mM triethylammonium phosphate buffer), isocratic | 39 | 78–10,000 | [84] |
| 9 Cathinones | Blood | PP | Prodigy Phenyl-3 (150 mm, 2.0 mm ID, 5 μm) (Phenomenex) | 0.1 % formic acid, methanol | 0.5–3 | 10–400 | [82] |
| 7 Cathinones | Hair | LLE | Kintex PFP (50 mm, 2 mm ID, 2.6 μm) (Phenomenex) | 5 mM ammonium formate (pH 3.5), methanol (5 mM ammonium formate) | 10–50 pg/mg | [85] | |
| Butylone | Blood, liver | SPE | Allure PFP (50 mm, 2.1 mm ID, 5 μm) (Restek) | 0.02 % formic acid (2 mM of ammonium formate), acetonitrile | 25 (blood) | 50–2,000 (blood) | [86] |
| 4-Methylethcathinone | Blood, urine | LLE | Zorbax SB-C18 (50 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.96 (blood), 0.68 (urine) | 10–1,000 | [87] |
| MDPV, α-PVP, α-PBP | Hair | LLE (Extrelut) | Phenyl-hexyl (150 mm, 2.1 mm ID, 3 μm) (Agilent) | 10 mM ammonium formate (0.1 % formic acid, pH 3.3)/methanol (65:35), isocratic | 0.02 ng/10-mm | 0.05–50 ng/10-mm | [74] |
| Mephedrone | Blood | PP | Zorbax SB-C18 (50 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.08 | 1–100 | [88] |
| MDPV, mephedrone | Blood, plasma, urine | SPE | Kintex PFP (50 mm, 2.1 mm ID, 1.8 μm) (Phenomenex) | 2 mM ammonium formate (2 % formic acid), acetonitrile (0.1 % formic acid) | 2 | 5–2,000 | [89] |
| Mephedrone | Blood, urine | LLE | Zorbax SB-C18 (150 mm, 2.1 mm ID, 3.5 μm) (Agilent) | 0.1 % formic acid, methanol (0.1 % formic acid) | 1 (blood), 2 (urine) | 20–2,000 | [90] |
| 10 Cathinones | Blood, other specimens | LLE | Zorbax XDB-C18 (150 mm, 4.6 mm ID, 5 μm) (Agilent) | 5 mM ammonium acetate, methanol/acetonitrile | – | 5–200 | [91] |
| MDPV | Hair | SPE | Zorbax Eclipse Plus C18 (50 mm, 2.1 mm, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile | 2.0 pg/mg | 2–3,000 pg/mg | [75] |
| MDPV | Blood | PP | Zorbax SB-C18 (50 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.5 | 5–500 | [92] |
| Buphedrone | Blood | PP | Zorbax SB-C18 (50 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.3 | 1–1,000 | [93] |
| 3,4-Dimethylmethcathinone, metabolites | Urine | PP (hydrolysis) | L-column2 ODS (150 mm, 1.5 mm ID, 5 μm) (Chemicals Evaluation and Research Institute) | 10 mM ammonium formate buffer (pH 5), methanol | – | 10–5,000 | [77] |
| MDPV, metabolites | Plasma | PP (hydrolysis) | Synergy polar-RP (100 mm, 2 mm ID, 2.5 μm) (Phenomenex) | 0.1 % formic acid, acetonitrile (0.1 % formic acid) | 0.1 | 0.25–1,000 | [94] |
| α-PBP | Blood, urine, tissues | QuEChERS | Zorbax Eclipse Plus C18 (100 mm, 2.1 mm ID, 1.8 μm) (Agilent) | 10 mM ammonium formate (0.1 % formic acid), acetonitrile |
0.05 (blood, urine), 0.1 (tissues) | 8.6–4,280 | [95] |
| 3,4-Dimethylmethcathinone, metabolites | Blood, urine | QuEChERS | Shim-pack XR-ODS III (50 mm, 2.0 mm ID, 1.6 μm) (Shimadzu); L-column2 ODS (150 mm, 1.5 mm ID, 5 μm) (Chemical Evaluation and Reaearch Institute) | 10 mM ammonium formate, methanol; 10 mM ammonium formate (pH 5.0), methanol | 1.03 (blood), 1.37 (urine) | 5–400 | [96] |
| MDPV metabolittes | Urine | PP, LLE, SPE (hydrolysis) | Atlantis T3 (150 mm, 2.1 mm) (Waters) | 10 mM ammonium formate buffer (0.1 % formic acid), acetonitrile (0.1 % formic acid) | [97] | ||
| α-PVP, metabolites | Urine | PP (hydrolysis) | L-column2 ODS (150 mm, 1.5 mm ID, 5 μm) (Chemicals Evaluation and Research Institute) | 10 mM ammonium formate (pH 5), methanol | – | 10–10,000 | [79] |
| PV9 | Blood, urine | QuEChERS | Zorbax Eclipse Plus C18 (100 mm, 2.1 mm ID, 1.8 µm) (Agilent) | 10 mM ammonium formate (0.1 % formic acid), acetonitrile | 0.05 | 10–1,000 | [98] |
In the same way as identification by GC–MS, other information obtained by TOFMS or tandem MS is needed to clarify the molecular structure. An authentic drug or library database is needed to identify the drugs. Moreover, the probable fragmentation pathways of cathinones are described in the previous articles [61, 65, 66, 99]. These data are helpful in identifying the drugs.
As described in the section on synthetic cannabinoids, we occasionally encounter a dubious product that contains more than several milligrams of an unknown cathinone-like compound. In such a case, GC–MS, LC–MS, high-resolution MS (or TOFMS), and finally NMR spectroscopy are used to clarify the detailed chemical structure of the compound.
Concentrations in the cases of abuse
Synthetic cannabinoids
The common method of consumption of synthetic cannabinoids is smoking, which is the same as for conventional cannabis. The maximum concentrations of synthetic cannabinoids in serum are reached in less than 10 min after smoking [38]. The drugs absorbed in the body are metabolized smoothly, and the concentrations decrease rapidly. Moreover, there is also a report that cannabinoids accumulate in the adipose tissue because of their high lipophilicity [52]. Therefore, detection of the drug from serum is usually difficult. Synthetic cannabinoids absorbed in the human body are metabolized to hydroxyl or carboxyl derivatives of the aromatic ring or N-alkyl side chain [100]. It is difficult to identify the parent drug and its metabolites in blood by GC–MS alone because the fragmentation of the metabolites is similar to that of the parent drug and analogs. Moreover, the concentration of the unchanged synthetic cannabinoids in blood is very low, and the number of metabolites that are commercially available is small. Low sensitivity is a limitation for the determination of synthetic cannabinoids in blood by GC–MS.
Although the concentration is influenced by the sampling time after drug intake and by the intake amount, concentrations of these drugs in serum were reported in the range of 0.1–190 ng/ml in poisoning cases [46]. In fatal cases, the concentrations of the drugs in blood were 0.1–199 ng/ml for JWH-018 and 0.1–68.3 ng/ml for JWH-073 [50], 12 ng/ml for AM-2201 [100], 1.1–1.5 ng/ml for 5F-PB-22 [53], and 12.4 ng/ml for MAM-2201 [52].
Cathinones
Unlike synthetic cannabinoids, the most common method of consumption of cathinones are insufflation (snorting) or ingestion. Inhalation, sublingual and rectal administration, and intramuscular or intravenous injection have also been reported. Unlike synthetic cannabinoids, the concentration of cathinones in blood is thought to vary because of the many modes of administration used by abusers. Only the blood concentration at one point and at several points have been quantified, and there is no report on continuous monitoring of the profile of the drug concentration in blood. The fatal concentration of the drug in blood was reported to be around 400 ng/ml [75]. The stability of cathinones in blood samples is clearly influenced by pH, as well as in the final extracts. In blood samples preserved with NaF/potassium oxalate, the measured concentrations of cathinone, methcathinone, ethcathinone, mephedrone, and flephedrone declined by ca. 30 % after 2 days of storage at 20 °C [82].
Some groups have studied the metabolic pathways of cathinones [68, 94, 101–103]. Unlike synthetic cannabinoids, the parent cathinones are detected easily in biological materials and are selected as the target because the unchanged parent drugs are rapidly excreted in urine. Cathinones are ionized in the body, and the reabsorption rate is low in the kidney because of low hydrophobicity. The excretion profile of α-PBP and α-PVP in human urine was determined after an intravenous injection, and the elimination half-life in urine was approximately 12 h. Moreover, the excreted amount in urine was influenced by urinary pH, like a psycho-stimulant [104]. To analyze these drugs in biological materials, it is necessary to remove endogenous substances from each sample and enrich the content of the drug. As shown in Table 3, LLE is usually used for extraction of the drugs from biological materials. The quantification of the metabolites is important to predict the hazardous properties of the metabolites. However, because there are few metabolites marketed, no detailed study about their pharmacological activity or toxicity has been conducted.
In fatal cases, the concentrations of the drugs in blood were: 560–3,300 [72], 272 [105], and 60–1,120 ng/ml [106] for methylone; 1.2–22 [107], 5.1 [108], and 5.5 µg/ml [88] for mephedrone; 55.2 ng/ml for α-PBP [95]; 486 [73] and 654 ng/ml [109] for α-PVP; 180 ng/ml [98] for PV9; 170 [70], 82 [110], 1,200 [74], 440 [75], 17–38 [92], and 700 ng/ml [111] for MDPV.
Conclusions
The number of abusers of synthetic cannabinoids and cathinones has increased remarkably worldwide. The chemical structures of the distributed drugs are skillfully changed so that the drugs may pass through screenings for detection. Simple screening methods are required for detection of these drugs in seized and biological materials. There are currently no commercial kits or devices for the routine screening of these drugs. Colorimetric, immunochemical, and chromatographic methods have been introduced in this review; a suitable method must be chosen for each laboratory. Although various human sample matrices are available for testing, urine and blood are of the first choices. However, many of these drugs, especially unchanged synthetic cannabinoids, exist in urine and blood for only a short period. Therefore, other matrices that can prove the consumption of these drugs, such as hair and saliva, are likely to receive more attention in the future.
Acknowledgments
The authors are thankful to Professors Akira Ishii, Chief Editor, and Osamu Suzuki, Emeritus Chief Editor, Forensic Toxicology, for providing us an opportunity to write this review.
Conflict of Interest
There are no financial or other relations that could lead to a conflict of interest.
Abbreviations
- A-796260
[1-[2-(4-Morpholinyl)ethyl]-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
- A-834735
[1-[(Tetrahydro-2H-pyran-4-yl)methyl]-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
- AB-001
Adamantan-1-yl(1-pentyl-1H-indol-3-yl)methanone
- AB-005
[1-[(1-Methyl-2-piperidinyl)methyl]-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
- AB-CHMINACA
N-[(1S)-1-(Aminocarbonyl)-2-methylpropyl]-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide
- AB-FUBINACA
N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide
- AB-PINACA
N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide
- ADB-FUBINACA
N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide
- ADBICA
N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-pentyl-1H-indole-3-carboxamine
- ADB-PINACA
N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide
- AM-1220
[1-[(1-Methylpiperidin-2-yl)methyl]-1H-indol-3-yl]-(naphthalen-1-yl)methanone
- AM-1248
Adamantan-1-yl[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]methanone
- AM-1241
(2-Iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl]methanone
- AM-2201
[1-(5-Fluoropentyl)-1H-indol-3-yl]-1-naphthalenylmethanone
- AM-2233
(2-Iodophenyl)[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]-methanone
- AM-679
(2-Iodophenyl)(1-pentyl-1H-indol-3-yl)methanone
- AM-694
1-[(5-Fluoropentyl)-1H-indol-3-yl]-(2-iodophenyl)methanone
- AMB
Methyl (1-pentyl-1H-indazole-3-carbonyl)-l-valinate
- APICA
N-(1-Adamantyl)-1-pentyl-1H-indole-3-carboxamide
- APINACA
N-(1-Adamantyl)-1-pentyl-1H-indazole-3-carboxamide
- Cathinone
2-Amino-1-phenylpropan-1-one
- CI
Chemical ionization
- EI
Electron ionization
- ELISA
Enzyme-linked immunosorbent assay
- ESI
Electrospray ionization
- FDU-PB-22
Naphthalen-1-yl 1-(4-fluorobenzyl)-1H-indole-3-carboxylate
- 5F-PB-22
1-(5-Fluoropentyl)-8-quinolinyl ester-1H-indole-3-carboxylic acid
- FUB-PB-22
Quinolin-8-yl-1-(4-fluorobenzyl)-1H-indole-3-carboxylate
- GC
Gas chromatography
- GC–MS
Gas chromatography–mass spectrometry
- GC–MS-MS
Gas chromatography–tandem mass spectrometry
- HU-210
3-(1,1′-Dimethylheptyl)-6aR,7,10,10aR-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol
- JWH-015
1-Naphthalenyl(2-methyl-1-propyl-1H-indol-3-yl)methanone
- JWH-018
1-Naphthalenyl(1-pentyl-1H-indol-3-yl)methanone
- JWH-019
1-Naphthalenyl(1-hexyl-1H-indol-3-yl)methanone
- JWH-030
1-Naphthalenyl(1-pentyl-1H-pyrrol-3-yl)methanone
- JWH-073
1-Naphthalenyl(1-butyl-1H-indol-3-yl)methanone
- JWH-200
1-Naphthalenyl[1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl]methanone
- JWH-203
2-(2-Chlorophenyl)-1-(1-pentyl-1H-indol-3-yl)ethanone
- JWH-250
2-(2-Methoxyphenyl)-1-(1-pentyl-1H-indol-3-yl)ethanone
- JWH-251
2-(2-Methylphenyl)-1-(1-pentyl-1H-indol-3-yl)ethanone
- JWH-307
[5-(2-Fluorophenyl)-1-pentyl-1H-pyrrol-3-yl](naphthalene-1-yl)methanone
- LC
Liquid chromatography
- LC–MS
Liquid chromatography–mass spectrometry
- LC–MS-MS
Liquid chromatography–tandem mass spectrometry
- LLE
Liquid–liquid extraction
- LOD
Limit of detection
- LOQ
Limit of quantification
- MAM-2201
[1-(5-Fluoropentyl)-1H-indol-3-yl](4-methyl-1-naphthalenyl)methanone
- MDPBP
3′,4′-Methylenedioxy-α-pyrrolidinobutiophenone
- MDPPP
3′,4′-Methylenedioxy-α-pyrrolidinopropiophenone
- MDPV
3,4-Methylenedioxypyrovalerone
- MN-18
N-1-Naphthalenyl-1-pentyl-1H-indazole-3-carboxamide
- MOPPP
4′-Methoxy-α-pyrrolidinopropiophenone
- NM-2201
Naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate
- NMR
Nuclear magnetic resonance
- NNEI
N-1-Naphthalenyl-1-pentyl-1H-indole-3-carboxamide
- MPBP
4′-Methyl-α-pyrrolidinobutiophenone
- MPHP
4′-Methyl-α-pyrrolidinohexanophenone
- MPPP
4′-Methyl-α-pyrrolidinopropiophenone
- MRM
Multiple reaction monitoring
- MS
Mass spectrometry
- MS-MS
Tandem mass spectrometry
- NPB-22
8-Quinolinyl 1-pentyl-1H-indazole-3-carboxylate
- α-PBP
α-Pyrrolidinobutiophenone
- α-PHP
α-Pyrrolidinohexanophenone
- PP
Protein precipitation
- α-PPP
α-Pyrrolidinopropiophenone
- PTFE
Polytetrafluoroethylene
- PV8
1-Phenyl-2-(pyrrolidin-1-yl)heptan-1-one
- PV9
1-Phenyl-2-(pyrrolidin-1-yl)octan-1-one
- α-PVP
1-Phenyl-2-(pyrrolidin-1-yl)pentan-1-one, α-pyrrolidinovalerophenone
- PX1
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide
- QUPIC
Quinolin-8-yl 1-pentyl-1H-indole-3-carboxylate
- QUCHIC
Quinolin-8-yl 1-(cyclohexylmethyl)-1H-indole-3-carboxylate
- RCS-4
(4-Methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone
- SDB-005
Naphthalen-1-yl 1-pentyl-1H-indazole-3-carboxylate
- SIM
Selected ion monitoring
- SPE
Solid-phase extraction
- SPME
Solid-phase microextraction
- SRM
Selected reaction monitoring
- THJ-018
1-Naphthalenyl(1-pentyl-1H-indazol-3-yl)methanone
- THJ-2201
[1-(5-Fluoropentyl)-1H-indazol-3-yl](naphthalen-1-yl)methanone
- TLC
Thin-layer chromatography
- TOFMS
Time-of-flight mass spectrometry
- UV
Ultraviolet
- UR-144
(1-Pentyl-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone
- XLR-11
[1-(5-Fluoropentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
- XLR-12
(2,2,3,3-Tetramethylcyclopropyl)[1-(4,4,4-trifluorobutyl)-1H-indol-3-yl]methanone
References
- 1.Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell JC. Psychoactive substances—some new, some old: a scan of the situation in the US. Drug Alcohol Depend. 2014;134:71–77. doi: 10.1016/j.drugalcdep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y. Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol. 2013;31:44–53. [Google Scholar]
- 4.Chung H, Choi H, Heo S, Kim E, Lee J. Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol. 2014;32:82–88. [Google Scholar]
- 5.Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a 3-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233:416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Barratt MJ, Cakic V, Lenton S. Patterns of synthetic cannabinoid use in Australia. Drug Alcohol Rev. 2013;32:141–146. doi: 10.1111/j.1465-3362.2012.00519.x. [DOI] [PubMed] [Google Scholar]
- 7.Caudevilla-Gálligo F, Ventura M, Indave Ruiz BI, Fornís I. Presence and composition of cathinone derivatives in drug samples taken from a drug test service in Spain (2010–2012) Hum Psychopharmacol. 2013;28:341–344. doi: 10.1002/hup.2296. [DOI] [PubMed] [Google Scholar]
- 8.Presley BC, Jansen-Varnum SA, Logan BK. Analysis of synthetic cannabinoids in botanical materials: a review of analytical methods and findings. Forensic Sci Rev. 2013;25:27–46. [PubMed] [Google Scholar]
- 9.Elsohly MA, Gul W, Wanas AS, Radwan MM. Synthetic cannabinoids: analysis and metabolites. Life Sci. 2014;97:78–90. doi: 10.1016/j.lfs.2013.12.212. [DOI] [PubMed] [Google Scholar]
- 10.Favretto D, Pascali JP, Tagliaro F. New challenges and innovation in forensic toxicology: focus on the “New Psychoactive Substances”. J Chromatogr A. 2013;1287:84–95. doi: 10.1016/j.chroma.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs RCA. A structure–reactivity relationship driven approach to the identification of a color test protocol for the presumptive indication of synthetic cannabimimetic drugs of abuse. Forensic Sci Int. 2014;242:135–141. doi: 10.1016/j.forsciint.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Zaitsu K, Katagi M, Nakanishi K, Shima N, Kamata H, Kamata T, Nishioka H, Miki A, Tatsuno M, Iwamura T, Sato T, Tsuchihashi H, Suzuki K. Comprehensive analytical methods of the synthetic cannabinoids appearing in the illicit drug market (in Japanese with English abstract) Jpn J Forensic Sci Tech. 2011;16:73–90. [Google Scholar]
- 13.Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the US. J Forensic Sci. 2012;57:1168–1180. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- 14.Arntson A, Ofsa B, Lancaster D, Simon JR, McMullin M, Logan B. Validation of a novel immunoassay for the detection of synthetic cannabinoids and metabolites in urine specimens. J Anal Toxicol. 2013;37:284–290. doi: 10.1093/jat/bkt024. [DOI] [PubMed] [Google Scholar]
- 15.Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3:466–478. doi: 10.1002/dta.268. [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Heo S, Choe S, Yang W, Park Y, Kim E, Chung H, Lee J. Simultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC–MS. Anal Bioanal Chem. 2013;405:3937–3944. doi: 10.1007/s00216-012-6560-z. [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products. Forensic Toxicol. 2012;30:114–125. [Google Scholar]
- 18.Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y. New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products. Forensic Toxicol. 2013;31:93–100. [Google Scholar]
- 19.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31:223–240. [Google Scholar]
- 20.Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol. 2014;32:105–115. [Google Scholar]
- 21.Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014;32:266–281. [Google Scholar]
- 22.Cox AO, Daw RC, Mason MD, Grabenauer M, Pande PG, Davis KH, Wiley JL, Stout PR, Thomas BF, Huffman JW. Use of SPME-HS-GC–MS for the analysis of herbal products containing synthetic cannabinoids. J Anal Toxicol. 2012;36:293–302. doi: 10.1093/jat/bks025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst L, Krüger K, Lindigkeit R, Schiebel HM, Beuerle T. Synthetic cannabinoids in “spice-like” herbal blends: first appearance of JWH-307 and recurrence of JWH-018 on the German market. Forensic Sci Int. 2012;222:216–222. doi: 10.1016/j.forsciint.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Kneisel S, Westphal F, Bisel P, Brecht V, Broecker S, Auwärter V. Identification and structural characterization of the synthetic cannabinoid 3-(1-adamantoyl)-1-pentylindole as an additive in ‘herbal incense’. J Mass Spectrom. 2012;47:195–200. doi: 10.1002/jms.2059. [DOI] [PubMed] [Google Scholar]
- 25.Harris DN, Hokanson S, Miller V, Jackson GP. Fragmentation differences in the EI spectra of three synthetic cannabinoid positional isomers: JWH-250, JWH-302, and JWH-201. Int J Mass Spectrom. 2014;368:23–29. [Google Scholar]
- 26.DeRuiter J, Smith FT, Abdel-Hay K, Clark CR. Analytical differentiation of 1-alkyl-3-acylindoles and 1-acyl-3-alkylindoles: isomeric synthetic cannabinoids. Anal Chem. 2014;86:3801–3808. doi: 10.1021/ac500316x. [DOI] [PubMed] [Google Scholar]
- 27.Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Morzherin Y, Shafran Y. 3-Naphthoylindazoles and 2-naphthoylbenzoimidazoles as novel chemical groups of synthetic cannabinoids: chemical structure elucidation, analytical characteristics and identification of the first representatives in smoke mixtures. Forensic Sci Int. 2014;242:72–80. doi: 10.1016/j.forsciint.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Ibáñez M, Bijlsma L, van Nuijs ALN, Sancho JV, Haro G, Covaci A, Hernández F. Quadrupole-time-of-flight mass spectrometry screening for synthetic cannabinoids in herbal blends. J Mass Spectrom. 2013;48:685–694. doi: 10.1002/jms.3217. [DOI] [PubMed] [Google Scholar]
- 29.Grigoryev A, Kavanagh P, Melnik A, Savchuk S, Simonov A. Gas and liquid chromatography–mass spectrometry detection of the urinary metabolites of UR-144 and its major pyrolysis product. J Anal Toxicol. 2013;37:265–276. doi: 10.1093/jat/bkt028. [DOI] [PubMed] [Google Scholar]
- 30.Tsujikawa K, Yamamuro T, Kuwayama K, Kanamori T, Iwata YT, Inoue H. Thermal degradation of a new synthetic cannabinoid QUPIC during analysis by gas chromatography–mass spectrometry. Forensic Toxicol. 2014;32:201–207. [Google Scholar]
- 31.Sekuła K, Zuba D, Stanaszek R. Identification of naphthoylindoles acting on cannabinoid receptors based on their fragmentation patterns under ESI–QTOFMS. J Mass Spectrom. 2012;47:632–643. doi: 10.1002/jms.3004. [DOI] [PubMed] [Google Scholar]
- 32.Kneisel S, Auwärter V. Analysis of 30 synthetic cannabinoids in serum by liquid chromatography-electrospray ionization tandem mass spectrometry after liquid–liquid extraction. J Mass Spectrom. 2012;47:825–835. doi: 10.1002/jms.3020. [DOI] [PubMed] [Google Scholar]
- 33.Scheidweiler KB, Huestis MA. Simultaneous quantification of 20 synthetic cannabinoids and 21 metabolites, and semi-quantification of 12 alkyl hydroxy metabolites in human urine by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2014;1327:105–117. doi: 10.1016/j.chroma.2013.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wohlfarth A, Scheidweiler KB, Chen X, Liu H, Huestis MA. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC–MS/MS and library search. Anal Chem. 2013;85:3730–3738. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kneisel S, Auwärter V, Kempf J. Analysis of 30 synthetic cannabinoids in oral fluid using liquid chromatography-electrospray ionization tandem mass spectrometry. Drug Test Anal. 2013;5:657–669. doi: 10.1002/dta.1429. [DOI] [PubMed] [Google Scholar]
- 36.Kneisel S, Speck M, Moosmann B, Corneillie TM, Butlin NG, Auwärter V. LC/ESI–MS/MS method for quantification of 28 synthetic cannabinoids in neat oral fluid and its application to preliminary studies on their detection windows. Anal Bioanal Chem. 2013;405:4691–4706. doi: 10.1007/s00216-013-6887-0. [DOI] [PubMed] [Google Scholar]
- 37.Hutter M, Kneisel S, Auwärter V, Neukamm MA. Determination of 22 synthetic cannabinoids in human hair by liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2012;903:95–101. doi: 10.1016/j.jchromb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Teske J, Weller JP, Fieguth A, Rothämel T, Schulz Y, Tröger HD. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2010;878:2659–2663. doi: 10.1016/j.jchromb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Kacinko SL, Xu A, Homan JW, McMullin MM, Warrington DM, Logan BK. Development and validation of a liquid chromatography–tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. J Anal Toxicol. 2011;35:386–393. doi: 10.1093/anatox/35.7.386. [DOI] [PubMed] [Google Scholar]
- 40.Yeakel JK, Logan BK. Blood synthetic cannabinoid concentrations in cases of suspected impaired driving. J Anal Toxicol. 2013;37:547–551. doi: 10.1093/jat/bkt065. [DOI] [PubMed] [Google Scholar]
- 41.Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwärter V. Development and validation of a liquid chromatography–tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 42.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381–6388. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. LC–MS/MS method for the quantitation of metabolites of eight commonly used synthetic cannabinoids in human urine—an Australian perspective. J Chromatogr B. 2012;897:22–31. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Yanes EG, Lovett DP. High-throughput bioanalytical method for analysis of synthetic cannabinoid metabolites in urine using salting-out sample preparation and LC–MS/MS. J Chromatogr B. 2012;909:42–50. doi: 10.1016/j.jchromb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 47.Hermanns-Clausen M, Kneisel S, Hutter M, Szabo B, Auwärter V. Acute intoxication by synthetic cannabinoids—four case reports. Drug Test Anal. 2013;5:790–794. doi: 10.1002/dta.1483. [DOI] [PubMed] [Google Scholar]
- 48.Musshoff F, Madea B, Kernbach-Wighton G, Bicker W, Kneisel S, Hutter M, Auwärter V. Driving under the influence of synthetic cannabinoids (“Spice”): a case series. Int J Legal Med. 2014;128:59–64. doi: 10.1007/s00414-013-0864-1. [DOI] [PubMed] [Google Scholar]
- 49.Hutter M, Broecker S, Kneisel S, Auwärter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in herbal mixtures using LC–MS/MS techniques. J Mass Spectrom. 2012;47:54–65. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- 50.Shanks KG, Dahn T, Terrell AR. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in postmortem whole blood casework. J Anal Toxicol. 2012;36:145–152. doi: 10.1093/jat/bks013. [DOI] [PubMed] [Google Scholar]
- 51.Adamowicz P, Zuba D, Sekuła K. Analysis of UR-144 and its pyrolysis product in blood and their metabolites in urine. Forensic Sci Int. 2013;233:320–327. doi: 10.1016/j.forsciint.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Saito T, Namera A, Miura N, Ohta S, Miyazaki S, Osawa M, Inokuchi S. A fatal case of MAM-2201 poisoning. Forensic Toxicol. 2013;31:333–337. [Google Scholar]
- 53.Behonick G, Shanks KG, Firchau DJ, Mathur G, Lynch CF, Nashelsky M, Jaskierny DJ, Meroueh C. Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22. J Anal Toxicol. 2014;38:559–562. doi: 10.1093/jat/bku048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazzarino M, de la Torre X, Botrè F. A liquid chromatography–mass spectrometry method based on class characteristic fragmentation pathways to detect the class of indole-derivative synthetic cannabinoids in biological samples. Anal Chim Acta. 2014;837:70–82. doi: 10.1016/j.aca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Wood DM, Dargan PI. Mephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicity. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:227–233. doi: 10.1016/j.pnpbp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 56.Uchiyama N, Kawamura M, Kamakura H, Kikura-Hanajiri R, Goda Y. Analytical data of designated substances (Shitei-yakubutsu) controlled by the pharmaceutical affairs law in Japan, part II: color test and TLC (in Japanese with English abstract) Yakugaku Zasshi. 2008;128:981–987. doi: 10.1248/yakushi.128.981. [DOI] [PubMed] [Google Scholar]
- 57.Dal Cason TA. The characterization of some 3,4-methylenedioxycathinone (MDCATH) homologs. Forensic Sci Int. 1997;87:9–53. [Google Scholar]
- 58.Swortwood MJ, Hearn WL, DeCaprio AP. Cross-reactivity of designer drugs, including cathinone derivatives, in commercial enzyme-linked immunosorbent assays. Drug Test Anal. 2014;6:716–727. doi: 10.1002/dta.1489. [DOI] [PubMed] [Google Scholar]
- 59.Ellefsen KN, Anizan S, Castaneto MS, Desrosiers NA, Martin TM, Klette KL, Huestis MA. Validation of the only commercially available immunoassay for synthetic cathinones in urine:randox drugs of abuse V biochip array technology. Drug Test Anal. 2014;6:728–738. doi: 10.1002/dta.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. Am J Drug Alcohol Abuse. 2012;38:616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- 61.Zuba D. Identification of cathinones and other active components of legal highs by mass spectrometric methods. Trends Anal Chem. 2012;32:15–30. [Google Scholar]
- 62.Matsuta S, Katagi M, Nishioka H, Kamata H, Sasaki K, Shima N, Kamata T, Miki A, Tatsuno M, Zaitsu K, Tsuboi K, Tsuchihashi H, Suzuki K. Structural characterization of cathinone-type designer drugs by EI mass spectrometry (in Japanese with English abstract) Jpn J Forensic Sci Tech. 2014;19:77–89. [Google Scholar]
- 63.Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test Anal. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- 64.Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Test Anal. 2011;3:569–575. doi: 10.1002/dta.204. [DOI] [PubMed] [Google Scholar]
- 65.Fornal E. Identification of substituted cathinones: 3,4-methylenedioxy derivatives by high performance liquid chromatography–quadrupole time of flight mass spectrometry. J Pharm Biomed Anal. 2013;81–82:13–19. doi: 10.1016/j.jpba.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Jankovics P, Váradi A, Tölgyesi L, Lohner S, Németh-Palotás J, Koszegi-Szalai H. Identification and characterization of the new designer drug 4′-methylethcathinone (4-MEC) and elaboration of a novel liquid chromatography–tandem mass spectrometry (LC–MS/MS) screening method for seven different methcathinone analogs. Forensic Sci Int. 2011;210:213–220. doi: 10.1016/j.forsciint.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Westphal F, Junge T. Ring positional differentiation of isomeric N-alkylated fluorocathinones by gas chromatography/tandem mass spectrometry. Forensic Sci Int. 2012;223:97–105. doi: 10.1016/j.forsciint.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS. J Mass Spectrom. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- 69.Strano-Rossi S, Cadwallader AB, de la Torre X, Botrè F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2706–2714. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- 70.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the US. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 71.Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography–mass spectrometry. Ther Drug Monit. 2011;33:257–263. doi: 10.1097/FTD.0b013e318208b693. [DOI] [PubMed] [Google Scholar]
- 72.Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, 3rd, Garg U, Pietak BR. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36:444–451. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- 73.Saito T, Namera A, Osawa M, Aoki H, Inokuchi S. SPME–GC–MS analysis of α-pyrrolidinovaleorophenone in blood in a fatal poisoning case. Forensic Toxicol. 2013;31:328–332. [Google Scholar]
- 74.Namera A, Urabe S, Saito T, Torikoshi-Hatano A, Shiraishi H, Arima Y, Nagao M. A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of α-pyrrolidinobutiophenone and α-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol. 2013;31:338–343. [Google Scholar]
- 75.Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem tissue distribution of MDPV following lethal intoxication by bath salts. J Anal Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- 76.Wright TH, Cline-Parhamovich K, Lajoie D, Parsons L, Dunn M, Ferslew KE. Deaths involving methylenedioxypyrovalerone (MDPV) in Upper East Tennessee. J Forensic Sci. 2013;58:1558–1562. doi: 10.1111/1556-4029.12260. [DOI] [PubMed] [Google Scholar]
- 77.Shima N, Katagi M, Kamata H, Matsuta S, Nakanishi K, Zaitsu K, Kamata T, Nishioka H, Miki A, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K. Urinary excretion and metabolism of the newly encountered designer drug 3,4-dimethylmethcathinone in humans. Forensic Toxicol. 2013;31:101–112. [Google Scholar]
- 78.Uralets V, Rana S, Morgan S, Ross W. Testing for designer stimulants: metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol. 2014;38:233–241. doi: 10.1093/jat/bku021. [DOI] [PubMed] [Google Scholar]
- 79.Shima N, Katagi M, Kamata H, Matsuta S, Sasaki K, Kamata T, Nishioka H, Miki A, Tatsuno M, Zaitsu K, Ishii A, Sato T, Tsuchihashi H, Suzuki K. Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol. 2014;32:59–67. [Google Scholar]
- 80.Swortwood MJ, Boland DM, DeCaprio AP. Determination of 32 cathinone derivatives and other designer drugs in serum by comprehensive LC-QQQ-MS/MS analysis. Anal Bioanal Chem. 2013;405:1383–1397. doi: 10.1007/s00216-012-6548-8. [DOI] [PubMed] [Google Scholar]
- 81.Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography–high resolution mass spectrometry. Anal Bioanal Chem. 2013;405:9437–9448. doi: 10.1007/s00216-013-7386-z. [DOI] [PubMed] [Google Scholar]
- 82.Sørensen LK. Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B. 2011;879:727–736. doi: 10.1016/j.jchromb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210:195–200. doi: 10.1016/j.forsciint.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- 85.Rust KY, Baumgartner MR, Dally AM, Kraemer T. Prevalence of new psychoactive substances: a retrospective study in hair. Drug Test Anal. 2012;4:402–408. doi: 10.1002/dta.1338. [DOI] [PubMed] [Google Scholar]
- 86.Rojek S, Kłys M, Strona M, Maciow M, Kula K. “Legal highs”—toxicity in the clinical and medico-legal aspect as exemplified by suicide with bk-MBDB administration. Forensic Sci Int. 2012;222:e1–e6. doi: 10.1016/j.forsciint.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 87.Gil D, Adamowicz P, Skulska A, Tokarczyk B, Stanaszek R. Analysis of 4-MEC in biological and non-biological material-three case reports. Forensic Sci Int. 2013;228:e11–e15. doi: 10.1016/j.forsciint.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 88.Adamowicz P, Tokarczyk B, Stanaszek R, Slopianka M. Fatal mephedrone intoxication—a case report. J Anal Toxicol. 2013;37:37–42. doi: 10.1093/jat/bks085. [DOI] [PubMed] [Google Scholar]
- 89.Johnson RD, Botch-Jones SR. The stability of four designer drugs: MDPV, mephedrone, BZP and TFMPP in three biological matrices under various storage conditions. J Anal Toxicol. 2013;37:51–55. doi: 10.1093/jat/bks138. [DOI] [PubMed] [Google Scholar]
- 90.Cosbey SH, Peters KL, Quinn A, Bentley A. Mephedrone (methylmethcathinone) in toxicology casework: a Northern Ireland perspective. J Anal Toxicol. 2013;37:74–82. doi: 10.1093/jat/bks094. [DOI] [PubMed] [Google Scholar]
- 91.Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J Anal Toxicol. 2013;37:135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- 92.Adamowicz P, Gil D, Skulska A, Tokarczyk B. Analysis of MDPV in blood—determination and interpretation. J Anal Toxicol. 2013;37:308–312. doi: 10.1093/jat/bkt025. [DOI] [PubMed] [Google Scholar]
- 93.Zuba D, Adamowicz P, Byrska B. Detection of buphedrone in biological and non-biological material—two case reports. Forensic Sci Int. 2013;227:15–20. doi: 10.1016/j.forsciint.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 94.Anizan S, Ellefsen K, Concheiro M, Suzuki M, Rice KC, Baumann MH, Huestis MA. 3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography–high resolution mass spectrometry. Anal Chim Acta. 2014;827:54–63. doi: 10.1016/j.aca.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Postmortem distribution of α-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Leg Med. 2014;16:241–246. doi: 10.1016/j.legalmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Usui K, Aramaki T, Hashiyada M, Hayashizaki Y, Funayama M. Quantitative analysis of 3,4-dimethylmethcathinone in blood and urine by liquid chromatography–tandem mass spectrometry in a fatal case. Leg Med. 2014;16:222–226. doi: 10.1016/j.legalmed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 97.Bertol E, Mari F, Berto RB, Mannaioni G, Vaiano F, Favretto D. A mixed MDPV and benzodiazepine intoxication in a chronic drug abuser: determination of MDPV metabolites by LC–HRMS and discussion of the case. Forensic Sci Int. 2014;243:149–155. doi: 10.1016/j.forsciint.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in a fatal poisoning case. Forensic Toxicol. 2014;32:243–250. [Google Scholar]
- 99.Bijlsma L, Sancho JV, Hernández F, Niessen WMA. Fragmentation pathways of drugs of abuse and their metabolites based on QTOF MS/MS and MSEE accurate-mass spectra. J Mass Spectrom. 2011;46:865–875. doi: 10.1002/jms.1963. [DOI] [PubMed] [Google Scholar]
- 100.Patton AL, Chimalakonda KC, Moran CL, McCain KR, Radominska-Pandya A, James LP, Kokes C, Moran JH. K2 toxicity: fatal case of psychiatric complications following AM2201 exposure. J Forensic Sci. 2013;58:1676–1680. doi: 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Springer D, Fritschi G, Maurer HH. Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4′-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography–mass spectrometry. J Chromatogr B. 2003;796:253–266. doi: 10.1016/j.jchromb.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Sauer C, Peters FT, Haas C, Meyer MR, Fritschi G, Maurer HH. New designer drug alpha-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom. 2009;44:952–964. doi: 10.1002/jms.1571. [DOI] [PubMed] [Google Scholar]
- 103.Tyrkkö E, Pelander A, Ketola RA, Ojanperä I. In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem. 2013;405:6697–6709. doi: 10.1007/s00216-013-7137-1. [DOI] [PubMed] [Google Scholar]
- 104.Namera A, Konuma K, Kawamura M, Saito T, Nakamoto A, Yahata M, Ohta S, Miyazaki S, Shiraishi H, Nagao M. Time-course profile of urinary excretion of intravenously administered α-pyrrolidinovalerophenone and α-pyrrolidinobutiophenone in a human. Forensic Toxicol. 2014;32:68–74. [Google Scholar]
- 105.Kovács K, Tóth AR, Kereszty EM. A new designer drug: methylone related death. Orv Hetil. 2012;153:271–276. doi: 10.1556/OH.2012.29310. [DOI] [PubMed] [Google Scholar]
- 106.Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, Holler JM. Distribution of methylone in four postmortem cases. J Anal Toxicol. 2012;36:434–439. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- 107.Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202:e62–e63. doi: 10.1016/j.forsciint.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 108.Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic Sci Int. 2011;206:e93–e95. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 109.Hasegawa K, Suzuki O, Wurita A, Minakata K, Yamagishi I, Nozawa H, Gonmori K, Watanabe K. Postmortem distribution of α-pyrrolidinovalerophenone and its metabolite in body fluids and solid tissues in a fatal poisoning case measured by LC–MS–MS with the standard addition method. Forensic Toxicol. 2014;32:225–234. [Google Scholar]
- 110.Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci. 2013;58:1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]


